Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
2020-07-06NiveditaDeySoumenBhattacharjee
Nivedita Dey, Soumen Bhattacharjee
Research Paper
Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
Nivedita Dey, Soumen Bhattacharjee
()
Present work was undertaken to screen some drought tolerant indigenous aromatic rice cultivars (IARCs), commonly cultivated in West Bengal, India, based on their capacity to produce osmolytes, redox-sensitive phenolic acids and flavonoids, as contrivances for redox-regulation under drought stress. Polyethylene glycol induced post imbibitional dehydration stress mediated changes in redox regulatory properties of the germinating seeds of the four IARCs (Jamainadu, Tulaipanji, Sitabhog, Badshabhog), which were assessed in terms of changes in prooxidant accumulation (localization of reactive oxygen species (ROS) by confocal microscopy, DCFDA (2′,7′-dichlorofluorescin diacetate)oxidation,O2-and H2O2accumulation), cumulative antioxidative defense (radical scavenging property and total thiol content), ROS scavenging phenolic acids (gallic acid, protocatechuic acid, gentisic acid, para-hydroxy benzoic acid, chlorogenic acid, caffeic acid, syringic acid, salicylic acid, sinapic acid and p-coumaric acid) and flavonoids (catechin, naringin, rutin, quercetin, kaempferol, myricetin and apigenin). The capability of germinating seeds to accumulate osmolytes (like glycinebetaine, proline, soluble carbohydrates and K+ion) and polyphenolic compounds was also correlated with their corresponding redox status and redox biomarkers (conjugated diene, hydroperoxide, thiobarbituric acid reactive substances and free carbonyl content) produced under the same conditions. The results in general showed that accumulation of osmolytes along with the redox-sensitive phenolics and flavonoids conferred the ability to maintain the redox homeostasis under drought stress for the tolerant IARCs (Badshabhog and Tulaipanji).
aromatic rice; drought; polyphenolic compounds; osmolytes; redox regulation
Drought or scarcity of water is one of the prime abiotic stress factors that significantly cause metabolic dysfunction, impart physiological limit and hamper the production of rice (Boyer, 1982; Petrov et al, 2015; Zu et al, 2017; Yang et al, 2019). The reduction of soil water significantly reduces the cereal production roughly by 10% on average (Lesk et al, 2016). Further, high temperature associated with water stress leads to an array of physiological, biochemical and molecular changes in plants, causing significant loss of yield (Fageria, 2007; Jagadish et al, 2007; Ramesh et al, 2017).
Though indigenous aromatic rice cultivars (IARCs) are cultivated in different agro-climatic zones with comparatively fewer inputs, studies pertaining to their stress-responsiveness, particularly towards abiotic stresses, are seldom found (Deb, 2005; Bhattacharjee and Dey, 2018). India, particularly West Bengal, still exhibits a rich biodiversity of IARCs with more than seventy cultivars cultivated in different agro-climatic zones (Deb, 2005), but for effective cultivation of IARCs under drought, it is absolutely necessary for proper reliable drought screening.
Dehydration stress, depending on its severity and duration, induces stomatal closure, subsequently causingreduction in the rate of photosynthetic carbon reduction cycle (PCRC) (Samarah, 2016). A reduction in PCRC, accompanied by enhancement of photosyntheticcarbon oxidation cycle, induced pseudo-cyclic electron flow due to lower regeneration of NADP+, causing loss of redox homeostasis. Excess generation of reactive oxygen species (ROS), depending on the duration and severity, always causes plethora of oxidative damages to the crop, subsequently reducing their field performances.
One of the important metabolic strategies that crops adopt to endure drought stress is to fine tune their metabolism with mobilization of metabolites for osmotic adjustment and cellular defense (Chaves and Oliveira, 2004). The complex metabolic shifts enabling the survival of the crop under drought stress is the accumulation of non-toxic compatible cytosolutes conferring osmoprotection (Rhodes and Samaras, 1994; Koyro et al, 2012; Ranganayakulu et al, 2013). The osmoprotectants without interfering normal metabolism can mitigate oxidative damages caused by elevated titre of ROS under drought (Ashraf and Foolad, 2007; Hasanuzzaman et al, 2013; Zivcak et al, 2016). In fact, there are reports that these osmoprotectants which sustain ion homeostasis can very well work as antioxidants (Hossain and Fujita 2010; Hasanuzzaman et al, 2013; Kaya et al, 2013). Apart from inducing competent enzymatic and nonenzymatic defense systems,plants often up-regulate the synthesis of osmoprotectants, primarily for maintaining ion homeostasis and turgor. But, some recent works find a close relationship between the accumulation of osmoprotectants and antioxidative defense (Kaya et al, 2013). Osmoprotectantslike glycinebetaine, proline, soluble carbohydrate, reducing carbohydrate etc. have been found to up-regulate the activities of antioxidative defense enzymes, which in turn detoxify ROS and maintain redox homeostasis.
The accumulation of the compatible cytosolutes is regarded as a basic strategy for the protection and survival of plants during the situation of water loss under temperature stress (Janska et al, 2010; Gupta et al, 2013). These osmoprotectants can stabilize and protect the structure of antioxidative enzymes and other proteins, maintain membrane integrity and scavenge ROS by up-regulation of the capacity of antioxidant enzymes under drought stress (Tausz et al, 2004; Farooq et al, 2008; Hefny and Abdel-Kader, 2009; Rasheed et al, 2011; Kumar et al, 2012). Proline, which is an important indicator of stress in rice, can also act as an indicator of water status (Tang et al, 2008; Silvente et al, 2012). Proline in elevated level is also associated with genotypes which are better adapted to intense environmental stresses (Sahebi et al, 2018) by combating with toxicity of over-accumulated proline itself (Rizhsky et al, 2004). Pyrroline-5- carboxylate synthetase (P5CS), a bi-functional enzyme, plays a key role in plant intracellular accumulation of proline. The first two steps of proline biosynthesis in plants are catalysed by P5CS (Parida et al, 2008). Under stress, P5CS allows synthesis of proline and turns off the synthesis during recovery from stress by the mechanism of feedback inhibition, which leads to enhanced proline catabolism (Kishor et al, 1995; Hong et al, 2000; Yamada et al, 2005).
During water scarcity, higher availability of carbohydrates is also associated with drought stress tolerance and acclimation (Liu and Huang, 2000). They not only act as signaling molecules at lower concentrations, but also act as ROS scavengers at higher concentrations. They act as antioxidants in plants under stress (Sugio et al, 2009; Lang-Mladek et al, 2010). Glycinebetaine (GB) accumulation in plants shows improvement in their ability to tolerate abiotic stress conditions, like unavailability of water (Sakamoto and Murata, 1998; Holmstrӧm et al, 2000; Park et al, 2004; Waditee et al, 2005). GB produced in chloroplasts is more effective showing higher tolerance of plants against stress than that in the cytosol (Park et al, 2007). It works by protecting photosystem II and mitigating oxidative membrane damage (Chen and Murata, 2011).
Apart from the synthesis of compatible cell cytosolutes, drought and other forms of abiotic stress also up- regulate synthesis of polyphenolic compounds. These compounds are produced from phenylpropanoid, shikkimic acid and pentose phosphate pathway, and are affected largely by drought and extremes of temperature (Velderrain-Rodríguez, 2014; Lin et al, 2016). Flavonoids, which include flavonones, anthocyanins, proanthocyanidines, isoflavonoids and flavones along with some phenolic acids, are found to be induced by abiotic stimuli to modulate the redox status of the tissue through their antioxidant function (Velderrain- Rodríguez, 2014; Lin et al, 2016; Quan and Xuan, 2018).
Therefore, apart from the conventional antioxidative system comprising of enzymatic antioxidative defense and low molecular weight antioxidants, plants may exploit both the osmoprotectants and phenolic compounds secondarily for redox-regulation. The present work was undertaken to assess whether these compounds (glycinebetaine, proline, soluble carbohydrate, reducing carbohydrate, redox sensitive flavonoids and phenolic acids) evoke any influence on redox-regulation under drought stress in four IARCs, commonly cultivated in West Bengal, in order that these metabolic parameters can be used as redox biomarkers for the selection of drought tolerant crops.
MATERIALS AND METHODS
Plant growth and PEG-6000 induced post imbibitional dehydration stress (PIDS) treatment
Four IARCs (Jamainadu, Tulaipanji, Sitabhog and Badshabhog), generally cultivated in drought prone Rarh regions of West Bengal, India, are selected as experimental materials. Seeds of all the rice cultivars were collected from Chinsurah Rice Research Institute, Government of West Bengal, India. At first, seeds of each cultivar were washed with distilled water. Then, seeds were surface-sterilized in 0.2% HgCl2solution for 5 min. Finally, they were washed with distilled water thrice. After 48 h of imbibition in sterile distilled water in darkness at (25 ± 2) ºC, the seeds were sown on moist filter paper in different petri plates and were placed in seed germinator cum stability chamber. Dehydration stress of different magnitudes was imposedto three different water-imbibed seed lots of each cultivarwith -0.344, -0.851 and -1.619 MPa polyethylene glycol (PEG)-6000 for 7 d from the day of seed sowing. For untreated control set, water- imbibed seeds were sown on moist filter paper in petri plates directly. All the seed lots were allowed to grow at (25 ± 2) ºC with 14 h photo period [light intensity, 270 µmol/(m2∙s)] and relative humidity of 78% ± 2%. For all biochemical analysis, 7-day-old seedlings were used.
Extraction and estimation of total ROS
Total ROS generation was estimated spectro- fluorometrically by performing anassay. Rice seedling tissue (7-day-old, 30 mg) was placed in 8 mL of 40 mmol/L Tris-HCl buffer (pH 7.0) in the presence of 100 µmol/L of 2′,7′-dichlorofluorescindiacetate (DCFDA, Sigma Aldrich, USA) at 30ºC. Supernatants were removed after 60 min, and fluorescence was monitored in a spectrofluorometer (F-4500 FL, Hitachi, Japan) with excitation at 504 nm and emission at 525 nm (Simontacchi et al, 1993). To differentiate ROS from other long-lived substances which are able to react with DCFDA, additional controls were performed. For additional controls, seedling tissues were incubated without DCFDA for 60 min, and then, tissues were removed followed by addition of DCFDA for 60 min before fluorescence was determined. These fluorescence values were subtracted from all readings to assess the fluorescence that depended on ROS.
Extraction and estimation of total radical scavenging activity
For determination of 2,2-diphenyl-1-pycryl hydrazyl (DPPH) free radical scavenging activity, the process of Mensor et al (2001) was followed with little modification. Sample (1.5 g) was treated with liquid nitrogen and extracted with 30 mL of 80% methanol at 28 ºC for 24 h in shaking incubator. Extracts were centrifuged at 3 500 r/min for 20 min at 4 ºC. Supernatants were collected and filtered, and filtrate was used for DPPH radical scavenging activity. For estimating the radical scavenging activity, 1 mL sample was mixed with 3 mL of DPPH solution (0.04 mg DPPH/mL ethanol) and incubated for 30 min in darkness, and then absorbance was taken at 517 nm. Total antioxidant capacity (TAC) was calculated as:
TAC (%) = 1 – (A–A) /A× 100
WhereAis the absorbance of 1 mL sample and 3 mL DPPH,Ais the absorbance of1 mL sample and 3 mL ethanol, andAis the absorbance of1 mL ethanol and 3 mL DPPH.
Extraction and estimation of H2O2
Hydrogen peroxide estimation was done by following the procedure of MacNevin and Uron (1953). Fresh tissue of 7-day-old seedlings of experimental rice cultivars was extracted with cold acetone (5 mL acetone for 1 g tissue). After filtration through Whatman No.1 filter paper, distilled water was added to the extract to make the volume up to 10 mL. Then, 1 mL of 5% titanic sulphate was dissolved in 20% H2SO4and added to the extract. The mixture was then centrifuged at 6 000 r/min for 10 min, after adding 2 mL NH4OH. Collected pellet was washed with acetone (thrice) and again centrifuged for 10 min at 5 000 r/min. After dissolving the pellet in 3 mL of 2 mol/L sulfuric acid (H2SO4), absorbance was taken at 420 nm.
Extraction and estimation of total thiol content
Total thiol content (TTC) was estimated by following the process of Tietze (1969). Rice tissue of 7-day-old seedlings (500 mg) was extracted with 3% trichloroacetic acid (TCA) solution. After a brief centrifugation, the supernatant was diluted 10-fold by 100 mmol/L phosphate buffer (pH 7.5). Then, 0.2 mmol/L NADPH, 0.5 mmol/L 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 0.5 U/mL glutathione reductase were added. Finally, the thiol content was measured by absorbance at 412 nm.
Localization of H2O2 by confocal microscopy
The sample preparation was done by following the method of Kaur et al (2016). Root segments (1 cm) of 7-day-old seedlings were dipped immediately in DCFDA-Tris-HCl solution (10 µmol/L) and kept at room temperature. After 2 h, samples were washed thrice with autoclaved Milli-Q water and slides were prepared with 20% glycerol. Accumulation of H2O2was identified by DCFDA staining, and observed using a confocal microscopy with the Leica application suite X software (microscope model number was Leica TCS SP8, 488 nm laser was used for excitation, emission was observed at 505–530 nm with objective 20X) in root tissue of seedlings (7-day-old) raised from highest magnitude of post-imbibitional dehydration stress (-1.619 MPa) as well as the untreated control. Green fluorescence indicates presence of H2O2.
Estimation of organic and inorganic osmolytes
GB was estimated by the method of Grieve and Grattan (1983). Finely ground dried seedling (7-day-old) was stirred with 20 mL distilled water for 24 h and then filtered. Then, filtrate was diluted with equal volume of 1 mol/L H2SO4, made into aliquots of 0.5 mL in micro centrifuge tubes and cooled over ice for 1 h. To each of these aliquots, 0.2 mL cold I2-KI reagent was added. The reactant were stirred gently and stored over night at 4 ºC. Then, the samples were centrifuged at 10 000 r/min for 15 min at 4 ºC. The precipitated per iodide crystals were dissolved in 1,2-dichloroethane. After 2 h, absorbance was measured at 365 nm.
Proline was estimated by the procedure of Bates et al (1973). Tissue of 7-day-old seedlings (200 mg) was homogenized with 3% sulfosalicylic acid and centrifuged at 4 000 r/min for 10 min. Then, the assay mixture containing 2 mL supernatant, 2 mL glacial acetic acid and 2 mL ninhydrin reagent was kept in hot water bath at 100 ºC for 1 h. Then, the test tubes were kept inside the chiller of refrigerator for 10 min. Again, they were kept at room temperature for 2 min, and finally the chromophore was extracted with tolune. Absorbance was then measured at 520 nm.
Estimation of reducing carbohydrate
Reducing carbohydrate was estimated by following the Nelson-Somogyi method (Gusakov et al, 2011). Tissue of 7-day-old seedlings (100 mg) was extracted with 80% ethanol twice. Supernatant, collected after centrifugation at 5 000 r/min for 10 min, was evaporatedby keeping it on a water bath at 80 ºC. Then, sugar wasdissolved by adding 10 mL distilled water. Assay mixture containing 2 mL supernatant and 1 mL alkaline copper tartrate (solution mixture of sodium carbonate, sodium bicarbonate, potassium sodium tartrate, sodium sulphate and copper sulphate) was kept in boiling water for 10 min. After cooling, arsenomolybolic acid (mixture of ammonium molybdate, sulfuric acid and disodium hydrogen arsenate) was added to the mixture, and then volume was made up to 10 mL with distilled water. Finally, after 10 min, absorbance was taken at 620 nm.
Estimation of soluble carbohydrate
Soluble carbohydrate was estimated according to McCready et al (1950) with some necessary modifications.Tissue of 7-day-old seedlings (100 mg) was homogenizedwith 5 mL of 80% ethanol. After centrifugation at 5 000 r/min for 10 min, supernatant was collected and diluted to 10 mL with distilled water for the estimation of soluble carbohydrate. The diluted supernatant (1 mL) and anthrone reagent (3 mL) were mixed in cold condition. The mixture was then kept in hot water bath for 15 min and again cooled down to 25 ºC. Absorbance was measured at 610 nm.
Estimation of K+ ion
It was experimented according to Allison et al (1954) using flame photometer. Dried tissue samples of 7-day-old seedlings were boiled for 30 min with 65% nitric acid and after cooling down, again boiled by adding 70% perchloric acid till the fume appeared. Equal volume of distilled water was added after cooling. Solutions were filtered. Filtrates were taken, and volumes were made up to 50 mL each by adding distilled water. K+ion level was estimated by flame photometer.
Extraction and estimation of proline metabolizing enzymes
Proline dehydrogenase (PDH) activity was measured by the method of Rena and Splittstoesser (1975). Tissues of 7-day-old seedlings were homogenised with phosphate buffer, and supernatant containing enzyme extract was taken. Then, enzyme extract was added in the reaction mixture containing 20 mmol/L L-proline, 100 mmol/L Na2CO3-NaHCO3(pH 10.3) and 10 mmol/L NAD+and subsequently incubated at 28 ºC for 15 min. Finally, absorbance was measured at 340 nm. One unit of PDH activity is defined as the amount of enzyme catalyzing the formation of 1 µmol NADH per min.
Activity of P5CS was assayed by following the method of Hayzer and Leisinger (1980). The enzyme was extracted with 50 mmol/L Tris-HCl (pH 7.1) and then assay mixture was made by using 50 mmol/L Tris-HCl buffer (pH 7.1), 50 mmol/L-glutamate, 20 mmol/L MgCl2, 10 mmol/L ATP and 100 mmol/L hydroxylamine-HCl. The initiation of reaction was taken place by the addition of enzyme extract. Next, the reaction mixture was incubated for 15 min at 37 ºC. Then, 1 mL of stop buffer (6.0 g trichloro acetic acid and 2.5 g FeCl3in a final volume of 100 mL by using 2.5 mol/L HCl) was added to stop the reaction. After that, the mixture was centrifuged at 8 500 r/min for 15 min, and absorbance was taken at 535 nm against a blank. The blank was identical to the reaction mixture, lacking ATP. The amount of produced γ-glutamyl hydroxamate complex was measured using the molar extinction coefficient of 250 mol/cm. Enzyme activity was defined as the amount of enzyme required to release 1 µmol γ-glutamyl hydroxamate per minute.
Quantitative assessment of phenolic acids and flavonoids by RP-HPLC (Reversed phase-high performance liquid chromatography)
Samples were prepared through soxlet-mediated hydroethanolic extraction of dry-powdered seedlings (7-day-old) followed by rotary vacuum evaporation for concentrating the samples, and 20 µL solution was taken for HPLC.
Dionex Ultimate 3000 liquid chromatograph including a diode array detector with 5 cm flow cell and with Chromeleon system manager as data processor was used for HPLC analyses. Separation was achieved by a reversed-phase Acclaim C18 column (5 micron particle size, 250 mm × 4.6 mm). Filtration of all extracted solutions was done through HPLC filter [0.45 μm membrane filter (Millipore)]. The mobile phase contains methanol (Solvent A) and 0.5% acetic acid solution (Solvent B), and the column was thermostatically controlled at 25 ºC and the injection volume was kept at 20 μL. A gradient elution was performed by varying the proportion of solvent A to solvent B. By the measurement of the integrated peak area, the quantifications of phenolic acids and flavonoids in the sample extracts were carried out. The contents were calculated using the calibration curve by plotting peak area against concentration of the respective standard sample. Standard stock solutions of 21 phenolic acids and flavonoids, including gallic acid, protocatechuic acid, gentisic acid, p-hydroxy benzoic acid, catechin, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferullic acid, sinapic acid, salicylic acid, naringin, rutin, ellagic acid, myricetin, quercetin, naringenin, apigenin and kaempferol, were prepared in methanol at 10 μg/mL. Filtration of all standard solutions was carried out through HPLC filter 0.45 μm membrane filter (Millipore, USA).
Extraction and estimation of biomarkers of oxidative stress
To estimate membrane lipid peroxidation, test for thiobarbituric acid reactive substances (TBARS) was performed using the procedure of Heath and Packer (1968). Seedling tissue (7-day-old) (200 mg) was homogenized in 5 mL of 0.1% trichloroacetic acid(TCA) and then centrifuged at 10 000 r/min for 15 min and finally supernatant was taken. In 1 mL of supernatant, 3 mL of 5% TCA containing 1% thiobarbituric acid (TBA) was added and heated in a hot water bath for 30 min and cooled quickly in cold water bath. It was finally centrifuged at 10 000 r/min for 10 min. The absorbance of the supernatant was measured at 530 nm. The concentration of TBARS was measured from its extinction coefficient of 155 μmol/cm. The non-specific turbidity was corrected by subtracting A600from A530. The concentration of TBARS was measured from its extinction coefficient of 155 μmol/cm. TBARS content is finally expressed in nmol/g dry mass of tissue.
Lipoxygenase was estimated according to Peterman and Siedow (1985). Enzyme was extracted by centrifugation at 5 000 r/min and re-centrifugation at 17 000 r/min in cold using 50 mmol/L sodium- phosphate buffer (pH 6.5). Then, the assay mixture was made containing enzyme extract, 1.30 mmol/L linoleic acid and 1.65 mmol/L sodium-phosphate buffer (pH 6.5). After incubation of the assay mixture for 1 h at 25 ºC, absorbance was taken at 234 nm.
Hydroperoxide was estimated by following Devasagayam et al (2003) with some necessary modifications, and it was extracted with 150 mmol/L Tris-HCl (pH 6.8). Theassay mixture contained 0.25 mmol/L H2SO4, 250 mmol/L ammonium ferrous sulphate, 100 mmol/L xylenol orange, 4 mmol/L butylated hydroxytoluene (BHT) (in 90% methanol) and an aliquot of sample extract. After incubation at room temperature for 30 min, triphenyl phosphine (100 mmol/L) was added to the reaction mixture to specify the reduction of hydroperoxide, distinguished from hydrogen peroxide (Nourooz-Zadehet al, 1994). Then the absorbance was taken at 560 nm.
The process of Buege and Aust (1978) was followed for the estimation of conjugated diene. Rice tissue of 7-day-old seedlings was extracted with chloroform : methanol mixture (2 : 1) followed by vigorous vortex mixing and then centrifuged for 10 min at 2 000 r/min. After centrifugation, the lower chloroform layer was collected and dried at 45 ºC under steam of nitrogen. The obtained residue was dissolved in 7 mL cyclo-hexane, and absorbance was taken at 230 nm.
Determination of germination performances and post germinative growth performances
Germination performances (Rubio-Casal et al, 2003; Kader, 2005; Bhattacharjee, 2008) of PIDS-raised IARCs were assessed in terms of coefficient of velocity of germination (CVG), germination rate index (GRI), mean germination time (MGT), and mean daily germination (MDG) as:
= ∑/ ∑() × 100
= ∑∑× 100
=∑() / ∑× 100
=/
Here,is No. of seeds germinated on day,is No. of days from sowing,is total No. of germinated seeds, andis total No. of days.
Statistical analysis
Each experiment was carried out twice at different times with three replicates. Results were calculated as mean ± standard error. For the-test, statistical analysis of the data for significance, two-paired samples for means was applied using Microsoft Excel 2010, which shows the significant variations between untreated control and different magnitude of PIDS raised seedlings.
RESULTS
Differential accumulation of osmolytes (organic and inorganic)
When application of PEG-6000 of different magnitudes (-0.344, -0.851 and -1.619 MPa) was imposed in post-imbibitional stage to four IARCs, cultivar- and dose-dependent changes in the accumulation of osmolytes (proline, glycinebetaine, soluble carbohydrate, reducing carbohydrate and K+ion) were noticed (Figs. 1 and 2). When compared between the cultivars, Tulaipanji and Badshabhog exhibited significant increment of all four organic osmolytes under the same magnitude of PIDS. On the contrary, the accumulations of glycinebetaine, soluble carbohydrate, reducing carbohydrate and proline were found to be reduced over their corresponding untreated control for Jamainadu and Sitabhog. Proline concentration was reduced for Sitabhog, whereas Jamainadu exhibited a marginal but significant increase in proline concentration (Figs. 1 and 2). A comparative estimation of the inorganic osmolyte K+also revealed almost same pattern of Tulaipanji with exhibiting greater accumulation of K+over their untreated control under different magnitude of PIDS. On the contrary, Jamainadu and Sitabhog exhibited a dose-dependent reduction in the K+accumulation. Badshabhog exhibited a marginal but significant increase of the same under the different magnitudes of PIDS (Fig. 1).
Proline being a bona fide osmolyte and antioxidant, its metabolism was studied in terms of two important marker enzymes, P5CS and PDH. The activities of P5CS and PDH were found to increase and decrease for Tulaipanji and Badshabhog, respectively (Fig. 2). On the contrary, Jamainadu exhibited a significant decline and increment in the activities of P5CS and PDH under different magnitudes of PIDS, respectively, and Sitabhog exhibited a significant increment in P5CS activity in dose-dependent manner under PIDS and also a significant rise in the activity of PDH (Fig. 2), corroborating well with the data of proline accumulation. Therefore, from the results pertaining to the accumulation of proline, glycinebetaine, soluble carbohydrate and reducing carbohydrate, it can be said that a cultivar-specific differences in the accumulation of osmolytes under the different magnitude of PIDS was observed, with Tulaipanji and Badshabhog showing the greater capability to accumulate those organic osmolytes under stress. Sitabhog and Jamainadu suffered significant loss of those osmolytes under the same magnitude of PIDS. The activities of proline metabolising enzymes further confirmed the better ability of proline synthesis and inhibition of proline degradation under PIDS for Tulaipanji and Badshabhog as compared to Sitabhog and Jamainadu, corroborating well with the data of proline accumulation.

Fig. 1. Differential osmolyte accumulation (organic and inorganic).
A, Accumulation of glycinebetaine. B, Soluble carbohydrate content. C, Reducing carbohydrate content. D, Accumulation of K+ion.
PIDS, Post imbibitional dehydration stress.
Data are Mean ± SE (= 3). * and **, Significant differences at the 0.05 and 0.01 levels (-test), respectively.

Fig. 2. Differential proline accumulation (A) and activities of pyrrolin-5-carboxylate synthetase (B) and proline dehydrogenase (C).
PIDS, Post imbibitional dehydration stress.
Data are Mean ± SE (= 3). * and **, Significant differences at the 0.05 and 0.01 levels (-test), respectively.
Changes in ROS-antioxidant interaction at metabolic interface
PIDS (-0.344, -0.851 and -1.619 MPa) caused changes in ROS-antioxidant interaction at metabolic interface during early germination of all the IARCs. The redox status pertaining to ROS-antioxidant interaction was estimated in terms of ROS accumulation (DCFDA oxidation and H2O2accumulation) and total antioxidant capacity (DPPH radical scavenging activity and total thiol content). The results in general showed a significant tilt towards prooxidants for Sitabhog and Jamainadu, whereas Tulaipanji and Badshabhog showed significant tilt towards antioxidant competence under PIDS (Fig. 3). The rise in endogenous level of accumulation of total ROS and H2O2in consonance with the reduction of radical scavenging properties and total thiol content for Jamainadu and Sitabhog imposed serious oxidative threat to the germinating juvenile seedlings raised from different magnitude of PIDS. Tulaipanji and Badshabhog, respectively not only controlled the endogenous level of ROS through the general up-regulation in the antioxidant competence but also reduced significantly.
Laser scan confocal microscopy was also done to further corroborate the data of spectrofluorometric and spectrophotometric ROS estimation of PIDS-raised seedlings of IARCs. The result showed significantly greater histochemical accumulation of ROS in the tissues of Jamainadu and Sitabhog, raised from -1.619 MPa PIDS as compared to their control (Fig. 4). Tulaipanji and Badshabhog exhibited significantly lower accumulation of ROS at cellular level detected histochemically by laser confocal microscopy under the same magnitude of PIDS, when compared to their untreated control (Fig. 4).
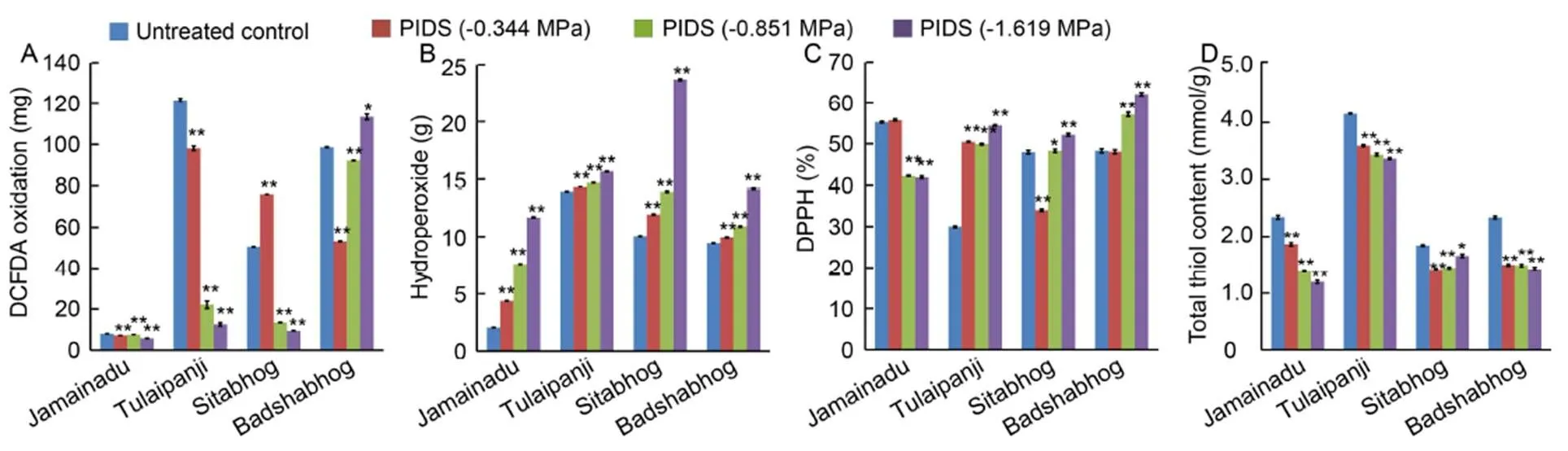
Fig. 3. Dehydration stress induced changes in internal redox cues assessed in terms of total reactive oxygen species(ROS) accumulation.
A, DCFDA (2′,7′-dichlorofluorescin diacetate) oxidation. B, Hydrogen peroxide accumulation. C, DPPH assay. D, Total thiol content.
PIDS, Post imbibitional dehydration stress; DCFDA, 2′,7′-dichlorofluorescin diacetate; DPPH,2,2-diphenyl-1-pycryl hydrazyl.
Data are Mean ± SE (= 3). * and **, Significant differences at the 0.05 and 0.01 levels (-test), respectively.
PIDS induced differential elicitation of redox- sensitive flavonoids and phenolic acids in four IARCs
In order to ascertain the metabolic role of polyphenolic compounds particularly flavonoids and phenolic acids in ROS-antioxidant interaction, necessary for redox- regulation of PIDS-raised seedlings of all the IARCs, RP-HPLC based identification and quantification of some important redox-sensitive flavonoids and phenolic acids were done. Here, we have identified and quantified seventeen redox-sensitive phenolic compounds through RP-HPLC, like catechin (Rt 40.50), naringin (Rt 70.78), rutin (Rt 72.40), quercetin (Rt 88.14), kaempferol (Rt 99.42), myricetin (Rt 77.90), apigenin (Rt 93.60), gallic acid (Rt 7.69), protocatechuic acid (Rt 7.56), gentisic acid (Rt 30.68), para-hydroxy benzoic acid (Rt 36.76), chlorogenic acid (Rt 43.37), caffeic acid (Rt 47.05), syringic acid (Rt 49.17), salicylic acid (Rt 69.53), sinapic acid (Rt 62.66) and p-coumaric acid (Rt 55.27) (Fig. 5). Badshabhog exhibited the highest individual level up-regulation of phenolic compounds tested for PIDS-raised seedlings over their untreated control. It showed significantly greater accumulation of flavonoids rutin (249.37% increment) and kaempferol (68.75% increment) along withsynthesis of the flavonoids myricetin and apigenin which were all together absent in untreated control seedlings. Naringin and quercetin suffered only marginal loss in PIDS- raised seedlings over their untreated control. The phenolic acids like chlorogenic acid, caffeic acid and p-coumaric acid similarly showed an up-regulation by 14.51%, 5.65% and 31800%, respectively, for PIDS- raised seedlings of Badshabhog over their untreated control. Sinapic acid, which was all together absent in control seedlings, showedexpression (Table 1).
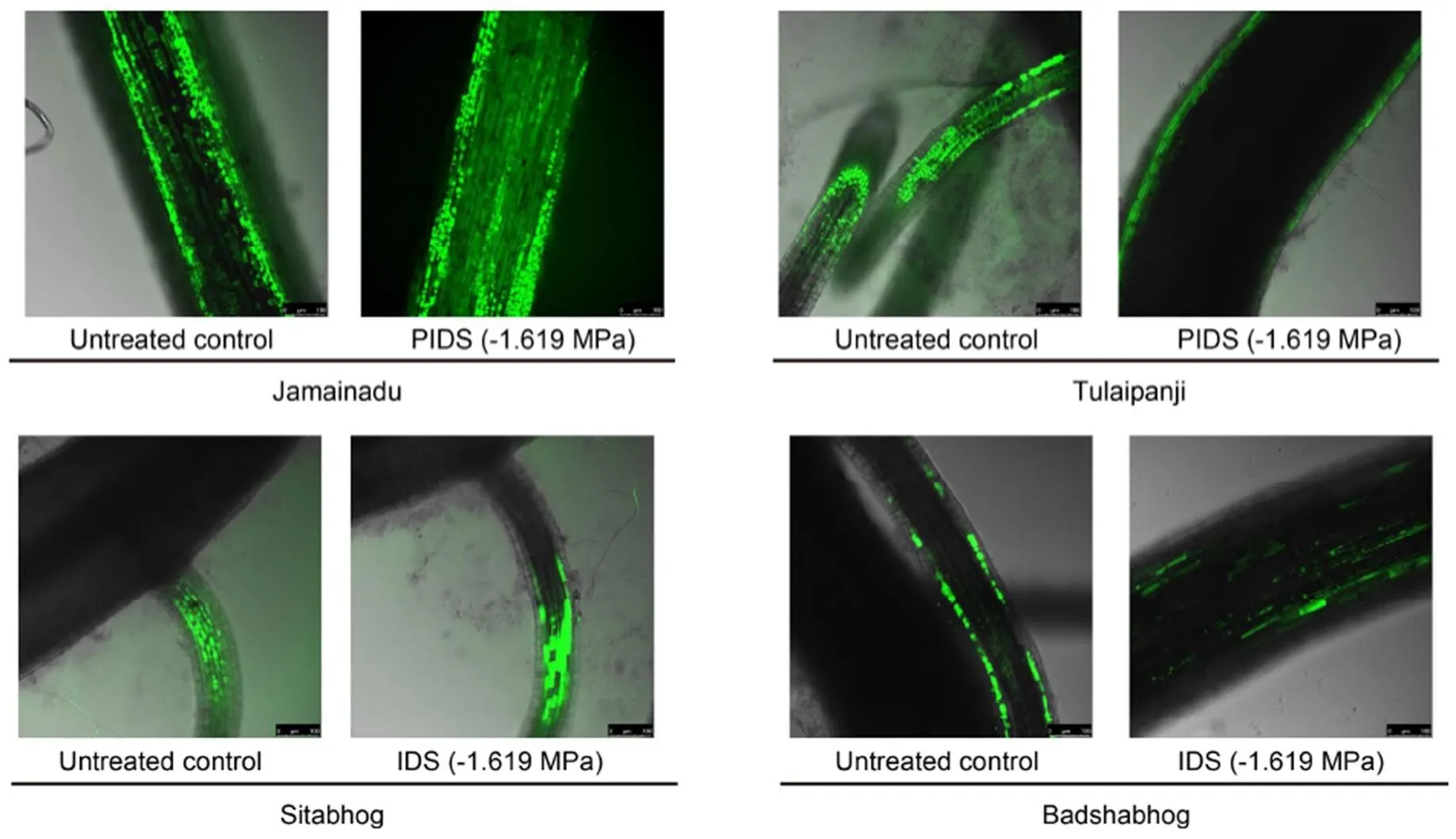
Fig. 4. Laser confocal microscopy showing accumulation of reactive oxygen species (H2O2) using Leica application suite X software.
PIDS, Post imbibitional dehydration stress.
Tulaipanji showed significant up-regulation in the accumulation of rutin, kaempferol, gentisic acid and chlorogenic acid by 4739.76%, 150.38%, 38.83% and 1103.45% respectively in PIDS-raised seedlings over their untreated control (Table 1). Sitabhog and Jamainadu showed down-regulation in the accumulation of almost all the flavonoids and phenolic acids quantified through RP-HPLC. Sitabhog particularly suffered significant loss in the accumulation of catechin, naringin, rutin, quercetin, myricetin, gallic acid, protocatechuic acid, para-hydroxy benzoic acid, chlorogenic acid, caffeic acid and syringic acid by 97.89%, 74.43%, 91.84%, 62.71%, 50.94%, 65.17%, 71.56%, 95.11%, 92.40%, 69.02% and 96.37%, respectively (Table 1). Gentisic acid, which was present in untreated control of Sitabhog in an appreciable amount (883.05 mg/g) got completely disappeared under PIDS. Similarly, Jamainadu also suffered significant loss of naringin, rutin, gallic acid, protocatechuic acid, gentisic acid, para-hydroxy benzoic acid and syringic acid by 40.58%, 94.44%, 53.94%, 93.56%, 53.93%, 80.67% and 58.75%, respectively (Table 1). Three phenolics, kaempferol, chlorogenic acid and caffeic acid got completely disappeared in PIDS-raised seedlings of Jamainadu. All the RP-HPLC based data convincingly support the fact that Badshabhog and Tulaipanji exhibited PIDS-induced elicitation and up-regulation in the synthesis of some important redox sensitive phenolic acids and flavonoids. Whereas, Sitabhog and Jamainadu showed an overall down-regulation in the accumulation of the redox-sensitive phenolic compounds.
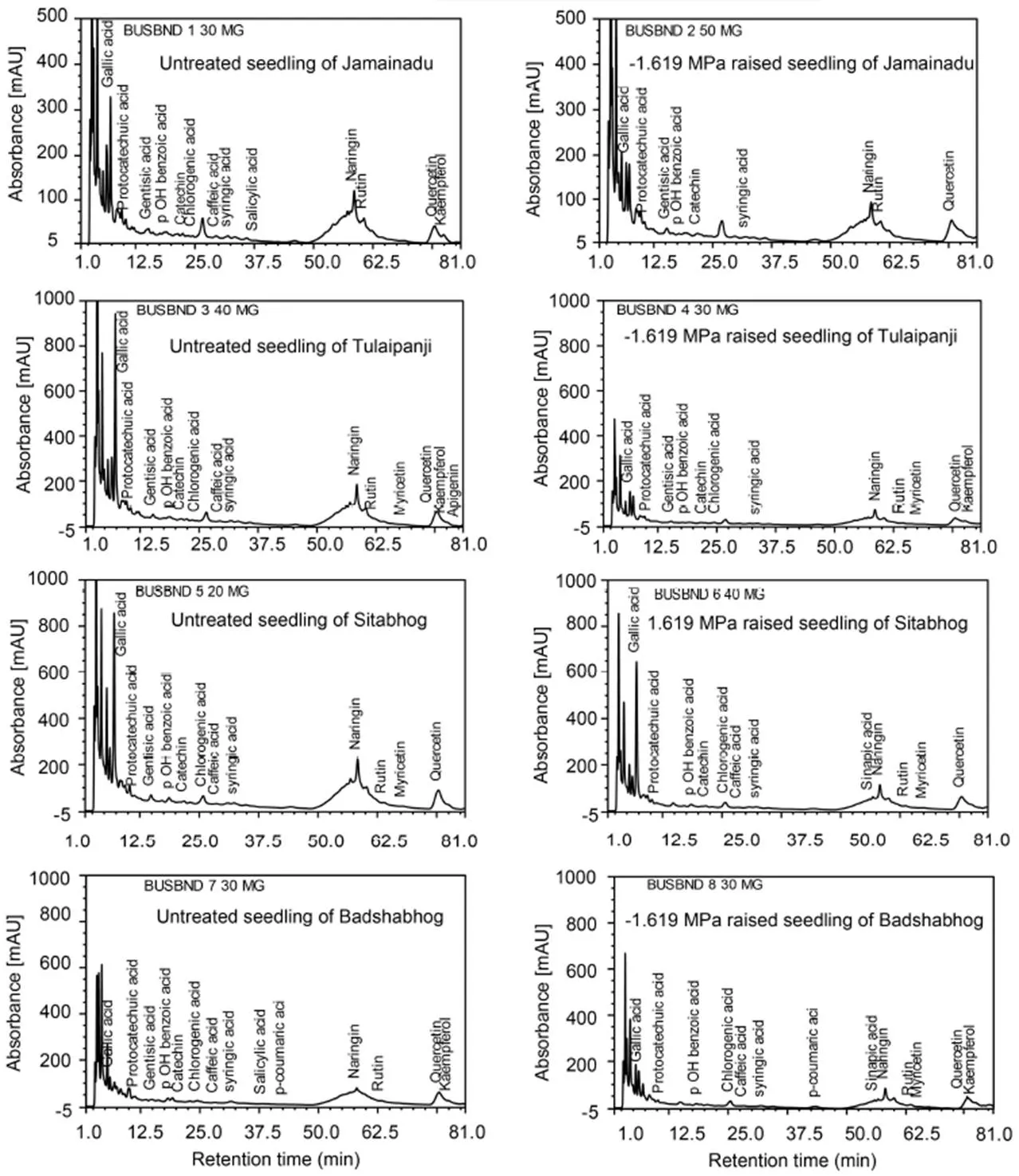
Fig. 5. Reversed phase-high performance liquid chromatography of 17 redox-sensitive flavonoids and phenolic acids.

Table 1. Changes in concentrations of flavonoids and phenolic acids associated under post imbibitional dehydration stress over their corresponding untreated control in seedlings (7-day-old) of four indigenous aromatic rice cultivars. ng/g
andrepresent down-regulation and up-regulation from untreated control, respectively.
PIDS induced differential changes in sensitive redox biomarkers and germination performances
The products of the parameters of oxidative lipid damage such as hydroperoxide, conjugated diene, TBARS and activity of lipoxygenase (LOX) were standardized as sensitive redox biomarkers for the assessment and the estimation of PIDS-induced loss of redox-homeostasis in all the four IARCs, and compared subsequently with the germination performances CVG, GRI, MGT and MDG. The results in general exhibited the impact of PIDS on newly assembled membrane system of the germinating seedlings of all the IARCs. We observed a significant rise in the accumulation of hydroperoxide, conjugated diene and TBARS for Sitabhog and Jamainadu in a dose dependent manner (Table 2). The activity of the enzyme LOX, responsible for enzymatic membrane lipid peroxidation (MLPO), also increased significantly in Sitabhog and Jamainadu in PIDS-raised seedlings over their untreated control (Table 2). Badshabhog and Tulaipanji, though suffered lipid peroxidation (accumulation of hydroperoxide, conjugated diene, TBARS and activity of LOX), when compared with Sitabhog and Jamainadu, accumulated significantly lower lipid peroxidation products under all doses of PEG-6000 treatment (Table 2). Even in some cases, the accumulation of the lipid peroxidation products was lesser or marginal. Therefore, the data of oxidative lipid damage was in concurrence with redox status of all the four IARCs.
DISCUSSION
The selection of metabolic and physiological markers for judicious screening of rice germplasms for their drought stress tolerance is extremely significant, though not absolutely a decisive approach in agricultural research. The integration of genomic and proteomic approaches, as in marker-assisted selection and stress proteome based screening along with important metabolic and biochemical data, always ensures a system level information of the complex drought response of plants, necessary for tolerance (Mir et al, 2012; Zagorchev et al, 2016; Zu et al, 2017). In this aspect, the effectiveness of drought stress induced oxidative stress tolerance involving elicitation of biosynthesis of phenolics and redox-sensitive compatible cell cytosolutes may be used as quality traits for biomarkers of stress tolerance (Abbas et al, 2014; Zhang et al, 2014; Lin et al, 2016; Zivcak et al, 2016; Swapna and Shylaraj, 2017).
MLPO,Membrane lipid peroxidation; CVG,Coefficient of velocity of germination; GRI, Germination rate index; MGT, Mean germination time; MDG,Mean daily germination.
Data are Mean ± SE (= 3). * and **, Significant differences at the 0.05 and 0.01 levels (-test), respectively.
In this study, four IARCs were screened for their drought resistant attributes based on their capacity to produce important redox sensitive phenolic acids and flavonoids. Badshabhog was selected as the lead germplasm for its ability to elicit the highest individual level synthesis of phenolics tested over their untreated control. The significantly greater accumulation of rutin and kaempferol along with thesynthesis of myricetin and apigenin under drought stress in Badshabhog offered the plant for better preparedness to combat oxidative stress induced by drought. Chlorogenic acid,-coumaric acid and caffeic acid also exhibit similar up-regulation necessary for redox- regulation (Velderrain-Rodríguez, 2014; Lin et al, 2016). Phenolic acid and sinapic acid were also producedunder drought stress in Badshabhog highlighting their role in redox-regulation under oxidative stress induced by drought (Quan and Xuan, 2018). Almost a similar trend was observed when we compared the ability of the cultivar Tulaipanji to elicit the synthesis of flavonoids and phenolic acids, with significantly greater accumulation of rutin, kaempferol, gentisic acid and chlorogenic acid etc. The significance of the synthesis of redox-sensitive flavonoids and phenolic acids under drought is primarily due to their ability to quench ROS and chelate transition metal ions necessary for Fenton reaction, thereby, restricting the accumulation of ROS, conferring better redox- regulation (Cao et al, 1997; Pandey et al, 2012; Mishra et al, 2013). We got a negative trend in the synthesis of seventeen phenolic acids and flavonoids under PIDS in Sitabhog and Jamainadu through RP-HPLC study. Sitabhog and Jamainadu particularly exhibited down-regulation in the accumulation of the redox sensitive flavonoids and phenolic acids like catechin, naringin, rutin, quercetin, myricetin, gallic acid, protocatechuic acid, para-hydroxy benzoic acid, chlorogenic acid, caffeic acid and syringic acid. The most noticeable event in this aspect is the complete disappearance of kaempferol, chlorogenic acid and caffeic acid for PIDS-raised seedlings of Jamainadu and gentisic acid for PIDS-raised seedlings of Sitabhog. The down-regulation in the synthesis of the redox- sensitive flavonoids and phenolic acids of Sitabhog and Jamainadu, significantly reduced their ability to combat oxidative stress induced by drought, thereby became susceptible to drought induced oxidative damages.
The role of the compatible cell cytosolutes like proline, glycinebetaine, soluble carbohydrate, reducing carbohydrate etc. under drought stress is considered to be of pivotal significance not only because of their role in stabilizing important cellular biomolecules or maintaining turgor pressure but also for their role in redox-regulation and prevention of oxidative damages (Koyro et al, 2012; Wani et al, 2013; Zivcak et al, 2016). In the present study, a comparative evaluation of four IARCs for their ability to produce osmolytes like proline, glycinebetaine, soluble carbohydrate and reducing carbohydrate revealed a clear distinction in their ability to produce the same components in the order Badshabhog > Tulaipanji > Sitabhog > Jamainadu. Further, the activities of P5CS and PDH were increased and decreased significantly for Badshabhog and Tulaipanji as compared to Sitabhog and Jamainadu. These osmoprotectants eliminate the negative effect of drought stress either by improving or up-regulating the antioxidative defense system and sustaining ion homeostasis (Ashraf and Foolad, 2007; Kaya et al, 2013; Singh et al, 2015). Hasanuzzaman et al (2014) and Zivcak et al (2016) reported osmolyte (proline, glycinebetaine and soluble carbohydrate) induced up-regulation in the activity of antioxidative enzyme to protect and restore enzymatic antioxidative defense system, responsible for maintaining redox homeostasis of plant cells. The up-regulation of proline biosynthesisdepends chiefly on the activities of P5CS (Nounjana et al, 2012). Since the accumulation of proline depends on its turnover, the activity of PDH is also important for its accumulation (Peng et al, 1996). Therefore, the cultivars maintaining or up-regulating the higher P5CS activity and reduced PDH activity are strategically better adapted for their redox-regulation under drought stress (Smirnoff and Cumes, 1989). Proline accumulation is also the part of signaling network associated with adaptive processes of the plant under drought (Brestic et al, 2014).
The osmolyte proline contributes significantly to drought stress tolerance through being an efficient scavenger of ROS (Smirnoff and Cumes, 1989), buffering cytosolic pH for redox maintenance (Hasanuzzaman et al, 2014), stabilizing of proteins and enzymes against oxidative deterioration (Verbruggen and Hermans, 2008). In soybean, rice and maize, over-expression ofgene exhibits improved drought and heat tolerance as compared to their wild type (de Rond et al, 2004; Liu et al, 2012; Nikolaeva et al, 2015). Similarly, the accumulation of GB is directly correlated with stabilization and maintenance of enzyme and membrane structure (Sakamoto and Murata, 2000). GB has protective role against oxidative stress as confirmed by several works (Park et al, 2004; Khan et al, 2015; Hisyam et al, 2017). Accumulation of sugars under drought stress also contributes significantly in redox- regulation through their ability to scavenge oxy-free radicals (Livingston et al, 2009; Koyro et al, 2012). Hence, the role of these compatible osmolytes under drought stress strongly supports their involvement in redox-regulation for better preparedness to combat drought-induced secondary oxidative stress in IARCs.
Therefore, the strategic changes in the accumulation of seventeen polyphenolic compounds, and the endogenous titer of osmolytes (proline, glycinebetaine, soluble carbohydrate and reducing carbohydrate) in Badshabhog and Tulaipanji make them better adopted to combat drought induced oxidative damages as compared to Sitabhog and Jamainadu during post- imbibitional stage of seedling establishment. Further, to corroborate this hypothesis, we also assessed some sensitive redox biomarkers, accumulation of reactive oxygen species and antioxidant capacity. The results strongly corroborated the fact that the IARCs capable of better accumulation of polyphenolic compounds and osmolytes exhibited better redox-regulatory property and hence suffered significantly lesser oxidative deterioration induced by PIDS (Basu et al, 2010; Tari et al, 2010; Faize et al, 2011; Zivcak et al, 2016).
Further, the positive correlation between the accumulation of phenolic compounds and their redox regulatory capacity in seedlings of four IARCs raised from PIDS strongly supports the role of polyphenolic compounds in the mitigation of oxidative stress (Rice- Evans et al, 1997). This group of chemicals characterized by the presence of more than one phenol unit per molecule may be flavonoids, phenolic acids or others. Out of the different groups of this polyphenolic compounds, flavonoids and phenolic acids are found to be redox-sensitive to cellular conditions and related to stress tolerance (Velderrain-Rodríguez et al, 2014; Lin et al, 2016). The structural chemistry of polyphenolic compounds is ideal for radical scavenging activity and often more effectivethan the bona fide antioxidant vitamins C and E (Rice-Evans et al, 1997). Significantly higher ability as electron donor and polyphenol derived radicals to stabilize and delocalize the unpaired electron (chain breaking reaction), and finally the capacity to chelate transition metal ions make these polyphenolic compounds ideal for redox- regulation under abiotic stress.
In earlier studies, efficiencies of antioxidative defense systems, particularly the elaborate enzymatic anti- oxidative defense network, have been held responsible for combating drought stress during early germination in rice (Wahid et al, 2007; Hossain et al, 2013; Chakraborty and Bhattacharjee, 2015; Chakraborty et al, 2019). However, present study exhibited the close connection between the accumulation of osmolytes andpolyphenolic compounds with the redox-regulatory properties in the four IARCs. The significantly better capacity for the maintenance of flavonoids and phenolic acids indirectly play pivotal role under PIDS to combat oxidative damage in, Badshabhog and Tulaipanji, confirming once again the role of these redox sensitive polyphenolic compounds to combat dehydration stress during post germinative seedling establishment stage (Steffensena et al, 2011). Similarly, the accumulation of osmolytes like glycinebetaine, proline, soluble carbohydrate and reducing carbohydrateseems to have positive correlation in preventing oxidative damages triggered by PIDS through their capacity to scavenge reactive oxygen species, protecting important cellular macromolecules from oxidative deterioration (Turkan and Demiral, 2009; Koyro et al, 2012; Zivcak et al, 2016; Swapna and Shylaraj, 2017).
Though the maintenance of osmolyte has long being proposed as first line of defense against drought stress but the action of osmolytes needs to be complemented by other ROS scavenging secondary metabolites for efficient redox regulation. We suggest polyphenolic compounds as the most promising secondary antioxidant defense system that complement with osmolyte for better redox regulation under drought stress in IARCs. When compared the IARCs, a distinct exhibition of differential redox controlling attributes in the PIDS-raised seedlings was observed. ROS-osmolyte/polyphenolic compounds interaction was found to act at metabolic interface for the generation of favorable internal redox cue in the order Badshabhog > Tulaipanji > Sitabhog > Jamainadu, determining their germination phenotype and ability of seedling establishment. Moreover, the work also made an effort to standardize the important redox regulated traits for screening of rice germplasms differing in sensitivity towards drought stress which may be targeted by plant breeders for selection or genetic manipulation for improving drought stress tolerance.
Acknowledgements
The authors acknowledge University of Burdwan, West Bengal, India, for State Funded Research Fellowship (Grant No. 136/35, 31.07.2014), Government of West Bengal, India.
Abbas S R, Ahmad S D, Sabir S M, Shah A H. 2014. Detection of drought tolerant sugarcane genotypes () using lipid peroxidation, antioxidant activity, glycine-betaine and proline contents., 14(1): 233–243.
Allison L E, Bernstein L, Bower C A, Brown J W, Fireman M, Hatcher J T, Hayward H E, Pearson G A, Reeve R C, Richards L A, Wilcox L V. 1954. Diagnosis and improvement of saline and alkali soils.: Richards L A. Agriculture Handbook No. 60, Washington, United States: Department of Agriculture: 96.
Ashraf M, Foolad M R. 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance., 59(2): 206–216.
Basu S, Roychoudhury A, Saha P P, Sengupta D N. 2010. Differential antioxidative responses ofrice cultivars to drought stress., 60(1): 51–59.
Bates L S, Waldren R P, Teare I D. 1973. Rapid determination of free proline for water stress studies., 39: 205–207.
Bhattacharjee S. 2008. Calcium-dependent signaling pathway in the heat-induced oxidative injury in., 52: 137.
Bhattacharjee S, Dey N. 2018. Redox metabolic and molecular parameters for screening drought tolerant indigenous aromatic rice cultivars., 24(1): 7–23.
Boyer J S. 1982. Plant productivity and environment., 218: 443–448.
Brestic M, Zivcak M, Olsovska K, Kalaji H M, Shao H B, Hakeem K R. 2014. Heat signaling and stress responses in photosynthesis.: Hakeem K R, Rehman R, Tahir I. Plant Signalling: Understanding the Molecular Cross-Talk. New Delhi: Springer: 241–256.
Buege J A, Aust S D. 1978. Microsomal lipid peroxidation., 52: 302–310.
Cao G H, Sofic E, Prior R L. 1997. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships., 22(5): 749–760.
Chakraborty A, Bhattacharjee S. 2015. Differential competence of redox-regulatory mechanism under extremes of temperature determines growth performances and cross tolerance in tworice cultivars., 176: 65–77.
Chakrabarty A, Banik N, Bhattacharjee S. 2019. Redox-regulation of germination during imbibitional oxidative and chilling stress in anrice cultivar (L., Cultivar Ratna)., 25(3): 649–665.
Chaves M M, Oliveira M M. 2004. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture., 55: 2365–2384.
Chen T H H, Murata N. 2011. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications., 34(1): 1–20.
De Ronde J A, Cress W A, Krüger G H, Strasser R J, van Staden J. 2004. Photosynthetic response of transgenic soybean plants, containing angene, during heat and drought stress., 161(11): 1211–1224.
Deb D. 2005. Seeds of Tradition, Seeds of Future: Folk Rice Varieties from East India. New Delhi: Research Foundation for Science Technology & Ecology: 12–14.
Devasagayam T P A, Boloor K K, Ramasarma T. 2003. Methods for estimating lipid peroxidation: An analysis of merits and demerits., 40(5): 300–308.
Fageria N K. 2007. Yield physiology of rice., 30: 843–879.
Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, Clemente-Moreno M J, Alcobendas R, Artlip T, Hernandez J A. 2011. Involvement of cytosolic ascorbate peroxidase and Cu/Zn- superoxide dismutase for improved tolerance against drought., 62(8): 2599–2613.
Farooq M, Basra S M A, Wahid A, Cheema Z A, Cheema M A, Khaliq A. 2008. Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (L.)., 194(5): 325–333.
Grieve C M, Grattan S R. 1983. Rapid assay for determination of water soluble quaternary ammonium compounds., 70: 303–307.
Gupta K, Dey A, Gupta B. 2013. Plant polyamines in abiotic stress responses., 35: 2015–2036.
Gusakov A V, Kondratyeva E G, Sinitsyn A P. 2011. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities., 2011: 283658.
Hasanuzzaman M, Nahar K, Alam M M, Roychowdhury R, Fujita M. 2013. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants., 14(5): 9643–9684.
Hasanuzzaman M, Alam M M, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. 2014. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (L.) varieties., 2014: 757219.
Hayzer D J, Leisinger T. 1980. The gene-enzyme relationships of proline biosynthesis in., 118(2): 287–293.
Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation., 125(1): 189–198.
Hefny M, Abdel-Kader D Z. 2009. Antioxidant-enzyme system as selection criteria for salt tolerance in forage sorghum genotypes (L. Moench).: Ashraf M, Ozturk M, Athar H R. Salinity and Water Stress. The Netherlands: Springer: 25–36.
Hisyam B, Alam M A, Naimah N, Jahan M S. 2017. Roles of glycinebetaine on antioxidants and gene function in rice plants under water stress., 16: 132–140.
Holmstrӧm K O, Somersalo S, Mandal A, Palva T E, Welin B. 2000. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycinebetaine., 51: 177–185.
Hong Z, Lakkineni K, Zhang Z, Verma DP. 2000. Removal of feedback inhibition of delta(1)-pyrroline-5 carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress., 122(4): 1129–1136.
Hossain M A, Fujita M. 2010. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress., 16: 19–29.
Hossain M A, Mostofa G M, Fujita M. 2013. Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (L.) seedlings., 2: 1–13.
Jagadish S V K, Craufurd P Q, Wheeler T R. 2007. High temperature stress and spikelet fertility in rice (L.)., 58(7): 1627–1635.
Janska A, Marsik P, Zelenkova S, Ovesna J. 2010. Cold stress and acclimation-what is important for metabolic adjustment?, 12(3): 395–405.
Kader M A. 2005. A comparison of seed germination calculation formulae and the association interpretation of resulting data., 138: 65–75.
Kaur N, Sharma I, Kirat K, Pati P K. 2016. Detection of reactive oxygen species inL. (rice)., 6(24): 1–9.
Kaya C, Ashraf M, Dikilitas M, Tuna A L. 2013. Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients: A field trial., 7: 249–254.
Khan M S, Ahmad D, Khan M A. 2015. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance., 18(4): 257–266.
Kishor P B K, Hong Z, Miao G H, Hu C A A, Verma D P S. 1995. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants., 108(4): 1387–1394.
Koyro H W, Ahmad P, Geissler N. 2012. Abiotic stress responses in plants: An Overview.: Ahmad P, Prasad M N V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. New York, Springer: 1–28.
Kumar M, Bijo A J, Baghel R S, Reddy C R K, Jha B. 2012. Selenium and spermine alleviates cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidant system and DNA methylation., 51: 129–138.
Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W, Jonak C, Hauser M T, Luschnig C. 2010. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in., 3(3): 594–602.
Lesk C, Rowhani P, Ramankutty N. 2016. Influence of extreme weather disasters on global crop production., 529: 84–87.
Lin D R, Xiao M S, Zhao J J, Li Z H, Xing B S, Li X D, Kong M Z, Li L Y, Zhang Q, Liu Y W, Chen H, Qin W, Wu H J, Chen S Y. 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes., 21(10): 1374.
Liu J, Zhang F, Zhou J J, Chen F, Wang B S, Xie X Z. 2012. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice., 78(3): 289–300.
Liu X Z, Huang B R. 2000. Carbohydrate accumulation in relation to heat stress tolerance in two creeping Bentgrass cultivars., 125(4): 442–447.
Livingston D P, Hincha D K, Heyer A G. 2009. Fructan and its relationship to abiotic stress tolerance in plants., 66(13): 2007–2023.
MacNevin W M, Uron P F. 1953. Spectrum of hydrogen peroxide from organic hydroperox-ides., 25: 1760–1761.
McCready R M, Guggolz J, Silviera V, Owens H S. 1950. Determination of starch and amylose in vegetables., 22(9): 1156–1158.
Mensor L L, Menezes F S, Leitao G G, Reis A S, dos Santos T C, Coube C S, Leitao S G. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method., 15(2): 127–130.
Mir R R, Zaman-Allah M, Sreenivasulu N, Trethowan R, Varshney R K. 2012. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops., 125(4): 625–645.
Mishra A, Kumar S, Pandey A K. 2013. Scientific validation of the medicinal efficacy of., 2013: 292934.
Nikolaeva M K, Maevskaya S N, Voronin P Y. 2015. Activities of antioxidant and osmoprotective systems and photosynthetic gas exchange in maize seedlings under drought conditions., 62: 314–321.
Nounjana N, Nghiab P T, Theerakulpisuta P. 2012. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes., 169(6): 596–604.
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff S P. 1994. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine., 220(2): 403–409.
Pandey A K, Mishra A K, Mishra A. 2012. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant., 58(1): 142–147.
Parida A K, Dagaonkar V S, Phalak M S, Aurangabadkar L P. 2008. Differential responses of the enzymes involved in proline biosynthesisand degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery., 30: 619–627.
Park E J, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen T H. 2004. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage., 40(4): 474–487.
Park E J, Jeknic´ Z, Pino M T, Murata N, Chen T H. 2007. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress., 30(8): 994–1005.
Peng Z, Lu Q, Verma D P S. 1996. Reciprocal regulation of Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants., 253(3): 334–341.
Peterman T K, Siedow J N. 1985. Behavior of lipoxygenase during establishment, senescence, and rejuvenation of soybean cotyledons., 78(4): 690–695.
Petrov V, Hille J, Mueller-Roeber B, Gechev T S. 2015. ROS- mediatedabiotic stress-induced programmed cell death in plants., 6: 69.
Quan N T, Xuan T D. 2018. Foliar application of vanillic and p- hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexinmomilactones in rice., 64(13): 1831–1846.
Ramesh R, Ramesh T, Rao P R, Shankar V G, Bhave M H V. 2017. High temperature stress effected the biochemical parameters of rice (L.) varieties and hybrids., 5(4): 1478–1490.
Ranganayakulu G S, Veeranagamallaiah G, Sudhakar C. 2013. Effect of salt stress on osmolyte accumulation in two groundnut cultivars (L.) with contrasting salt tolerance., 7: 586–592.
Rasheed R, Wahid A, Farooq M, Hussain I, Basra S M A. 2011. Role of proline and glycine betaine pretreatments in improving heat tolerance of sprouting sugarcane (sp.) buds., 65: 35–45.
Rena A B, Splittstoesser W E. 1975. Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons., 14(3): 657–661.
Rhodes D, Samaras Y. 1994. Genetic control of osmoregulation in plants.: Strange K. Cellular and Molecular Physiology of Cell Volume Regulation. Boca Raton: CRC Press: 347–367.
Rice-Evans C, Miller N, Paganga G. 1997. Antioxidant properties of phenolic compounds., 2(4): 152–159.
Rizhsky L, Liang H J, Shuman J, Shulaev V, Davletova S, Mittler R. 2004. When defense pathways collide: The response ofto a combination of drought and heat stress., 134(4): 1683–1696.
Rubio-Casal A E, Castillo J M, Luque C J, Figueroa E M. 2003. Influence of salinity on germination and seeds viability of two primary colonizers of Mediterranean salt pans., 53(2): 145–154.
Sahebi M, Hanafi M M, Rafii M Y, Mahmud T M M, Azizi P, Osman M, Abiri R, Taheri S, Kalhori N, Shabanimofrad M, Miah G, Atabaki N. 2018. Improvement of drought tolerance in rice (L.): Genetics, genomic tools, and the WRKY gene family., 2018: 3158474.
Sakamoto A, Murata A N. 1998. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold., 38: 1011–1019.
Sakamoto A, Murata N. 2000. Genetic engineering of glycinebetainesynthesis in plants: Current status and implications for enhancement of stress tolerance., 51(342): 81–88.
Samarah N H. 2016. Understanding how plants respond to drought stress at the molecular and whole plant levels.: Hossain M A, Wani S H, Bhattacharjee S, Burritt D J, Phan Tran L S. Drought Stress Tolerance in Plants. Switzerland: Springer Nature: 1–37.
Silvente S, Sobolev A P, Lara M. 2012. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress., 7(6): e38554.
Swapna S, Shylaraj K S. 2017. Screening for osmotic stress responsesin rice varieties under drought condition., 24(5): 253–263.
Simontacchi M, Caro A, Fraga C G, Puntarulo S. 1993. Oxidative stress affects-tocopherol content in soyabean embryonic axes upon imbibitions., 103(3): 949–953.
Singh S, Srivastava P K, Kumar D, Tripathi D K, Chauhan D K, Prasad S M. 2015. Morpho-anatomical and biochemical adapting strategies of maize (L.) seedlings against lead and chromium stresses., 4(3): 286–295.
Smirnoff N, Cumes Q J. 1989. Hydroxyl radicals scavenging activity of compatible isolates., 28(4): 1057–1060.
Steffensena S K, Rinnan A, Mortenseng A G, Laursen B, de Troiani R M, Noellemeyer E J, Janovska D, Dusek K, Delano-Frier J, Taberner A, Christophersen C, Fomsgaard I S. 2011. Variations in the polyphenol content of seeds of field grown Amaranthus genotypes., 129(1): 131–138.
Sugio A, Dreos R, Aparicio F, Maule A J. 2009. The cytosolic protein response as a sub component of the wider heat shock response in., 21(2): 642–654.
Tang R S, Zheng J C, Jin Z Q, Zhang D D, Huang Y H, Chen L G. 2008. Possible correlation between high temperature induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (L.)., 54: 37–43.
Tari I, Kiss G, Deér A K, Csiszár J, Erdei L, Gallé Á, Gémes K, Horváth F, Poór P, Szepesi Á, Simon L M. 2010. Salicylic acid increased aldose reductase activity and sorbitol accumulation in tomato plants under salt stress., 54: 677–683.
Tausz M, Sircelj H, Grill D. 2004. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid?, 55: 1955–1962.
Tietze F. 1969. Enzymatic method for quantitative determination of nanogram amounts of total and oxidised glutathione: Application to mammalian blood and other tissues., 27(3): 502–522.
Turkan I, Demiral T. 2009. Recent developments in understanding salinity tolerance., 67(1): 2–9.
Velderrain-Rodríguez G R, Palafox-Carlos H, Wall-Medrano A, Ayala-Zavala J F, Chen C Y O, Robles-Sanchez M, Astiazaran- García H, Alvarez-Parrilla E, González-Aguilar G A. 2014. Phenolic compounds: Their journey after intake., 5: 189–197.
Verbruggen N, Hermans C. 2008. Proline accumulation in plants: A review., 35(4): 753–759.
Waditee R, Bhuiyan M N H, Rai V, Aoki K, Tanaka Y, Hibino T, Suzuki S, Takano J, Jagendorf A T, Takabe T, Takabe T. 2005. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance inand., 102(5): 1318–1323.
Wahid A, Perveen M, Gelani S, Basra S M A. 2007. Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins., 164(3): 283–294.
Wani S H, Singh N B, Haribhushan A, Mir J I. 2013. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine., 14(3): 157–165.
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi- Shinozaki K, Shinozaki K, Yoshiba Y. 2005. Effects of free proline accumulation in petunias under drought stress., 56: 1975–1981.
Yang X L, Wang B F, Chen L, Li P, Cao C G. 2019. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, andquality., 9: 3742.
Zagorchev L, Teofanova D, Odjakova M. 2016. Ascorbate-glutathione cycle: Controlling the redox environment for drought tolerance.: Hossain M A, Wani S H, Bhattacharjee S, Burritt D J, PhanTran L S. Drought Stress Tolerance in Plants. Springer International Publishing: 187–226.
Zhang N, Liu B L, Ma C Y, Zhang G D, Chang J, Si H J, Wang D. 2014. Transcriptome characterization and sequencing-based identification of drought-responsive genes in potato., 41(1): 505–517.
Zivcak M, Brestic M, Sytar O. 2016. Osmotic adjustment and plant adaptation to drought stress.: Hossain M A, Wani S H, Bhattacharjee S, Burritt D J, Phan Tran L S. Drought Stress Tolerance in Plants. Springer International Publishing: 105–143.
Zu X F, Lu Y K, Wang Q Q, Chu P F, Miao W, Wang H Q, La H G. 2017. A new method for evaluating the drought tolerance of upland rice cultivars., 5(6): 488–498.
19 May 2019;
26 December 2019
Soumen Bhattacharjee (sbhattacharjee@bot.buruniv.ac.in; soumen1995@yahoo.com)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.008
(Managing Editor: Fang Hongmin)
杂志排行
Rice Science的其它文章
- Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
- Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases
- RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
- RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
