Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases
2020-07-06PreethiVijayaraghavareddyYinXinyouPaulStruikUdayakumarMakarlaSheshshayeeSreeman
Preethi Vijayaraghavareddy, Yin Xinyou, Paul C. Struik, Udayakumar Makarla Sheshshayee Sreeman
Research Paper
Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases
Preethi Vijayaraghavareddy1,2, Yin Xinyou2, Paul C. Struik2, Udayakumar Makarla1, Sheshshayee Sreeman1
(; )
Rice yield reduction due to water limitation depends on its severity and duration and on the phenological stage of its occurrence. We exposed three contrasting rice genotypes, IR64, UPLRi7 and Apo (adapted to lowland, upland and aerobic conditions, respectively), to three water regimes (puddle, 100% and 60% field capacity) in pots during the vegetative (GSI), flowering (GSII) and grain filling (GSIII) stages. Stress at all the three stages significantly reduced yield especially in lowland genotype IR64. Effect of water limitation was more severe at GSII than at the other two stages. Stress at GSI stage reduced both source activity (leaf area and photosynthetic rate) and sink capacity (tiller number or panicle number per pot). When stress was imposed at GSII, spikelet fertility was most affected in all the three genotypes. In both GSII and GSIII, although leaf area was constant in all the three water regimes, estimated relative whole-plant photosynthesis was strongly associated with yield reduction. Reduced photosynthesis due to stress at any given stage was found to have direct impact on yield. Compared to the other genotypes, Apo had deeper roots and maintained a better water relation, thus, higher carbon gain and spikelet viability, and ultimately, higher biomass and productivity under water-limited conditions. Therefore, screening for these stage-dependent adaptive mechanisms is crucial in breeding for sustained rice production under water limitation.
water limitation; phenology; upland; aerobic;; yield
Rice is a major staple food crop, providing 35%–80% of total calories required for populations of Asia (IRRI, 1997; Yang et al, 2009). It is mainly grown in semi-aquatic environments in paddies, and rice production in such a system requires 3000–4000 L of water to produce 1 kg of grains (Bouman, 2009). Most rice production is in irrigated lowland ecosystems in Asia. However, the industrial and household requirements are rapidly withdrawing fresh water away from agriculture in this region. This urgently necessitates efforts towards devising water saving agronomies as well as improving (or at least sustaining) rice productivity under water-limited conditions (Bouman and Tuong, 2001).
Many efforts have been made to modify cultivation practices, like SRI (system of rice intensification) (Uphoff et al, 2010), upland (Nishimura et al, 2008), rainfed lowland (Zeigler and Puckridge, 1995) and aerobic (Kadiyala et al, 2012) systems, to reduce water consumption in rice production. The emphasis in these systems has generally been to decrease the ‘E’ component of ‘ET’ (Evapo-Transpiration). However, these systems have one thing in common: yields are reduced compared to the irrigated lowland (paddy) ecosystem. Efforts have been made to identify genotypes with good yields in upland and aerobic conditions. Although these genotypes were selected for cultivation in water-limited conditions, yield penalties are still severe. Under water-limited conditions, reduced field capacity (FC) has a major impact on yield depending on crop growth stage that coincides with the stress, its intensity and duration. Understanding the physiological mechanisms that get affected under stress and assessing the variability in these mechanisms among various genotypes is crucial for further crop improvement.
Three growth stages, i.e. vegetative (GSI), flowering (GSII) and grain filling (GSIII) stages, are characterized as distinctive growth periods that are differentially influenced by biotic or abiotic stresses (Boonjung and Fukai, 1996; Sarvestani et al, 2008). At all phenological stages, water limitation potentially affects size and capacity of both source and sink for rice growth. Stress at GSI can impede leaf expansion (thus reducing source size) and inhibit tillering (thus reducing both sink and source). The carbon assimilatory capacity of the canopy remains small due to a reduced photosynthetic rate combined with the reduction in canopy leaf area. These factors can lead to lower final biomass production (Praba et al, 2009). GSII is the most critical stage of yield formation, and water limitation at this stage can adversely affect both carbon source and reproductive development, resulting in reduced yields (Jin et al, 2013). GSIII also represents a critical stage, during which reduction in the grain yield can be caused by reduced leaf photosynthesis as well as lower green leaf area by advanced leaf senescence.
Rice varieties that adapt to different ecosystems with diverse adaptive strategies to grow under water-limited conditions have been developed, with better extraction of water by roots and increased cellular-level tolerance (CLT) under stress (Raju et al, 2014), increased water use efficiency (WUE) (Sheshshayee et al, 2003) andreduced leaf transpiration through wax layers (Zhang et al, 2007). Plants with good root systems can maintain better tissue water potential with cooler canopy (Ramu et al, 2016) and that plants with high CLT have improved spikelet fertility in stress conditions (Raju et al, 2014). Significant progress has also been made in developing suitable cultivars for greater adaptation to diverse rice growing agronomic practices, which leads to the identification of UPLRi7 for upland conditions and Apo for aerobic cultivation (Ouyang et al,2017). IR64, a mega variety in Southeast Asian (Mackill et al, 2018), is predominantly grown in puddled conditions and is known to be sensitive to water limitation (Dharmappa et al, 2019).
The objective of this study was to understand the stress response of these three rice genotypes known to adapt to upland, aerobic and lowland rice ecosystems, respectively, and to assess their yielding ability under stress imposed at GSI, GSII and GSIII stages.
Materials and methods
Plant growth and stress conditions
Three rice genotypes, which were bred for lowland (IR64), upland (UPLRi7) and aerobic (Apo) conditions, were selected because they had similar phenology. For instance, all the three genotypes took 85–90 d to reach 50% flowering (Ouyang et al,2017). Pot experiments were conducted in the University of Agricultural Sciences, Bengaluru, India. Experiments were done in an open area under a mobile rain-out shelter, which protected plants against rainfall. Whenever required and during nights the shelter was drawn over the experimental area, thus pots were maintained at the specified water regime. Pots (24 L) were filled with equal amounts (20kg) of red soil with recommended fertilizers added. Direct sowing was followed, and thinning was done at 21 days after sowing (DAS) to maintain one plant per pot. Plants were well watered manually until the stress imposition. During treatments, three water regimes were maintained viz., puddle, 100% FC and 60% FC. Puddle treatment was created with standing water of 5 cm above the soil in the pot. 100% FC and 60% FC treatments were maintained following the gravimetric approach (Udayakumar et al, 1998): the pots were weighed daily using a hanging load cell balance (Essae-Teraoka, Japan), the reduction in weight of the pots as a result of evapo-transpiration was recorded, and on the early morning the pots were replenished with the exact same amount of water evapo-transpired in the preceding day to maintain a given FC. There were five replicates for each treatment and arranged in randomized-block design. Three separate experiments were conducted for each of the three phenological stages.
GSI experiment was conducted from January to April of 2016, in which the three water regimes were maintained at the time of maximum tillering stage (30 to 50 DAS).
GSII experiment was conducted from July to October 2016, in which the three water regimes were maintained particularly at the flowering (85 DAS). Water was withheld from flag-leaf stage, such that the required stress level was reached at the time of flowering, and maintained for 10 d.
GSIII experiment was conducted from July to October of 2017, in which the three water regimes were maintained after flowering (100 DAS) until grain maturity.
Relative water content
To assess the effect of different water regimes on tissue water status, relative water content (RWC) was measured. Ten leaf discs of 1cm2each were punched out of the second fully expanded leaf counted from the apex. Fresh weight was recorded immediately. The leaf discs were immersed in distilled water in a beaker for 6 h,and were gently blotted on a filter paper to remove the water adhering to the surface. Fresh weight of this tissue was taken to represent turgid weight. Then, the samples were dried in an oven (65 ºC–70ºC) until constant weight, and then dry weight was recorded. RWC (%) was estimated according to Babitha et al (2015).
Gas exchange
Leaf net-photosynthetic rate () and stomatal conductance (s) were measured with a portable gas-exchange system Li-6400 (LiCOR-Inc, Lincoln, Nebraska, USA). The ambient concentration of CO2in the leaf chamber of the Li-6400 was kept at 701.8 mg/m3, and the photosynthetically active radiation (PPFD) was 1500μmol/(m2·s). All measurements were made at 5 d after stress imposition on the second fully expanded leaf from the top for the GSI experiment, and on the flag leaf for the GSII and GSIII experiments.
Soil and plant analyzer develotrnent (SPAD) value
SPAD value was calculated by averaging nine readings per leaf using a portable, non-destructive chlorophyll meter (SPAD-502, Minolta, Japan). Mean of the measurements was calculated to arrive at a single value per pot. Measurements were recorded at 5 d after reaching 60% FC.
Morpho-physiological parameters
Plant height and number of tillers were recorded before harvest. Plant height was measured from surface of soil to tip of the longest leaf. Roots were separated from shoot to measure root length before drying. All the plant parts were oven-dried at 70ºC to record stem weight, leaf weight and root weight, and total biomass per pot (TBM) were calculated. Specific leaf area was calculated by dividing fresh leaf area by its dry biomass. Total leaf area (TLA) per pot was calculated by multiplying final dry leaf biomass with specific leaf area, which was determined from a subsample of leaves.
Yield parameters
Panicles were harvested from individual plants after maturity. Grains were manually separated from panicles. Spikelet fertility was calculated as the number of filled grains divided by the total number of grains.Weight of filled grains was recorded to obtain grain yield per pot.
Statistical analysis
Two-way ANOVA was conducted by using GenStat (15thedn) (http://www.genstat.co.uk/). The generated least significant difference for each parameter was used to check the significance level in all the three experiments. Standard correlation/regression procedures were performed using Microsoft Excel.
Results
Effect of stress at GSI (vegetative stage)
RWC, a simple proxy for water relations, decreased significantly as stress level increased in all the genotypes (Fig. 1-A). IR64 showed 10.1% and 17.1% reductions in RWC under 100% FC and 60% FC, respectively, relative to the puddle treatment, while UPLRi7(10.0% and 13.9%) and Apo (0.5% and 2.8%) showed significantly lower reductions in RWC (Fig. 1-A). SPAD value was not affected in any of the genotypes (Supplemental Fig. 1-A). Gas exchange parameters like stomatal conductance (s) and net assimilation rate () decreased with increasing stress in all the genotypes, albeit to different extents. Whiledecreased by 33.0% and 54.8% for IR64 under 100% FC and 60% FC compared to puddle, this reduction was much less for UPLRi7 (11.7% and 34.0%) and Apo (19.4% and 27.5%) (Fig.1-B). UPLRi7 maintained higher stomatal conductance compared to the other two genotypes and it was highest with 0.7 mol/(m2·s) under puddle condition.sdecreased significantly in all three genotypes with highest reduction for IR64 and UPLRi7 under 60% FC (Fig. 1-C). Apo recorded lowersunder the two stress regimes and a lower decrease insunder 60% FC compared to the other two genotypes (Fig. 1-C).
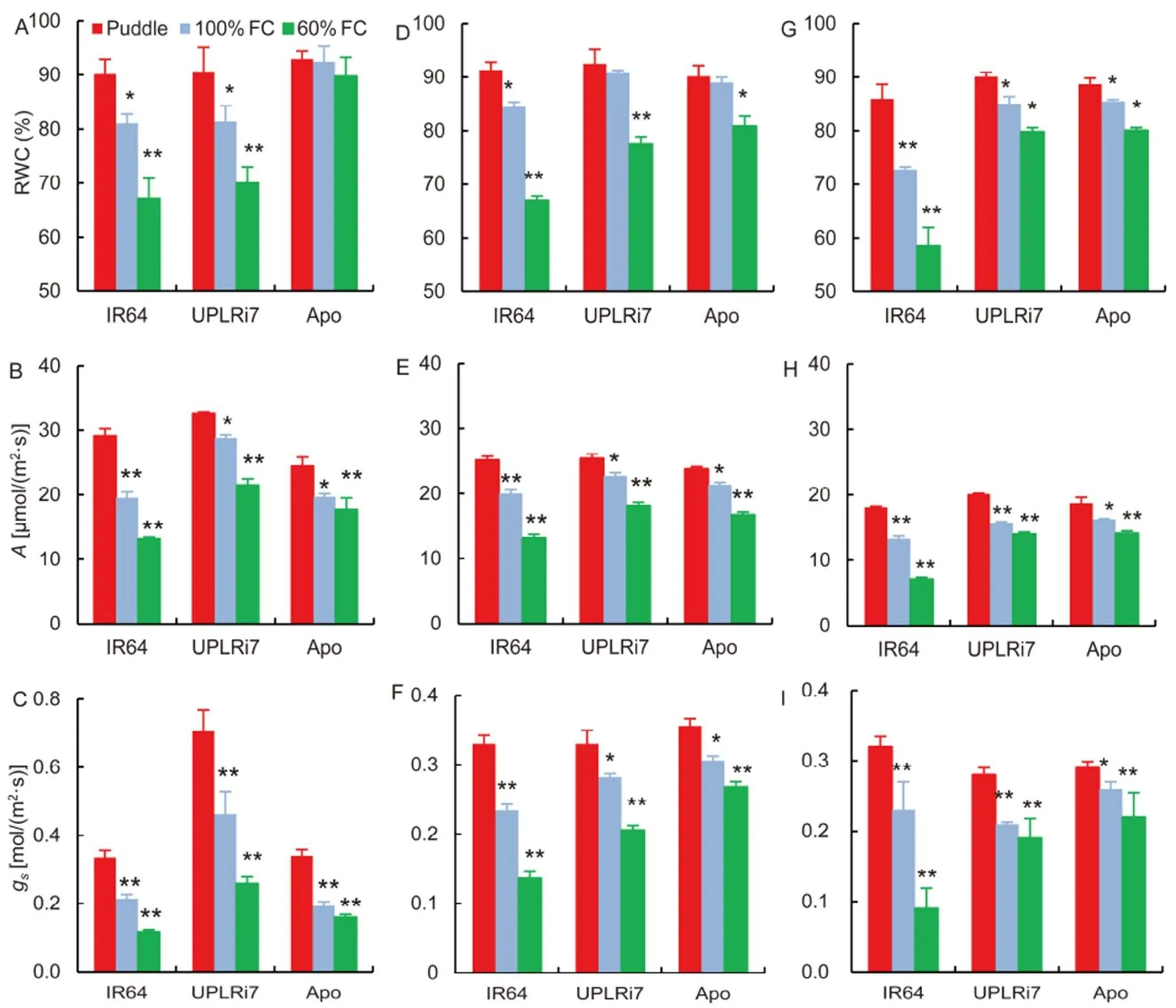
Fig. 1. Effects of water limitation on various physiological characteristics of three contrasting genotypes.
A,Relative water content (RWC) at the vegetative stage (GSI).B, Photosynthetic rate () at GSI.C,Stomatal conductance (g) at GSI. D, RWC at the flowering stage (GSII).E,at GSII.F,gat GSII. G, RWC at the grain filling stage (GSIII).H,at GSIII.I,Stomatal conductance (g) at GSIII.
FC, Field capacity. Data indicate Mean ± SE (= 5).* and ** indicate significant differences from puddle at the 0.05 and 0.01 levels within genotypes, respectively.
The reduction in soil moisture status resulted in a significant reduction in TLA in all the three genotypes (Table 1). UPLRi7 and IR64 showed a stronger reduction in TLA under 100% FC than Apo. Under 60% FC, all the genotypes showed a very strong reduction with IR64 showing the strongest one (57.7%) (Table 1). Both plant height and tiller number were sensitive to water limitation and IR64 showed a higher reduction than the other two genotypes. Although reduction in tiller number was low in Apo, plant height was significantly reduced compared to puddle conditions (Supplemental Table 1).Whereas, both IR64 and UPLRi7 showed significant reduction in tiller number and plant height under 100% FC and 60% FC. Stem weight decreased more in IR64 and Apo than in UPLRi7 with increased water limitation (Supplemental Table 1).
Reduction in plant height and tiller number closely matched the reduction in total biomass (TBM). Even under 100% FC, an overall reduction in TBM ranging from 16.8% to 25.0% was noticed (Table 1). Under 60% FC, IR64 recorded the strongest reduction in TBM. This effect of water limitation on physiological responses led to an average yield decrease of 13.2% and 27.6% under 100% FC and 60% FC, respectively (Table 2). IR64 recorded the highest reduction by 35.1% under 60% FC. Similarly, UPLRi7 was also sensitive to vegetative stress with a reduction of 17.7% under 100% FC and 31.7% under 60% FC. Apo showed the least reduction in yield under both 100% FC and 60% FC (6.0% and 16.2%, respectively).
Stress response at GSII (flowering stage)
There was a significant reduction in RWC under both 100% FC and 60% FC over puddle condition in all the genotypes. This reduction was strong in IR64, especially under 60% FC (Fig. 1-D). Reduction in chlorophyll content was highly significant under 60% FC for IR64 and UPLRi7 (Supplemental Fig. 1). Water limitation at GSII affected both assimilation rate and stomatal conductance (Fig. 2-E and -F). IR64 showed more than 45% reduction inandswhile both UPLRi7 and Apo being drought adaptive also showed >24% of reductions inandsunder 60% FC.
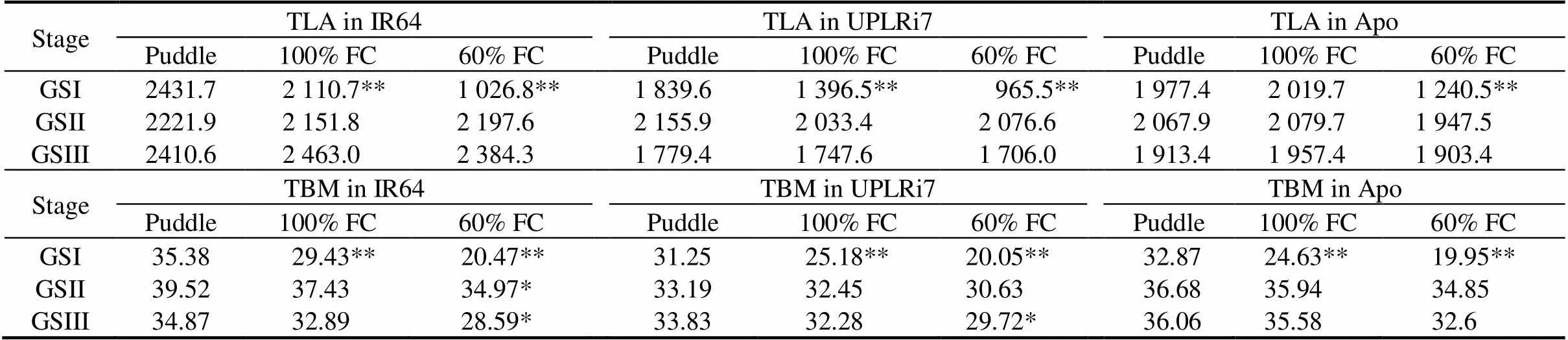
Table 1.Effects of water limitation on total leaf area (TLA, cm2/plant) and total biomass (TBM, g/plant).
GSI, Vegetative stage; GSII, Flowering stage; GSIII, Grain filling stage.
* and ** indicate significant differences from puddle at the 0.05 and 0.01 levels within genotypes, respectively.
As expected, water limitation during the GSII experiment had no considerable influence on plant height and tiller number (Supplemental Table 1). Similarly, TLA showed no significant changes in response to stress (Table 1). Reduction of TBM was marginal in all the genotypes under 60% FC. A mild water limitation (100% FC) under GSII significantly reduced yield. This reduction in yield was stronger when severity of stress increased to 60% FC. An overall yield loss of 22.3% and 44.4% was recorded under 100% FC and 60% FC, respectively. IR64 had 50.7% yield loss and UPLRi7 had 46.1%. Apo showed a significant reduction of 36.3% under 60% FC over the puddle condition (Table 2).
Stress effect at GSIII
RWC was significantly lower for IR64 than for UPLRi7 and Apo when imposed to stress. The latter two genotypes showed a marginal reduction (upto 6.5%) in RWC under 60% FC compared with the puddle treatment (Fig. 1-G). Under 60% FC, RWC of IR64 decreased by more than 19%. Gas exchange parameters recorded a similar trend. IR64 showed marked reduction in bothandsin both the water regimes compared to puddle treatment (Fig. 1-H and -I).
SPAD values were generally lower in IR64 than in UPLRi7 and Apo at all the phenological stages. Chlorophyll content of IR64 decreased significantly under both 100% FC and 60% FC (Supplemental Fig. 1). On the other hand, there was not much change in leaf area, plant height and tiller number in all the three genotypes (Table 1 and Supplemental Table 1). Shoot weight and TBM were significantly reduced in IR64 and UPLRi7 especially under 60% FC. An overall yield loss of 12.2% to 27.4% was recorded under 100% FC and 60% FC, respectively (Table 2). Under 60% FC, IR64 and UPLRi7 had the highest reduction (41.6% and 32.4%, respectively), whereas Apo had a marginal reduction in yield (9.8%).
Responses of spikelet fertility to stress at three stages
When stress was imposed during the GSI stage, a slight reduction in spikelet fertility was noticed (Fig. 2-A). A decrease in spikelet fertility was the most dominant yield component accounting for the observed yield reduction at GSII in all the three genotypes. IR64 had the greatest reduction in spikelet fertility followed by UPLRi7 (Fig. 2-B). Although Apo showed smaller reduction in spikelet fertility than the other genotypes, a reduction of 36.3% under 60% FC was observed irrespective of its adaptability to water-limited conditions. Stress at GSIII reduced the number of filled grains especially under 60% FC. IR64 had the highest reduction (18.0%) and Apo had the lowest reduction (9.5%) under 60% FC (Fig. 2-C).
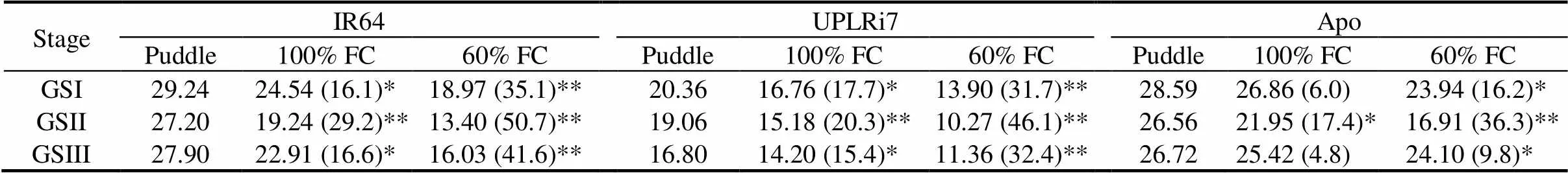
Table 2.Yield for the three contrasting genotypes at different growth stages and stress treatments. g/plant
GSI, Vegetative stage; GSII, Flowering stage; GSIII, Grain filling stage; FC, Field capacity.
* and ** indicate significant differences from puddle at the 0.05 and 0.01 levels within genotypes, respectively. Values in the parentheses are the reduced percent compared to the puddle condition.
Aerobic cultivar Apo had higher water extraction ability under stress
Significant effects of stress treatments at different growth stages on root traits were observed for all the three genotypes. In all the three growth stages, Apo and UPLRi7 maintained higher root growth compared to IR64 under stress. At GSI, root length was increased significantly in all the genotypes under 60% FC. Apo showed the highest increase of 40.8% and 42% under 100% FC and 60% FC, respectively (Fig. 2-D). UPLRi7 also showed a significant increase of 18.2% and 31.3% under 100% FC and 60% FC, respectively, compared to the puddle condition. Interestingly, cultivar with the highest root length showed the lowest root weight (Supplemental Fig. 2). UPLRi7 and Apo had the highest root length under 60% FC but root weight was significantly lower for these two genotypes than for IR64.
The increasing trend of root length in all the genotypes under stress conditions during GSI was not observed in GSII and GSIII. Root weight was marginally decreased by stress in GSII (Supplemental Fig. 2). At GSIII, IR64 and UPLRi7 showed marginal reduction in root length under 60% FC (Fig. 2-F).
Source to sink imbalance was the major contributor for yield loss at any given stage
At all the three growth stages, significant reduction in photosynthesis under 100% FC and 60% FC over puddle was noticed. Reductions inand yield were significantly related in all the three stages (Fig. 3-A). However, a strong linear relationship was shown between reduction in total leaf area and yield only at GSI stage (Fig. 3-B).
We computed an index as an indication of relative whole-plant photosynthesis by multiplying total leaf area with single leaf photosynthetic rate (the term ‘relative’ is used because the estimations are not absolute values which would require considering the vertical decrease ofwith lowering leaf positions). Reduction in this index was strongly related with reduction in yield in all the three phenological stages (Fig. 3-C).
Reproductive growth (sink) was examined along with photosynthetic rate (source activity) to explain yield loss at all the three stages. Reduction inand spikelet fertility were significantly correlated at GSII and GSIII (Fig. 3-D).
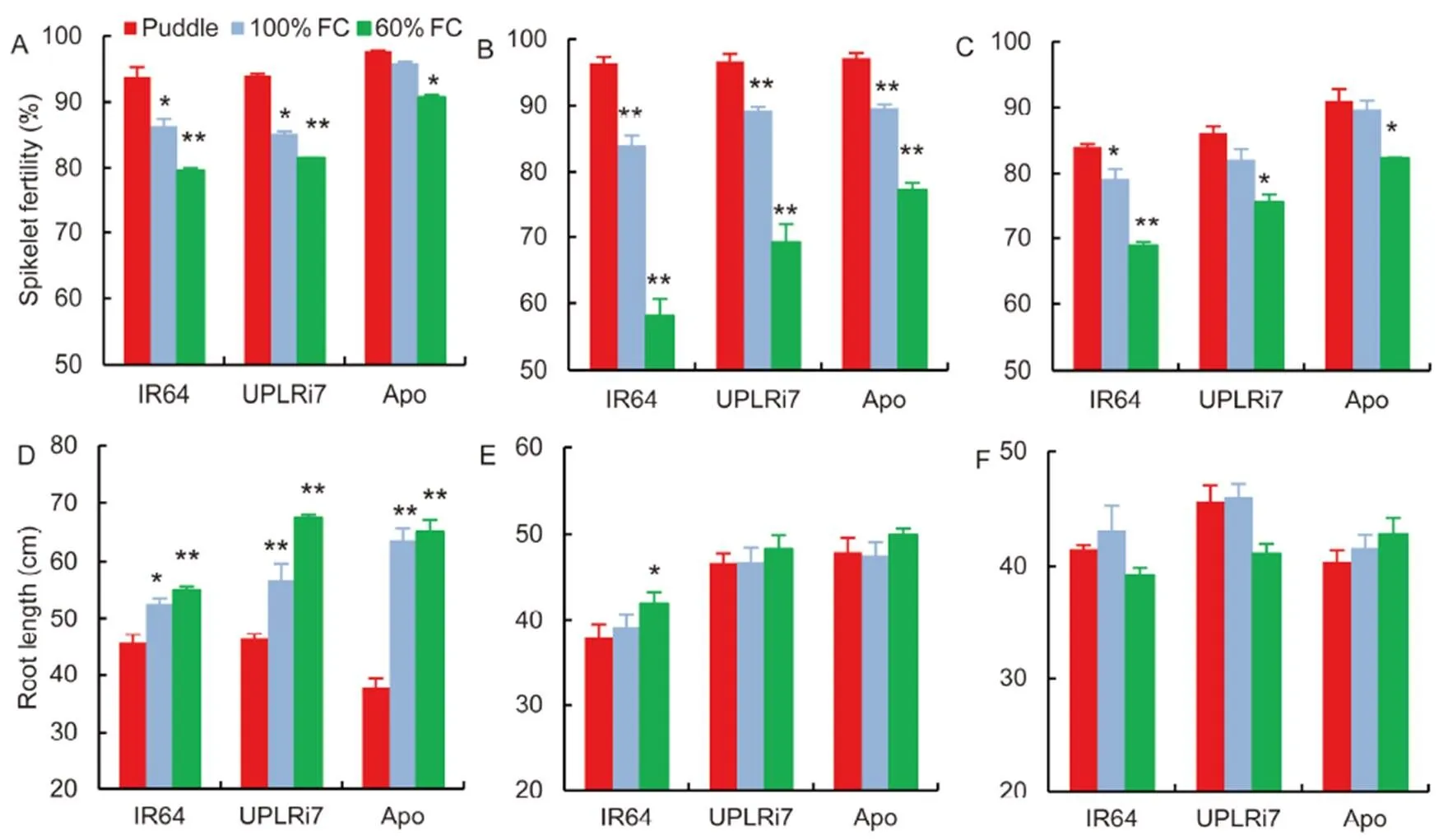
Fig.2. Effects of stress on spikelet fertility and root length among the genotypes.
A,Spikelet fertility at vegetative stage.B, Spikelet fertility at flowering stage.C,Spikelet fertilityat grain filling stage. D,Root length at vegetative stage.E, Root length at at flowering stage.F,Root length at at grain filling stage.
FC, Field capacity. Data indicate Mean ± SE (= 5). * and ** indicate significant differences from puddle at the 0.05 and 0.01 levels within genotypes, respectively.
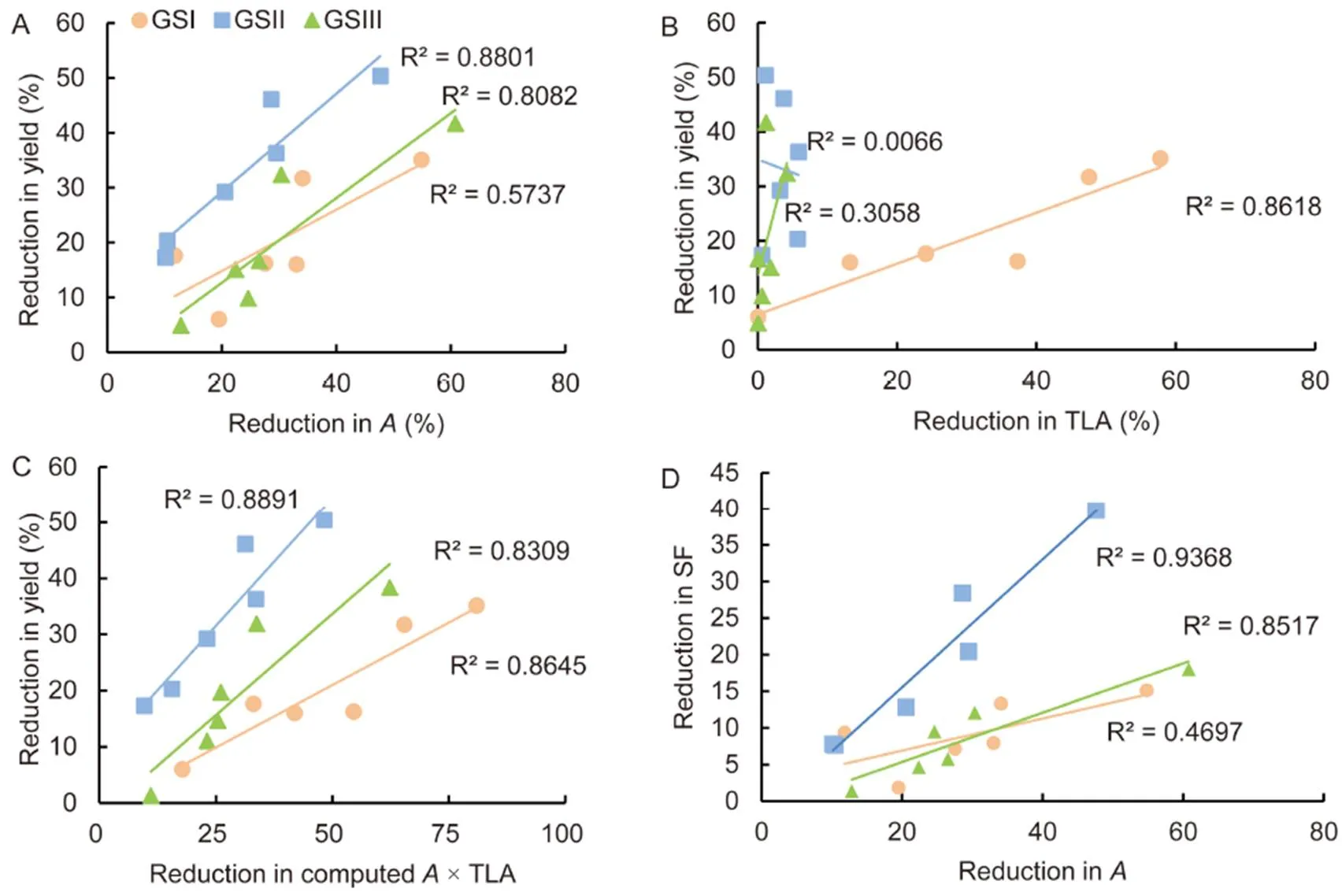
Fig. 3. Linear regression among reductions in several traits.
A, Linear regression of reductions in assimilation rate () and yield. B, Linear regression of reductions in total leaf area (TLA) and yield. C, Linear regression of reductions in computedand yield. D, Linear regression of reductions in assimilation rate () and spikelet fertility (SF).
GSI, Vegetative stage; GSII, Flowering stage; GSIII, Grain filling stage.
Reduction in yield over puddle was calculated for the means under 100% and 60% field capacity. ** indicates significance at the 0.01 level.
Discussion
Crop improvement strategies are aiming at identification of specific traits with precise phenotyping methods (Vijayaraghavareddy et al, 2017; Sheshshayee et al, 2018). But the major limitation is that response of plants is widely different at specific phenological stage. Further, these physiological responses could potentially vary between genotypes with known history of adaptation to specific agro-ecosystem.
Stress at any phenological stage reduces plant productivity
During all the phenological stages, reduced water availability significantly affected plant performance and productivity. Stress at any phenological stage reduced yields by 15.9% (100% FC) to 33.1% (60% FC), compared to puddle conditions (Table 2). For rice being semi-aquatic, even a mild water limitation reduces yield significantly (Yang et al, 2019). IR64 had the greater reduction in yield under 100% FC in all the three phenological stage because of its adaptability to lowland condition,whereas Apo, a genotype adapted to aerobic condition, showed lower reduction under 100% FC over puddle condition in all the three phenological stages. Though this trend was consistent under 60% FC, reduction in yield at GSII was significantly higher even for aerobic genotype Apo. Therefore, among the growth stages, GSII was most sensitive even for mild water limitation (Yang et al, 2019) with an overall yield loss of 22.3% and 44.4% under 100% FC and 60% FC, respectively.
Increasing root length assists to maintain tissue turgor
Maintenance of water relations under low soil moisture condition is an important drought adaptive strategy. In aerobic and water limited conditions, root traits are important for water uptake from deeper layers of soils (Raju et al, 2014). Increase in root length was observed at GSI (Fig. 2-D). But the influence of water limitation has no effect on root length at GSII and GSIII stages (Fig. 2-E and -F). The maximum root growth was observed at GSII and GSIII. It is most unlikely that a period of 15 d stress may have a significant influence on physical root length. However, GSI,which represents a period of active growth, showed a maximum increase in root length under water-limited conditions (Fig. 2-D). Root growth is known to be stimulated by soil moisture deficit and significant genetic variability has also been demonstrated for this response (Kadam et al, 2015), and this will be more pronounced if carbon assimilatory capacity and available carbon resources for remobilisation are not significant. Genotypes with better root system can maintain higher RWC due to efficient water mining ability (Nguyen et al, 1997; Raju et al, 2014). This could be an adaptive mechanism in upland genotype UPLRi7 and aerobic genotype Apo, hence showing significant increase in root length at GSI stage (Fig. 2-D).
Reduced spikelet fertility is the determining factor for reduced yield in all phenological stages
In GSI, the most prominent influence of stress was reduced leaf expansion and gas exchange. Decreases in canopy cover when plants experience stress during vegetative stage are well documented and have been attributed to reduced cell expansion and cell division (Boonjung and Fukai, 1996; Sarvestani et al, 2008; Praba et al, 2009). Reduction in turgor has a direct effect on carbon assimilation rates (Turk et al, 1980; Praba et al, 2009). The combined effect of reduced leaf area, tiller number and photosynthetic rate significantly impedes total carbon assimilatory capacity (source), which may lead to decreased spikelet fertility. Spikelet fertility was significantly lower at GSII stage than at the other stages (Fig. 2-B). Spikelet fertility at GSII stage is governed by a number of processes ranging from anther development, anther dehiscence, pollen viability, stigmatic receptivity, pollen efficiency, fertilization and ovule abortion, and all of these processes are extremely sensitive to stress (Shi et al, 2018). Besides, the spikelet water relations could further exacerbate the stress effect (O’Toole et al, 1984; Selote and Khanna-Chopra, 2004). Although a significant reduction in spikelet fertility was also noticed at GSIII (Fig. 2-C), factors like lack of availability of photosynthates for grain filling and negative source-sink interactions were the major contributors to reduce spikelet fertility at GSIII. Hence, yield losses arise from reduced source size resulting from a decrease in amount of photosynthates for grain filling, reduced number of spikelets and/or from reduction in efficiency of grain filling (Roitsch, 1999; Madani et al,2010).
Reduction in source availability will influence yield
Influence of source on growth and productivity can be viewed from two perspectives: reduction in canopy cover (Cakir, 2004) and reduction in photosynthetic rate per unit leaf area (Serraj et al, 1999). In other words, source influences growth and productivity because of a combination of these two factors collectively responsible for whole-plant photosynthesis. Yield reduction when stress coincides with grain filling (GSIII) could be resulted from lack of carbohydrates for grain filling and/or due to reduced synthesis of current photosynthates and their translocation (Madani et al, 2010). The reduction in leaf area during GSII and GSIII showed no any association with reduction in yield (Fig. 3-B). However, reduction in photosynthetic rate was strongly associated with reduction in yield (Fig. 3-A). Further, the reduction in the relative whole-plant photosynthesis showed a much stronger reduction in yield in all the stages. A change in source-sink interaction has been suggested as one of the major factors responsible for yield reduction under stress (Roitsch, 1999). Stress during the later phenological stages is well known to induce leaf senescence which decreases green leaf area and thus reduces canopy photosynthesis (Jagadish et al, 2015). However, we noticedno considerable reduction in green leaf area due to stress during GSII and GSIII stages (Table 1), which is against the normally noticed trend. There could be two possible reasons. Firstly, stress was provided for a period of 15 d, which may not be severe enough to cause changes in leaf area. Secondly, total leaf area was computed by multiplying leaf dry weight with specific leaf area. As we had only taken the green and functional leaves for recording leaf weight, this method can cause little error in determining functional leaf area.
Among the growth stages, moisture stress at GSII significantly affects the yield irrespective of adaptability of a genotype. Reductions in biomass, spikelet fertility and carbon gain seem to be major determining factors of yield under stress at GSI, GSII and GSIII stages, respectively. Reduction in yield was significantly high for lowland cultivar IR64 even at a 100% FC, which is a mild water limitation compared with puddle condition. Although the aerobic genotype Apo maintained much higher yield in water limited conditions (100% FC and 60% FC), there is a need of further improvement to reduce yield loss especially when stress induced at GSII stage. Hence, a genotype which maintained source to sink balance like Apo can be used for further crop improvement programmes. This information would therefore be most useful in improving the performance of rice crops which differ in their adaptability.
Acknowledgements
This work was supported by an anonymous private donor, via Wageningen University Fund, to the first author’s PhD fellowship. Dr C. G. van der Linden and Dr P. S. Bindraban are acknowledged for their valuable support.
Supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Effect of water limitation on total plant height, number of tillers and stem weight.
Supplemental Fig. 1. Effect water limitation on SPAD value at GSI, GSII and GSIII in rice genotypes.
Supplemental Fig. 2. Effect water limitation on root weight at GSI, GSII and GSIII in rice genotypes.
Babitha KC, Vemanna R S, Nataraja K N, Udayakumar M. 2015. Overexpression of EcbHLH57 transcription factor fromL. in tobacco confers tolerance to salt, oxidative and drought stress.,10(9): e0137098.
Boonjung H, Fukai S. 1996. Effects of soil water deficit at different growth stages on rice growth and yield under upland conditions: 2. Phenology, biomass production and yield.,48(1): 47–55.
Bouman B. 2009. How much water does rice use?, 69: 115–133.
Bouman B A M, Tuong T P. 2001. Field water management to save water and increase its productivity in irrigated lowland rice.,49(1): 11–30.
Çakir R. 2004. Effect of water stress at different development stages on vegetative and reproductive growth of corn., 89(1): 1–16.
Dharmappa P M, Doddaraju P, Malagondanahalli M V, Rangappa R B, Mallikarjuna N M, Rajendrareddy S H, Ramanjinappa R, Mavinahalli R P, Prasad T G, Udayakumar M, Sheshshayee S M. 2019. Introgression of root and water use efficiency traits enhances water productivity: An evidence for physiological breeding in rice (L.).,12(1): 14.
IRRI. 1997. Rice Almanac. 2nd edn. Los Banos, the Philippines: International Rice Research Institute.
Jagadish K S V, Kavi Kishor P B, Bahuguna R N, von Wiren N, Sreenivasulu N. 2015. Staying alive or going to die during terminal senescence: An enigma surrounding yield stability.,6: 1070.
Jin Y, Yang H X, Wei Z, Ma H, Ge X C. 2013. Rice male development under drought stress: Phenotypic changes and stage-dependent transcriptomic reprogramming.,6(5): 1630–1645.
Kadam N N, Yin X, Bindraban P S, Struik P C, Jagadish K S. 2015. Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water deficit stress than rice?,167(4): 1389–1401.
Kadiyala M D M, Mylavarapu R S, Li Y C, Reddy G B, Reddy M D. 2012. Impact of aerobic rice cultivation on growth, yield, and water productivity of rice-maize rotation in semiarid tropics.,104(6): 1757–1765.
Madani A, Shirani-Rad A, Pazoki A, Nourmohammadi G, Zarghami R, Mokhtassi-Bidgoli A. 2010. The impact of source or sink limitations on yield formation of winter wheat (L.) due to post-anthesis water and nitrogen deficiencies.,56(5): 218–227.
Mackill D J, Khush G S. 2018. IR64: A high-quality and high-yielding mega variety.,11(1): 18.
Nguyen H T, Babu R C, Blum A. 1997. Breeding for drought resistance in rice: Physiology and molecular genetics consideration., 37(5): 1426–1434.
Nishimura S, Yonemura S, Sawamoto T, Shirato Y, Akiyama H, Sudo S, Yagi K. 2008. Effect of land use change from paddy rice cultivation to upland crop cultivation on soil carbon budget of a cropland in Japan.,125: 9–20.
O’Toole J C, Hsiao T C, Namuco O S. 1984. Panicle water relations during water stress.,33(2): 137–143.
Ouyang W J, Struik P C, Yin X Y, Yang J C. 2017. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought.,68(18): 5191–5205.
Praba M L, Cairns J E, Babu R C, Lafitte H R. 2009. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat.,195(1): 30–46.
Raju B R, Narayanaswamy B R, Mohankumar M V, Sumanth K K, Rajanna M P, Mohanraju B, Udayakumar M, Sheshshayee M S. 2014. Root traits and cellular level tolerance hold the key in maintaining higher spikelet fertility of rice under water limited conditions.,41(9): 930–939.
Ramu V S, Swetha T N, Sheela S H, Babitha C K, Rohini S, Reddy M K, Tuteja N, Reddy C P, Prasad T G, Udayakumar M. 2016. Simultaneous expression of regulatory genes associated with specific drought-adaptive traits improves drought adaptation in peanut.,14(3): 1008–1020.
Roitsch T. 1999. Source-sink regulation by sugar and stress.,2(3): 198–206.
Sarvestani Z T, Pirdashti H, Sanavy S A M M, Balouchi H. 2008. Study of water stress effects in different growth stages on yield and yield components of different rice (L.) genotypes., 11(10): 1303–1309.
Selote D S, Khanna-Chopra R. 2004. Drought-induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles, 121(3): 462–471.
Serraj R, Sinclair T R, Purcell L C. 1999. Symbiotic N2fixation response to drought., 50(331): 143–155.
Sheshshayee M S, Bindumadhava H, Shankar A G, Prasad T G, Udayakumar M. 2003. Breeding strategies to exploit water use efficiency for crop improvement., 30(2): 253–268.
Sheshshayee M S, Vijayaraghavareddy P, Sreevathsa R, Rajendrareddy S, Arakesh S, Bharti P, Dharmappa P, Soolanayakanahally R. 2018. Introgression of physiological traits for a comprehensive improvement of drought adaptation in crop plants.,10(6): 92.
Shi W J, Li X, Schmidt R C, Struik P C, Yin X Y, Jagadish S V K. 2018. Pollen germination andfertilization in response to high-temperature during flowering in hybrid and inbred rice.,41(6): 1287–1297.
Turk K J, Hall A E, Asbell C W. 1980. Drought adaptation of cowpea: I. Influence of drought on seed yield.,72(3): 413–420.
Udayakumar M, Rao R C N, Wright G C, Ramaswamy G C, Ashok RS, Gangadhar G C, Hussain I S A. 1998. Measurement of transpiration efficiency in field conditions., 1: 69–75.
Uphoff N, KassamA, Harwood R. 2010. SRI as a methodology for raising crop and water productivity: Productive adaptations in rice agronomy and irrigation water management,9(1): 3–11.
Vijayaraghavareddy P, Vanitha P, Vemanna R, Sheshshayee M S, Makarla U. 2017. Quantification of membrane damage/cell death using Evan’s blue staining technique.,7(16): e2519.
Yang J C, Huang D F, Duan H, Tan G L, Zhang J H. 2009. Alternate wetting and moderate soil drying increases grain yield and reduces cadmium accumulation in rice grains.,89(10): 1728–1736.
Yang X L, Wang B F, Chen L, Li P, Cao C G. 2019. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality., 9(1): 3742.
Zeigler R S, Puckridge D W. 1995. Improving sustainable productivity in rice-based rainfed lowland systems of South and Southeast Asia.,35(3): 307–324.
Zhang J Y, Broeckling C D, Sumner L W, Wang Z Y. 2007. Heterologous expression of two Medicago truncatula putative ERF transcription factor genes, WXP1 and WXP2, inled to increased leaf wax accumulation and improved drought tolerance, but differential response in freezing tolerance.,64(3): 265–278.
12 June 2019;
24 October 2019
Sheshshayee Sreeman(msshesh1@uasbangalore.edu.in)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.009
(Managing Editor: Fang Hongmin)
杂志排行
Rice Science的其它文章
- Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
- Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
- RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
- RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
