Serum chitinase-3-like protein 1 is a biomarker of liver fibrosis in patients with chronic hepatitis B in China
2020-09-21XinJinBinFuZhengJieWuXioQinZhengJinHuHuLinFengJinLingLingTng
Xin Jin , Bin Fu , Zheng-Jie Wu , Xio-Qin Zheng , Jin-Hu Hu , Lin-Feng Jin ,Ling-Ling Tng , c , *
a State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b College of Medicine, Shaoxing University, Shaoxing 3120 0 0, China
c Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University, Shulan International Medical College, Hangzhou 310022, China
Keywords:
ABSTRACT
Introduction
Hepatitis B virus (HBV) can cause a chronic and continuous infection due to immune nonresponse in humans.In 2016, the worldwide prevalence of HBsAg was 3.9%, representing 350 million infected people [1].Sustained HBV infection causes liver inflammation, necrosis, fibrosis, cirrhosis and even hepatocellular carcinoma(HCC).Moreover, significant fibrosis is considered a trait reflecting the progressive nature of chronic HBV infection and requires antiviral treatment [2].Effective treatment can reverse fibrosis progression in the early stage [3].Early detection of liver fibrosis is therefore essential for early intervention [4 , 5].
Invasive liver biopsy is the gold standard for assessing necrosis, inflammation (grading) and fibrosis (staging).However, its invasiveness, potential complications, sampling error and many other factors limit its application [6].Furthermore, it is unrealistic to use liver biopsy to monitor disease progression.Numerous researchers have extensively attempted to develop noninvasive tools to evaluate the stage of hepatic fibrosis and monitor its progression [7-9].Transient elastography (TE) is a fast, reliable, repeatable, and noninvasive approach for evaluating liver fibrosis and monitoring its progression.However, many confounding factors affect the liver stiffness (LS) and therefore, impact the diagnostic accuracy of TE on liver fibrosis.These factors include obesity, acute edema, tissue inflammation and alcohol consumption [10].Traditional serological markers for liver assessment include hyaluronan,the n-terminal peptide of type III procollagen (PIIINP), laminin (LN)and type IV collagen (Col-IV).However, they tend to be influenced by liver inflammation [11].In addition, many noninvasive fibrosis tests are used in daily practice, such as the fibrosis index that is based on four factors (FIB-4) and the aspartate aminotransferase to platelet ratio index (APRI) [12 , 13].Previous studies have evaluated and validated the aforementioned noninvasive methods in hepatitis C virus [14 , 15]or HBV patients [16 , 17], but they also display some inevitable inefficiencies such as low diagnostic accuracy.It is imperative to find new noninvasive tools to evaluate hepatic fibrosis.
Chitinase-3-like protein 1 (CHI3L1) is a member of the "mammalian chitinase-like proteins" and is secreted as a 40-kDa glycoprotein [18].It was reported that CHI3L1 mRNA expression displayed a tissue-specific pattern [19].Moreover, hepatic CHI3L1 expression is related to the progression of liver diseases, with the highest levels reflecting irreversible cirrhosis [20].Thus, in this study, we aimed to assess the diagnostic value of serum CHI3L1 for liver fibrosis in patients with chronic HBV infections.
Methods
Study subjects
A total of 134 chronic hepatitis B (CHB) individuals who were treated at the First Affiliated Hospital of Zhejiang University School of Medicine from October 2016 to May 2018 were enrolled in this investigation.The inclusion criteria were as follows: (1) positive HBsAg for at least 6 months; (2) ALT levels ≤2 upper limit of normal (ULN = 50 U/L); and (3) age between 18 and 60 years.The exclusion criteria were as follows: (1) pregnancy; (2) severe diseases of the heart, lung, brain, kidney, blood system or other organs; (3) liver diseases due to other etiologies; and (4) HCC, extrahepatic neoplasia or intrahepatic cholangiocarcinoma.
The study protocol was reviewed and sanctioned by the Research Ethics Committee of The First Affiliated Hospital of Zhejiang University School of Medicine (No.2018-918).Informed consent was obtained from all the patients.Our study was registered with the Chinese Clinical Trial Registry (Number: ChiCTR190 0 022176,http://www.chictr.org/cn/).
Determination of serum CHI3L1
Blood samples were collected from patients who had undergone TE and were kept in a -80 °C freezer until measurements.Serum CHI3L1 concentration was determined using a CHI3L1 ELISA kit in accordance with the manufacturer's protocol (Hangzhou Proprium Biotech Co., Ltd., Hangzhou, China).
Liver biopsy
Fifty of 134 patients underwent liver biopsies.Liver biopsies were assessed by two experienced hepatopathologists blinded to the patients' clinical data.Liver biopsy samples were taken via the right intercostal space from the right liver lobe.All biopsy cores were at least 1 cm in length and consisted of at least three portal tracts.The liver biopsy specimens were fixed, paraffin-embedded,and stained with hematoxylin and eosin (HE).Histological grading of necroinflammatory activity (G) and staging of fibrosis (S) were determined according to Scheuer's classification [21].Necroinflammatory activity grade (G) was divided into; G0, no activity; G1,minimal activity; G2, mild activity; G3, moderate activity; and G4,severe activity.Liver fibrosis stages (S) were divided into no fibrosis(S0); enlarged, fibrotic portal fibrosis (S1); septum formation (S2);fibrosis with architectural distortion (S3); and cirrhosis (S4).
TE
TE was performed using FibroTouch (Wuxi Hisky Medical Technology Co., Ltd., Wuxi, China).The technical background and examination procedure have been described previously [22].Measurements were performed on the right lobe of the liver through the right intercostal space at a depth of 25 and 65 mm below the skin surface.Measurements were performed by two independent and experienced technicians.The median LS of 10 valid FibroTouch measurements (with their interquartile ranges) was expressed in kilopascals (kPa).Fibrosis was staged according to the METAVIR scoring system as follows: F0, ≤ 7.3 kPa; F1,>7.3 kPa but ≤ 9.7 kPa; F2,>9.7 kPa but ≤ 12.4 kPa; F3,>12.4 kPa but ≤17.5 kPa; and F4,>17.5 kPa.For this study, significant fibrosis was defined as a liver stiffness (LS)>9.7 kPa [2].
Clinical characteristics and noninvasive fibrosis tests
We collected demographic information and clinical data from the electronic medical record, including age, sex, and body mass index (BMI).Routine blood tests, liver biochemical tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT),hyaluronic acid (HA), etc.], as well as HBV biomarkers, were collected within the same period.
APRI and FIB-4 for the noninvasive evaluation of fibrosis were calculated using the following formulas:APRI = [(AST/ULN)/platelet(× 109/L]× 100 and FIB-4 =(age × AST)/[platelet(× 109/L) × ALT1/2 ].
Statistical analysis
The data were analyzed using GraphPad Prism version 7.0(GraphPad Software Inc., California, CA, USA), MedCalc version 16.8(MedCalc, Ostend, Belgium) and SPSS version 19.0 (SPSS Inc., New York, NY, USA).Continuous variables with a normal distribution were shown as the mean ±standard deviation (SD), whereas those not normally distributed were shown as the median (interquartile range).Intergroup comparisons were performed using the Kruskal-Wallis test, one-way ANOVA, Mann-WhitneyUtest or Student'st-test for continuous variables and theχ2test for categorical variables.The correlation coefficient was calculated using the Pearson correlation analysis.Receiver operating characteristic (ROC) curves and areas under the ROC curve (AUC) were calculated to evaluate CHI3L1 for liver fibrosis stages.The optimal cut-offvalue was chosen as that with the best sensitivity and specificity in the AUC analysis (Youden's index).APvalue of<0.05 was considered statistically significant.
Results
Patient characteristics
From October 2016 to May 2018, a total of 134 CHB patients(90 males and 44 females) were enrolled.The median age of the patients was 39 (32-48) years, the median LS was 7.8 (6.2-10.6)kPa, and the median concentration of CHI3L1 was 61.4 (48.6-84.8)ng/mL.All demographic and clinical characteristics of the 134 CHB patients are summarized in Table 1.
With reference to the LS value of 9.7 kPa [2], the 134 patients were categorized as those without significant hepatic fibrosis (<F2,n= 90, 67.16%) and those with significant fibrosis (≥ F2,n= 44, 32.84%).We found that patients with significant fibrosis were older than those without significant fibrosis (44 vs.36 years,P= 0.002).Patients with significant fibrosis showed higher levels of AST, ALP, GGT, and Col-IV but lower concentrations of albumin (ALB) than those without significant fibrosis (P<0.05).However, sex ratios, BMI, ALT levels and liver fibrosis markers (HA, PIIINP, LN) were not significantly different between the two groups(P>0.05).More importantly, serum CHI3L1 levels were found to be substantially higher in patients with significant hepatic fibrosis(≥F2) than in those without significant hepatic fibrosis (<F2) (81.8 vs.56.5 ng/mL,P<0.001, Table 2).
The association of serum CHI3L1 with LS
The Pearson correlation coefficients of serum CHI3L1 with other clinical parameters in subjects with chronic HBV infection are provided in Table 2.We found that CHI3L1 was correlated with sev-eral clinical parameters, including LS, APRI, and FIB-4.However, the highest correlation was found for LS (r= 0.580,P<0.001, Fig.1 A).

Table 1Demographic and clinical characteristics of chronic hepatitis B patients.

Table 2Pearson correlation coefficient of CHI3L1 with other clinical parameters in chronic hepatitis B patients.
We next compared the levels of CHI3L1 among the patients with different METAVIR scores (Fig.1 B).The median serum concentrations of CHI3L1 in the F0, F1, F2, F3 and F4 groups were 55.3(42.6-76.3) ng/mL, 59.1 (50.0-74.2) ng/mL, 62.3 (53.7-83.6) ng/mL,152.1 (82.5-201.8) ng/mL, and 154.1 (119.6-240.0) ng/mL, respectively.
The serum CHI3L1 levels were significantly increased as METAVIR scores were increased (F0-1 to F4) (P<0.001), and serum CHI3L1 levels were significantly different between fibrosis stages (F0 vs.F3 and F4; F1 vs.F3 and F4; F2 vs.F3 and F4;P<0.05).Furthermore, the difference in serum CHI3L1 levels was not significant between the groups at the early stage (F0, F1 and F2;P>0.05, Fig.1 B).These findings indicated that serum levels of CHI3L1 were correlated with the stage of liver fibrosis.
Correlation of serum CHI3L1 with other clinical parameters
To explore the relationship between serum CHI3L1 levels and other clinical parameters that reflected severe hepatic fibrosis in Table 1 , we conducted Pearson correlation analysis.The results revealed that increased levels of CHI3L1 in serum were positively correlated with AST levels (r= 0.499,P<0.001), ALP levels(r= 0.300,P<0.001), and GGT levels (r= 0.421,P<0.001), and were negatively correlated with ALB levels (r= -0.261,P= 0.003;Fig.2).
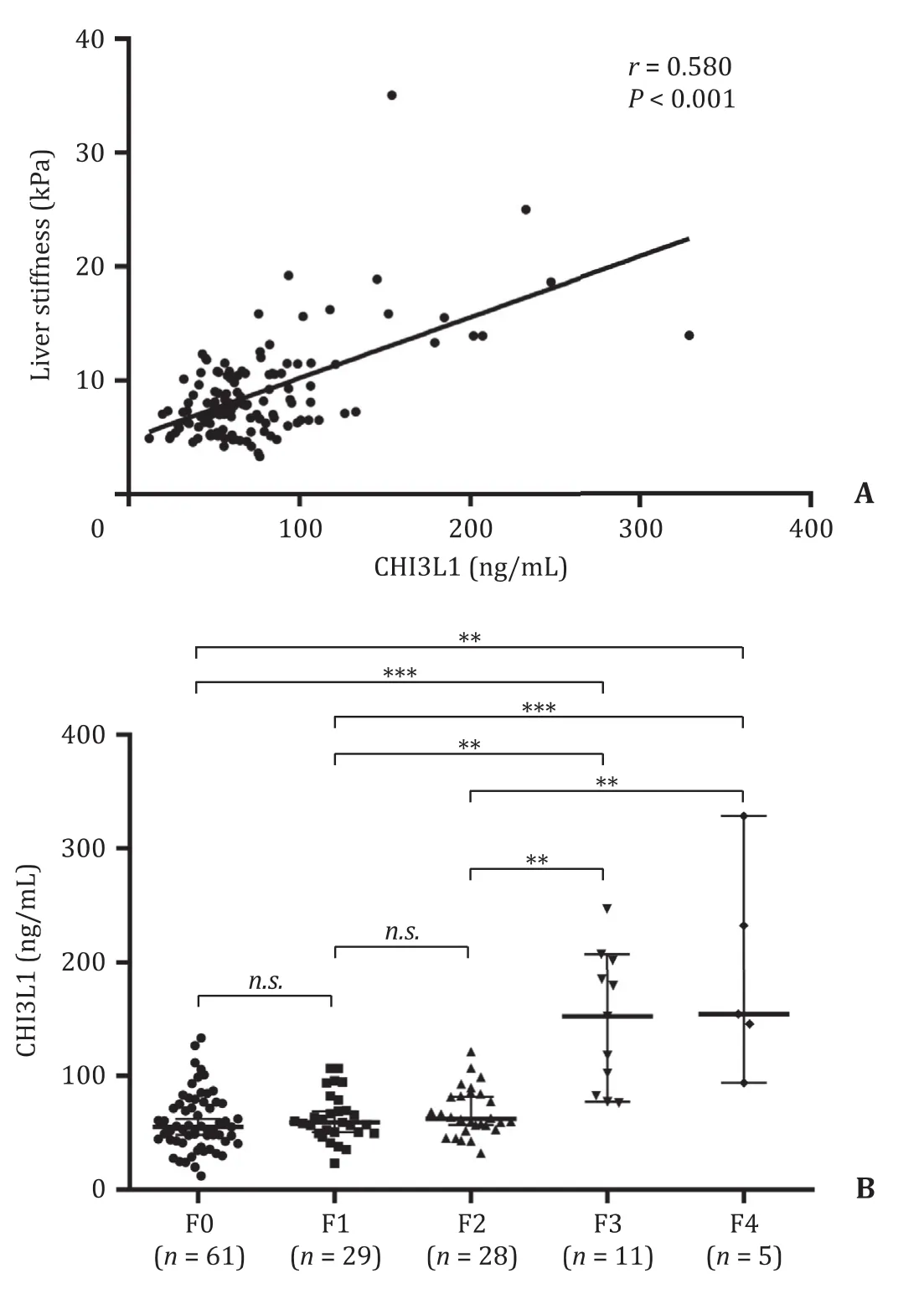
Fig.1.Serum CHI3L1 levels were significantly correlated with HBV related fibrosis.A: The correlation analysis between CHI3L1 and liver stiffness in patients with chronic HBV infection; B: Serum CHI3L1 levels in various stages of liver fibrosis by FibroTouch in chronic hepatitis B patients.Solid line: linear growth trend; R: correlation coefficient.n.s.: no significant.**: P < 0.01; ***: P < 0.001.CHI3L1: chitinase-3-like protein 1; HBV: hepatitis B virus.
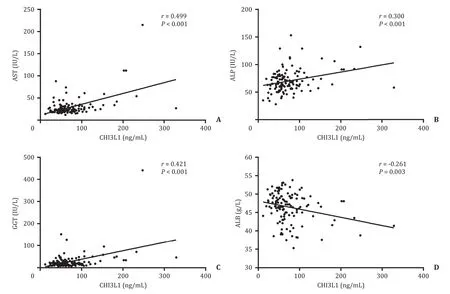
Fig.2.Correlation between serum CHI3L1 levels and other laboratory indexes in chronic hepatitis B patients.A : AST; B: ALP; C: GGT; D: ALB.Pearson correlation test was used for correlation analysis.Solid line: linear growth trend; R: correlation coefficient.AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma glutamyltranspeptidase; ALB: albumin; CHI3L1: chitinase-3-like protein 1.
The role of serum CHI3L1 in detecting significant liver fibrosis in CHB patients
Liver fibrosis ≥F2 is an indicator for the initiation of antiviral treatment for patients with chronic HBV infection based on several national and international HBV treatment guidelines [2].Moreover, a noninvasive method to separate patients with liver fibrosis ≥ F2 or<F2 is highly desirable, particularly for those with normal ALT, who are usually reluctant to undergo a liver biopsy.Therefore, we sought to explore the usefulness of serum CHI3L1 in discriminating no significant fibrosis (<F2) from significant fibrosis (≥F2).ROC curve analysis showed that the specificity and sensitivity of CHI3L1 in predicting significant fibrosis were 75.6%and 59.1%, respectively, and the cut-offvalue was 76.0 ng/mL.The AUC was 0.728 (95% CI: 0.637-0.820) (Fig.3).Given that significant fibrosis (≥ F2) was defined here by LS>9.7 kPa, the median serum concentration in individuals with ≥F2 was 81.9 ng/mL,which was greater than the median concentration in individuals with<F2 (56.5 ng/mL,P<0.001).Our data indicated that CHI3L1 levels could be used to differentiate liver fibrosis<F2 from ≥ F2.
Furthermore, the performance of FIB-4 and APRI noninvasive tests was compared using comparative ROC analysis.We found that CHI3L1 displayed a better performance than the APRI score(AUC = 0.583; 95% CI: 0.472-0.694;P= 0.036) in predicting significant hepatic fibrosis (≥F2) (Fig.3), but its performance did not significantly differ from that of the FIB-4 score (AUC = 0.631; 95%CI: 0.521-0.741;P= 0.178).Thus, serum CHI3L1 serves as a noninvasive marker for identifying chronic HBV patients with ≥F2 liver fibrosis and thus can be used as a reference to select patients requiring antiviral treatments.

Fig.3.ROC curves, sensitivity and specificity.ROC curve analysis of serum CHI3L1,APRI and FIB-4 in the prediction of significant fibrosis in chronic hepatitis B patients.ROC: receiver operating characteristic; CHI3L1: chitinase-3-like protein 1;APRI: aspartate aminotransferase to platelet ratio index; FIB-4: fibrosis index based on the four factors.
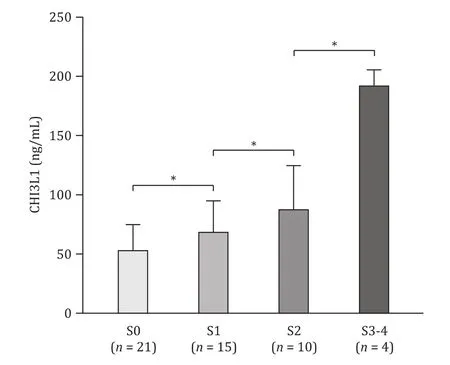
Fig.4.Correlation between serum CHI3L1 levels and liver biopsy fibrosis stage in chronic hepatitis B patients.Statistics analysis was performed by Mann-Whitney U test.*: P < 0.05.CHI3L1: chitinase-3-like protein 1.
CHI3L1 varies with liver biopsy fibrosis stage
Fifty CHB patients underwent liver biopsies in our study.According to liver fibrosis stages (S0-S4), 21 patients had no fibrosis(S0), 15 had mild fibrosis (S1), 10 were staged as S2, and four had S3 fibrosis or cirrhosis (S4).As shown in Fig.4 , the results showed that serum CHI3L1 levels were increased with fibrosis stage in patients with HBV-related liver diseases (S0 to S3-4) (P<0.05).
Discussion
CHI3L1, a gene located on chromosome 1q32.1 [23], is expressed by various cells, including monocytes, macrophages, neutrophils, hepatic stellate cells, chondrocytes and synovial cells.Current research on CHI3L1 focuses on cancer patients [24].Immunohistochemical analysis showed that CHI3L1 stained positive in areas with active fibrogenesis, and the degree of positive staining could reflect the fibrosis stage [25].In addition, CHI3L1 is a liverspecific or highly liver-enriched gene, and its expression is abundant.Previous studies have found that CHI3L1 mostly correlates with the natural history and severity of many liver diseases [26 , 27].It was reported that CHI3L1 could predict advanced fibrosis in patients with chronic hepatitis C [28]and nonalcoholic fatty liver disease [29].Previous literature showed that the CHI3L1 levels of healthy controls were lower than those of CHB patients [30].Thus, this study aimed to explore serum CHI3L1 as a noninvasive diagnostic marker in chronic HBV-infected patients.We showed that serum CHI3L1 levels were increased with fibrosis progression.CHI3L1 might be a potential biomarker for distinguishing patients with significant fibrosis from those without significant fibrosis in chronic HBV infection.
It is currently believed that liver fibrosis persistently and gradually decreases with long-term antiviral treatment in patients with chronic HBV infection.Thus, periodic liver fibrosis evaluation is necessary, especially in patients with significant fibrosis.However,many methods, including liver biopsy and TE, have limitations in some situations.CHI3L1 is a noninvasive measurement and can be detected repeatedly in easy-to-obtain specimens in the same patient.If serum CHI3L1 levels are significantly increased in patients with chronic HBV with normal or slightly increased ALT or there are inconsistencies with other noninvasive tools, liver biopsy is recommended to examine the severity of hepatic fibrosis, thereby monitoring disease progression and determining timely treatment.Thus, CHI3L1 has great potential for widespread use in patients with chronic HBV infection.
Previous studies on chronic HBV showed that patients with substantial hepatic fibrosis were older than those without significant hepatic fibrosis [31 , 32].Our study was in line with others.The progression of liver fibrosis is time-dependent given that the repeated replication of HBV causes tissue damage and repair, which eventually results in liver fibrosis.Patients with significant fibrosis have pronounced fibrous proliferation in the portal area, tissue damage, and bile duct damage.It was further revealed that the levels of AST, GGT and ALP were higher, whereas the level of ALB was lower in patients with significant liver fibrosis.These findings were in agreement with other studies [33-35].Moreover, Pearson correlation analysis revealed that serum CHI3L1 levels were strongly and positively correlated with these serological indexes, which reflected severe hepatic fibrosis.
Currently, the molecular mechanism of increased CHI3L1 expression in fibrosis is still under investigation.Numerous studies have indicated that CHI3L1 is a growth factor of fibroblasts as well as endothelial cells, and its expression in the active human liver fibrotic area is high [18 , 36 , 37].In particular, hepatic stellate cells(HSCs) contain CHI3L1 mRNA.CHI3L1 has been reported to trigger the proliferation of HSCs and facilitate the formation of fibrotic cells.In addition, CHI3L1 may activate several signaling pathways.For instance, CHI3L1 combines with interleukin-13 receptor a2 (IL-13Ra2) and activates Wnt/β-catenin signaling, macrophage mitogen-activated protein kinase, protein kinase B/AKT and TGF-β1 production [38].TGF-β1 promotes the production of hepatic fibrosis proteins [39].In addition, as a component of secreted extracellular matrix protein, CHI3L1 is involved in the formation of fibrosis and matrix remodeling [40].CHI3L1 mediates the formation of a positive feedback loop through signaling pathways, which accelerates the fibrosis process.
The limitation of the present study is that liver fibrotic status was mainly based on TE.As we know, some factors, such as liver congestion, hyperbilirubinemia, transaminitis, and prolonged prothrombin time, may impact the TE value [41].CHI3L1 may compensate the shortcomings of TE.
In summary, we found that CHI3L1 correlated well with LS.The increase of serum CHI3L1 levels was parallel with the escalation of METAVIR scores in patients with HBV-related liver diseases.The easy usage of CHI3L1 can serve as a routine laboratory test in monitoring disease progression to benefit chronic HBV patients.The combination of TE and CHI3L1 might reduce the necessity of liver biopsy.
Acknowledgments
We thank Professor Biao-Yang Lin for his kindly assistance.
CRediT authorship contribution statement
Xin Jin:Data curation, Formal analysis, Writing - original draft.Bin Fu:Data curation, Software, Investigation.Zheng-Jie Wu:Methodology, Investigation.Xiao-Qin Zheng:Resources, Validation.Jian-Hua Hu:Resources, Investigation.Lin-Feng Jin:Formal analysis, Visualization.Ling-Ling Tang:Conceptualization, Supervision,Funding acquisition, Writing - review & editing.
Funding
This study was supported by grants from the National Science and Technology Major Project (2017ZX10204401), and the Zhejiang Public Welfare Technology Applied Research Project(LGF18H160014).
Ethical approval
The study was approved by the Research Ethics Committee of The First Affiliated Hospital of Zhejiang University School of Medicine (No.2018-918).
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Endoscopic papillary large balloon dilation with or without sphincterotomy for large bile duct stones removal: Short-term and long-tem outcomes
- Two-in-one: A pooled analysis of primary hepatic neuroendocrine carcinoma combine d/collide d with hepatocellular carcinoma
- A ten-year experience of inferior vena cava reconstruction for malignancy: The importance of a multidisciplinary approach with hepatobiliary surgery
- New variation of median arcuate ligament compression causing hepatic arterial hypoperfusion during liver transplantation
- Echinococcus granulosus: A novel parenchymal sparing surgical treatment
- Role of selected criteria and preventive chemotherapy in tumor recurrence after liver transplantation
