Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
2020-07-06ZhuXiaoboZeMuChernMawshengChenXueweiWangJing
Zhu Xiaobo, Ze Mu, Chern Mawsheng, Chen Xuewei, Wang Jing
Review
Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
Zhu Xiaobo1, Ze Mu1, Chern Mawsheng2, Chen Xuewei1, Wang Jing1
(State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China, State Key Laboratory of Hybrid Rice / Key Laboratory of Major Crop Diseases & Collaborative Innovation Center for Hybrid Rice in Yangtze River Basin / Rice Research Institute, Sichuan Agricultural University)
Plant lesion mimic mutants () generally possess autoimmunity and hypersensitive response (HR)-like cell death in the absence of biotic or abiotic stress. They have attracted much attention because they are useful tools for deciphering the interaction between defense signaling and growth. Recent studies have identified more than 30involved in the plant immune response and cell death in rice. Genes underlying these, coding for diverse types of proteins, mainly regulate transcription, protein translation and modification, vesicular trafficking and catalyzation of metabolism. Here, we presented an overview of the most recent advances on the study ofin rice and proposed a perspective on potential utilization ofgenes in agriculture.
lesion mimic mutant; autoimmunity; cell death; growth regulation; rice
Plants are sessile organisms that are constantly exposed to adverse environmental factors, including various pathogens. To survive from pathogen attack, plants have developed a robust innate immune system. During plant-pathogen interaction, many molecular events are activated to reprogram plants to fight against pathogens. Typical immune responses usually include production of reactive oxygen species (ROS), synthesis of antimicrobial metabolites, activation of pathogenesis-related() genes, trafficking of related proteins or metabolites, protein modifications includingphosphorylation and ubiquitination, changes in hormone levels and ion fluxes, and reinforcement of cell wall (Nurnberger et al, 2004; Tsuda and Katagiri, 2010; Henry et al, 2013). As a consequence, rapid cell death, characterized as hypersensitive response (HR), occurs in and around the infection site to restrict further invasion of pathogens, preventing disease development(Mur et al, 2008). However, activation of such an immune response usually delays plant growth (Lorrain et al, 2003).
Many mutants have been identified in maize, rice,and other plants that exhibit spontaneous, HR-like cell death spots on their bodies even in the absence of pathogen infection, insect feeding, stress or mechanical damages (Hoisington et al, 1982; Lorrain et al, 2003; Huang et al, 2010). These mutants are usually named based on the cell death phenotypes, such as(),(),() and others (Lorrain et al, 2003; Cao et al, 2019). Here we employ more commonly used designationto represent these types of mutants. These mutants usually carry characteristics of activated immune response, such as ROS burst, elevated levels of salicylic acid (SA), ethylene (ET) or jasmonic acid (JA), in the absence of pathogen attack.
It has been a long time since the first ricemutant,(), was identified andcharacterized (Sekiguchi, 1965). From then on, an increasing number offrom rice have been reported. Huang et al (2010) firstly reviewed progress of riceresearch, mainly focusing onsources and phenotypes, genetic models, and the six genes cloned at that time. During the past ten years, at least another 27 new ricehave been identified. Theseusually present enhanced immune responses, elevated disease resistance and altered growth or developments (Supplemental Table 1), whichprovide new materials and tools for studying the crosstalk between immunity and growth.
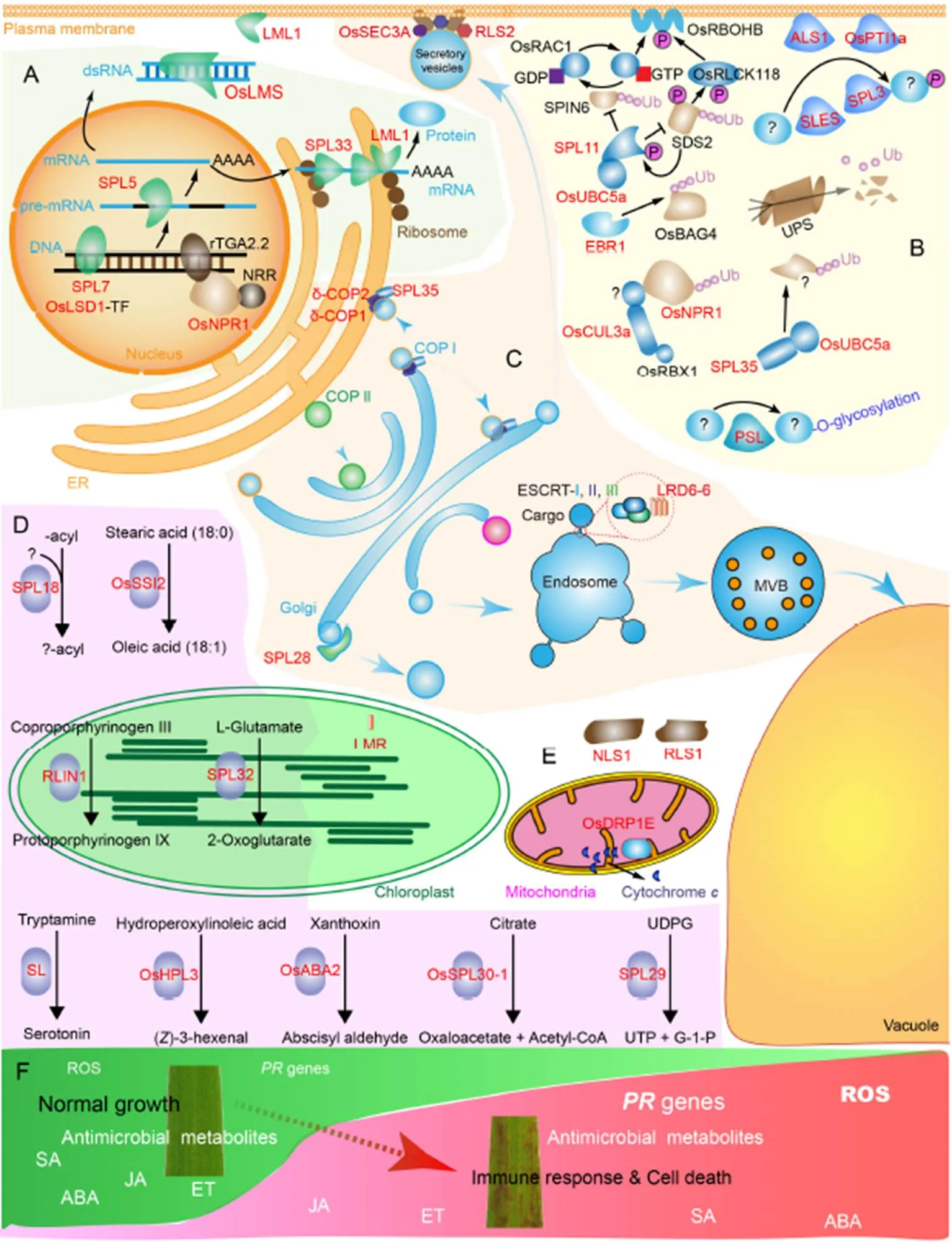
Fig. 1. Schematic illustration of pathways regulated by rice lesion mimic mutants (LMMs).
A,genes involved in transcription and translation processes. B, LMMs acting as regulators of protein post-translational modifications. C, LMMs function in efficient intracellular vesiculartrafficking. D, LMMs catalyze metabolic pathways. E, Other LMMs regulate immunity and cell death. F, Common downstream signaling pathways activated in.
Red color fonts indicate identified proteins causing LMM.
ER, Endoplasmic reticulum; NRR, Negative regulator of resistance; UPS,Ubiquitin proteasome system; PSL, Premature senescence leaf; LMR, Lesion mimic resembling; P, Phosphate; GDP, Guanosine diphosphate; GTP, Guanosine triphosphate; ROS, Reactive oxygen species; SA, Salicylic acid; JA, Jasmonic acid; ABA, Abscisic acid; ET, Ethylene; UDPG, Uridine 5-diphosphoglucose- glucose; UTP, Uridine triphosphate.
In this review, we mainly choose 33 rice LMM proteins for discussion. These LMMs mainly regulate four types of biological processes, including gene transcription and protein translation (Fig. 1-A), protein post-translational modifications (PTMs) (Fig. 1-B), vesicular trafficking (Fig. 1-C), and metabolic fluxes (Fig. 1-D). In addition, there are other regulators of immunity and cell death (Fig. 1-E). We discussed functions of thesegenes and the crosstalk between immunity and growth, and provide thoughts for the implication of breeding rice with high yield and disease resistance.
Shifts in factors regulating gene transcription or protein translation result in an LMM phenotype
Transcription is the first step for a gene to function in which the protein-coding DNA (gene) is transcribed by RNA polymerase II into a pre-mRNA. Pre-mRNA is subsequently spliced into a matured mRNA, which is used as a template to translate into a protein (Merchante et al, 2017). Transcription factors (TFs) including transcriptional activators and repressors play essential roles in the initiation of transcription and often determine the expression level of a gene (Merchante et al, 2017). The heat shock transcription factor SPOTTED LEAF (SPL) 7 represses immune response and cell death (Yamanouchi et al, 2002; Kojo et al, 2006). Themutant, containing a loss-of- function SPL7 protein with a base substitution in the heat stress transcription factor DNA-binding domain, displays HR-like cell death on leaves and accumulates more H2O2when incubated with elicitors prepared from cell wall extracts of the rice blast fungus(Yamanouchi et al, 2002; Kojo et al, 2006). However, the detailed mechanism of how SPL7 regulates immunity and cell death is currently unknown. Zinc finger protein LESION SIMULATING DISEASE 1 (OsLSD1) functions together with certain TFs in nucleus to regulate rice immune response and cell death (Wang et al, 2005; Wu et al, 2014). Down-regulation ofexpression by RNAi causes HR-like cell death. Interestingly, both elevation and inhibition of its mRNA levels lead to enhanced rice blast resistance, defining its unique and complex role in regulating immunity (Wang et al, 2005; Wu et al, 2014). NONEXPRESSOR OF PR GENES 1 (NPR1) is a transcriptional coactivator, a key protein controlling the establishment of systemic acquired resistance (SAR), an induced defense that spreads to the whole plant through long distance transport after exposure to various elicitors (Pajerowska-Mukhtar et al, 2013). Overexpression of/() in rice can constitutively activate immune response and HR-like cell death, resulting in enhanced host resistance to both rice blast and bacterial blight diseases (Chern et al, 2005b; Yuan et al, 2007). The bacterial blight resistance mediated by OsNPR1 is weakened by NEGATIVE REGULATOR OF RESISTANCE (NRR). NRR interacts with OsNPR1 in the nucleus and suppresses the OsNPR1-mediated activation of defense genes (Chern et al, 2005a). LikeNPR1, OsNPR1 can also interact with TGA (TGACGTCA motif-binding factor) transcription factors to activate immune related genes (Chern et al, 2005b). While OsNPR1 enhances resistance to biotrophicpathogens, it reduces resistance to herbivores (Yuan et al, 2007). This may be due to the role of OsNPR1 in mediating an antagonistic crosstalk between SA- and JA-dependent pathways in rice (Yuan et al, 2007).
mRNA splicing is another regulatory step. SPL5 is a putative splicing factor 3b subunit 3 (SF3b3) which is thought to be involved in pre-mRNA processing (Chen et al, 2009, 2012). Deficiency of SPL5 activates a large number of genes involved in biotic defense responses and ROS metabolism, resulting in spontaneous cell death and elevated resistance to both blast and bacterial blight (Chen et al, 2009; Ge et al, 2015; Jin et al, 2015).() is anotherwith HR-like cell death that displays enhanced resistance to rice blast.encodes a protein containing two double-stranded RNA binding motifs that is predicted to bind dsRNA (Undan et al, 2012). However, the substrates of OsLMS remain unidentified.
Eukaryotic translation elongation factor 1 alpha (eEF1A) has a pivotal role in protein synthesis by catalyzing GTP dependent binding of aminoacyl- tRNA to the acceptor site of the ribosome (Merchante et al, 2017). The eukaryotic translation termination factor 1 (eRF1) is also essential for protein synthesis by terminating and releasing the nascent polypeptide chains from the ribosome (Merchante et al, 2017). The rice eEF1A genes,and,and eRF1 gene(are reported to regulate plant immunity and cell death. Theand/mutants display lesion mimic spots on leaves and enhance resistance to both rice blast and bacterial blight (Wang et al, 2017; Zhao et al, 2017; Qin et al, 2018; Zhang et al, 2018). Because the proteins are localized both on endoplasmic reticulum (ER) and plasma membrane, eRF1 LML1 may possess other functions in addition to its role in translation termination (Qin et al, 2018). Interestingly, SPL33/LMM5.1 and LMM5.4 are two eEF1As which share 97.4% amino acid sequence identity and are functionally redundant (Wang et al, 2017; Zhao et al, 2017). Due to natural variations in their promoters, the expression patterns ofandare different inandrice. Inrice, the expression ofis undetectable and its function is masked by(Zhao et al, 2017). Therefore, only themutant, but not, displays the LMM phenotype inrice and the function of thelocus is subsequently discovered through crossingmutant with anrice (Wang et al, 2017; Zhao et al, 2017).
Alteration of protein PTMs can cause an LMM phenotype
PTMs of proteins are a common phenomenon that widely exist in living organisms. Ubiquitination, phosphorylation and glycosylation are three well- known PTMs which regulate protein stability, activity, interaction and signal transduction (Yin et al, 2019). Their roles in immune response and cell death have also been uncovered in the study of rice. SPL11 is an E3 ubiquitin ligase containing a U-box domain and an armadillo repeat domain. A single point mutation generates a premature stop codon in the SPL11 protein creating themutant, which displays spontaneous cell death and enhanced resistance to fungal and bacterial pathogens (Zeng et al, 2004). SPL11 ubiquitinates SPL11 INTERACTING PROTEIN 6 (SPIN6), a Rho GTPase-activating protein, to tag it for degradation through the 26S proteasome-dependent pathway (Liu et al, 2015). Strikingly, SPIN6 interacts with the small GTPase OsRac1 which is a critical defense component in rice immune signaling. SPIN6 can catalyze the GTP- bound OsRac1 into the GDP-bound state, thus regulating immunity and cell death in rice (Liu et al, 2015). The monocot-specific receptor-like kinase SPL11 CELL DEATH SUPPRESSOR 2 (SDS2) is also suggested to be a substrate of SPL11. SDS2 phosphorylates SPL11, which in turn ubiquitinates SDS2 for degradation (Fan et al, 2018). SDS2 can also phosphorylate the receptor-like cytoplasmic kinases OsRLCK118 and the phosphorylated OsRLCK118 trans-phosphorylates OsRbohB to activate it, leading to ROS generation and defense gene expression (Fan et al, 2018). SPIN6 and SDS2, two positive regulators of immunity, are accumulated to higher levels in themutant resulting in spontaneous HR-like cell death and enhanced resistance (Liu et al, 2015; Fan et al, 2018). Thus, the study of themutant revealed a complex signaling pathway linking many important cellular components. ENHANCED BLIGHT AND BLAST RESISTANCE 1 (EBR1), a RING-type E3 ubiquitin ligase, negatively regulates immunity and cell death in rice (You et al, 2016). Themutant displays spontaneous cell death and enhanced disease resistance to rice blast and bacterial blight (You et al, 2016). In themutant, OsBAG4, a bcl2-associated athanogene family protein and a substrate of EBR1, accumulates. Accumulation of OsBAG4 triggers cell death and autoimmunity in rice (You et al, 2016). As a master regulator of SAR, the stability of NPR1 is also regulated by ubiquitination mediated by the Cullin3- based RING E3 ubiquitin ligase (CRL3), composed of Cullin3 (CUL3), RBX1 and BTB proteins. In rice, OsCUL3a is a CUL3 protein that mediates the ubiquitination and degradation of OsNPR1 (Liu et al, 2017). Loss-of-function of OsCUL3a activates OsNPR1- mediated immunity and cell death (Liu et al, 2017). Recently, the gene encoding a CUE (coupling of ubiquitin conjugation to ER degradation) domain containing protein SPL35 is cloned as another E3 ubiquitin ligase from a new. The SPL35 protein is predominantly found in cytosol, ER and an unknown punctate compartment (Ma et al, 2019). SPL35 directly interacts with the E2 protein OsUBC5a, which also interacts with the SPL11 E3 ligase mentioned above (Bae and Kim, 2013). Knockdown oforleads to an LMM phenotype in rice, suggesting that the SPL35-OsUBC5a module is involved in immunity and cell death regulation. SPL35 is also found to interact with monoubiquitin through its CUE domain (Ma et al, 2019). However, the substrate of the SPL35-OsUBC5a module is currently unknown. Moreover, overexpression ofalso results in enhanced immune response and cell death, indicating a much complex network governed by SPL35 (Ma et al, 2019).
Many protein kinases are responsible for rice, indicating that protein phosphorylation is main step regulating immunity and cell death in rice. A mitogen- activated protein kinase kinase kinase (MAPKKK), named SPL3/ENHANCED DISEASE RESISTANCE 1 (OsEDR1)/ACCELERATED CELL DEATH AND RESISTANCE 1 (OsACDR1), is reported to regulate HR-like cell death formation (Kim et al, 2009; Shen et al, 2011; Wang S H et al, 2015). Overexpression ofin rice confers spontaneous HR-like cell death and enhanced disease resistance (Kim et al, 2009). Interestingly, loss-of-function ofalso activates immunity and cell death in rice (Shen et al, 2011; Wang S H et al, 2015). The LMM phenotype of/is likely resulting from the altered levels of hormones such as SA, JA, ET and abscisic acid (ABA) (Shen et al, 2011; Wang S H et al, 2015). However, how this MAPKKK regulates immunity and cell death through hormones remains unclear. SPOTTED LEAF SHEATH (SLES) is the second MAPKKK cloned from a rice. Thedisplays lesion mimic spots mainly on leaf sheaths, resulting in early leaf senescence and enhanced defense response (Lee et al, 2018). Two plasma membrane protein kinases, PTO-INTERACTING PROTEIN 1a (OsPTI1a) and APOPTOSIS LEAF AND SHEATH 1 (ALS1)/LIGHT INDUCED LESION MIMIC MUTANT 1 (LIL1) are implicated in the pathways of cell death and autoimmunity. OsPTI1a localizes to plasma membrane through its N-terminal palmitoylation (Matsui et al, 2015). Proper complex formation mediated by OsPTI1a at the plasma membrane is required for the negative regulation of plant immune responses and cell death in rice (Matsui et al, 2015). The immune response and cell death regulated by OsPTI1a is dependent on RAR1 which is a main regulator of R proteins involved in disease resistance (Takahashi et al, 2007). The cysteine-rich receptor like kinase ALS1/LIL1 is identified from two semi-dominant rice(Zhou et al, 2017; Du et al, 2019). The mutants display HR-like cell death spots on their leaf blades and leaf sheaths and constitutively activated defense response, resembling SA-, JA- and OsNPR1/NH1-mediated defense responses in rice (Zhou et al, 2017; Du et al, 2019). Overexpression of OsNPR1/NH1 readily leads to a lesion mimic phenotype and enhanced disease resistance, which is inducible by application of SAR inducer benzothiadiazole (BTH); deletion of two cysteine-rich receptor-like kinases (and) in a rice mutant () blocks this OsNPR1/NH1-mediated, BTH-inducible lesion mimic phenotype and enhances disease resistance, showing the involvement of receptor-like kinases in OsNPR1/NH1 function in rice (Chern et al, 2016).
In addition, glycosylation is also suggested to regulate rice immunity and cell death. The() gene encodes a putative core 2/I branching beta-1,6-N-acetylglucosaminyl transferase that is predicted to function in protein glycosylation. In themutant, the-glycosylation is impaired while the ET-related metabolic process is activated. Themutant exhibits spontaneous cell death and autoimmunity (Ke et al, 2019). This discovery highlights the importance of protein-glycosylation in cell death and immunity.
Modifications of intracellular vesicle trafficking lead to LMM phenotype
Plant cells usually contain many membrane-bounded compartments that interact with the cellular environment to regulate various aspects of plant growth (Paul et al, 2014). Vesicular transport pathways play essential roles in cargo (usually proteins, lipids and other biomolecules) trafficking between these compartments. Many types of vesicles have been identified to participate in vesicular transport in the endocytic and secretory pathways (Paul et al, 2014). The coat protein complex I (COPI), COPII and clathrin-coated vesicles and their adaptor complexes are important for sorting and directing the transportation of cargos (Paul et al, 2014; Gomez-Navarro and Miller, 2016). The multivesicular bodies (MVBs) and secretory vesicles are also important for cargo transportation. Here, we mainly focused on trafficking pathways and components which are involved in autoimmunity and spontaneous cell death in rice. For more details on how these vesicles are assembled and transported, readers are referred to other reviews (McMahon and Mills, 2004; Cui et al, 2016; Mei and Guo, 2018).
COPI-coated vesicles are involved in retrograde transport from the Golgi apparatus to ER (Gomez-Navarro and Miller, 2016). In rice, the coatomer subunits δ-COP1 and δ-COP2 interact directly with SPL35, a CUE domain-containing protein discussed above. Knockdown oforgene leads to constitutive immunity activation and HR-like cell death (Ma et al, 2019). SPL35 protein is found to distribute in some cytosolic punctate compartments which are likely the COPI-coated vesicles, providing evidence that the COPI trafficking pathway is involved in the regulation of HR-like cell death and immunity in rice (Ma et al, 2019). The clathrin- associated adaptor protein complex 1, medium subunit μ1 (AP1M1) protein SPL28, is localized to the Golgi apparatus and likely involved in the regulation of clathrin-associated adaptor protein complex 1 (AP-1)- mediated membrane-cytoplasm recycling during transport vesicle formation and vesicle uncoating in rice (Qiao et al, 2010). Themutant accumulates antimicrobial compounds and activates defense response to rice blast and bacterial blight pathogens, suggesting that the clathrin-coated vesicle pathway is also involved in HR-like cell death and immunity in rice (Qiao et al, 2010). The MVB-mediated trafficking pathway is regulated by an AAA type ATPase LRD6-6. Defect in its ATPase activity leads to autoimmunity and HR-like cell death (Zhu et al, 2016). LRD6-6 interacts with the endosomal sorting complexes required for transport of (ESCRT)-III components OsSNF7 and OsVPS2, and co-localizes with the MVB marker protein RabF1/ARA6. Moreover, the transportation of soluble vacuolar carboxypeptidase Y from ER to vacuoles, which is mediated by MVBs, is blocked in themutant (Zhu et al, 2016). Thus, activated immunity and enhanced cell death inis due to the inhibition of trafficking pathway mediated by MVBs(Zhu et al, 2016). The exocyst is an evolutionarily conserved protein complex composed of SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70 and EXO84 that are involved in exocytosis (Mei and Guo, 2018). Rice OsSEC3A binds to phosphatidylinositol and interacts with the SNAP25-type t-SNARE protein OsSNAP32, which is involved in rice blast resistance (Ma et al, 2018). Knockout ofcauses HR-like cell death and enhanced defense responses. OsSEC3A has a punctate distribution on the plasma membrane which matches its role (Ma et al, 2018). Loss-of-function of another exocyst complex subunit OsEXO70A1 in() also confers an LMM phenotype. Although the immune response of themutant is not surveyed, the secretory pathway is thus also believed to be involved in regulating rice immunity and cell death based on the phenotype (Tu et al, 2015).
Changes in several metabolic pathways in rice generate LMM phenotypes
Living organisms metabolize nutrients as sources of energy and building blocks for proteins, lipids, nucleic acids and carbohydrates to survive (Green et al, 2014). Dysfunction of several metabolic fluxes can cause accumulation of intermediate metabolites, leading to autoimmunity and cell death. Currently, at least nine key enzymes involved in different metabolic pathways have been identified in the study of ricethat confer autoimmunity and cell death. SPL18, an acyl transferase, likely contributes to disease resistance by potentiating disease-resistance signaling through its enzymatic activity (Mori et al, 2007). It may also act by producing phytoalexin-like secondary products. Therefore, whenmRNA accumulates to high levels, the immune response is activated and cell death occurs (Mori et al, 2007). Rice SUPPRESSOR OF SA INSENSITIVE 2 (OsSSI2) is a fatty acid desaturase that catalyzes the conversion of stearic acid (18:0) to oleic acid (18 : 1). Knockdownofaccumulates stearic acid (18 : 0), thus increases endogenous free SA levels and induces SA-responsive genes, leading to enhanced disease resistance and spontaneous HR-like cell death (Jiang et al, 2009). A novel defense pathway was also discovered in the study of rice() (Fujiwara et al, 2010).encodes a CYP71P1 protein which exhibits tryptamine 5-hydroxylase enzymatic activity and catalyzes the conversion of tryptamine to serotonin (Fujiwara et al, 2010). Ricemutant exhibits unique orange-colored lesions and accumulates tryptamine. Exogenous application of serotonin is capable of inducing defense responses and cell death mediated by the RacGTPase pathway and the Gα subunit of the heterotrimeric G protein (Fujiwara et al, 2010). The()/()exhibits cell death on leaf and enhanced disease resistance to bacterial blight pathogen (Wang J et al, 2015).encodes a putative coproporphyrinogen III oxidase functioning in the tetrapyrrole biosynthesis pathway, suggesting that this pathway is involved in rice immunity and cell death (Sun et al, 2011; Wang J et al, 2015). Hydroperoxylinoleic acid is catabolized by hydroperoxide lyase (HPL) to form ()-3-hexenal or by allene oxide synthase to form JA in the oxylipin pathway(Liu X Q et al, 2012). Loss-of-function of a rice HPL, OsHPL3/CYP74B2, leads to accumulation of JA but reduction of green leaf volatiles in the/mutant (Liu X Q et al, 2012; Tong et al, 2012). Thus, OsHPL3 modulates rice defense responses against different invaders, including pathogens and insects through the oxylipin pathway (Liu X Q et al, 2012; Tong et al, 2012). The UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) SPL29 catalyzes the decomposition of uridine 5′-diphosphoglucose-glucose (UDPG), an important signaling molecule involved in immunity (Xiao et al, 2018). Loss-of-function ofcan accumulate excessive UDPG, leading to a ROS burst, immune response and cell death (Wang Z H et al, 2015; Xiao et al, 2018). Anothergene,, encodes a chloroplast-localized ferredoxin dependent glutamate synthase, which is a key enzyme in the process of inorganic nitrogen assimilation (Sun et al, 2017). Themutant displays spontaneous cell death and elevated disease resistance which likely results from reduction of photorespiration rate and burst of ROS (Sun et al, 2017). Themutant exhibits spontaneous cell death and enhanced resistanceto rice bacterial and blast diseases. OsABA2, a xanthoxin dehydrogenase involved in ABA biosynthesis pathway, is responsible for this phenotype (Liao et al, 2018). Consistent with OsABA2 function, ABA level in themutant is significantly reduced (Liao et al, 2018). This discovery directly links biosynthesis of ABA, which causes senescence, with immunity and cell death. Recently, an ATP-citrate lyase (ACL), a subunit protein, SPL30, is reported to regulate immunity and cell death in rice (Ruan et al, 2018). Theharbors A-to-T substitution that converts an asparagine to tyrosine (N343Y), which causes significant degradation of SPL30N343Yin a ubiquitin- dependent manner and reduces the ACL enzymatic activity(Ruan et al, 2018). Further suppressor screens revealed thatis epistatic toin the pathogen response pathway, linkingto a previously known serotonin activated immune pathway (Ruan et al, 2018).
Other regulations contribute to rice LMM phenotype
Apart from the above pathways, other types of regulators controlling immunity and cell death in rice have been characterized. Resistance (R) proteins are key components in mediating defense responses. The semi-dominant mutants,() and, are found to display spontaneous cell death on leaf sheaths and constitutively activate defense responses (Tang et al, 2011). Thegene encodes a typical coiled-coil, nucleotide binding and leucine rich repeat domain (CC-NB-LRR)-containing R protein. Bothandmutations cause constitutive auto-activation of the NLS1 R protein leading to a SA- and NPR1-independent defense response(Tang et al, 2011). The conserved nucleotide-binding (NB) domain has been also referred to as NB-ARC (nucleotide-binding, Apaf-1, R proteins, and Ced-4) (van der Biezen and Jones, 1998). RAPID LEAF SENESCENCE 1 (RLS1), a NB-ARC domain- containing protein, is also found to regulate cell death in rice. Themutant displays yellow brown lesions and accelerated leaf senescence while immune responses inis not studied (Jiao et al, 2012).
Cytochromeplays an important role in mitochondria governing apoptosis in animals (Hoeberichts and Woltering, 2003). However, how cytochromeis released from the mitochondria to cytosol remains poorly understood. In the study of a ricemutant, the mitochondria-localized GTPase DYNAMIN- RELATED PROTEIN 1E (OsDRP1E) is found to negatively regulate cell death and immunity by controlling mitochondrial structure and cytochromerelease (Li Z Q et al, 2017). Mitochondrial morphology is changed in themutant and concentration of cytoplasmic cytochromeis increased. As a result, themutant exhibits spontaneous cell death and enhanced resistance to fungal and bacterial pathogens (Li Z Q et al, 2017). This discovery advances our knowledge on the relationship among cytochromerelease, immunity and cell death in rice.
Immune response activated and cell death occurred in lmms
The LMM phenotype is primarily characterized by the presence of brown-spots on rice leaf blades or leaf sheaths. These brown-spots are reminiscent of HR which is regarded as a form of rapid programmed cell death that occurs in host cells during plant-pathogen interaction (Coll et al, 2011). Molecular events such as burst of ROS, accumulation ofmRNAs and elevation of hormone levels are accompanied with HR (Coll et al, 2011). Indeed, these events autonomously happen ins, priming immune response resulting in HR-like cell death phenotypes. Almost alls accumulate elevated levels of ROS and transcripts ofgenes. A majority of the mutants, such as,,,and, also include imbalanced hormone levels, including SA, JA, ET and ABA (Tang et al, 2011; Tong et al, 2012; You et al, 2016; Liao et al, 2018; Xiao et al, 2018). The antimicrobial metabolites phytoalexins, serotonin and phenolic compounds also accumulate to high concentrations in some of thes, like,,,and(Fujiwara et al, 2010; Qiao et al, 2010; Jin et al, 2015; Wang S H et al, 2015; Zhu et al, 2016). The deposition of callose and increase of cell wall component lignin are also detected in these(Qiao et al, 2010; Zhu et al, 2016). All these are hallmarks of a typical immune response in plants.
Obviously, although the types ofgenes and their pathways are highly diverse, their downstream molecular events triggering the immune response and cell death are conserved (Fig. 1-F). Thus,are of great significance for deciphering upstream regulation of defense signaling pathways.
Cross-talks between immunity and growth in lmms
Normal growth can be suppressed on activation of the immune response, resulting in growth delay and yield penalties in plants (Yin et al, 2019). Thus, high yield with enhanced resistance is a highly desirable trait but also a major challenge in crop breeding.
The sustained activation of immune response results in cell death and huge influences on many aspects of plant growth in rice(Supplemental Table 1). It is very likely that adverse effects on normal growth ofmay result from the altered photosynthetic system caused by the immune response and cell death. This preliminary conclusion is based on the following observations: (1) Many, such as,and, carrying weak growth are reported to display abnormal degradation of chloroplasts and reduced photosynthetic capacity (Jiao et al, 2012; Sun et al, 2017; Xiao et al, 2018). (2) Mutants like,andshow stay-green or delayed leaf senescence phenotype which usually represents a longer durations of photosynthetic activity and growth closer to the wild type at certain stages even though some aspects of agronomic traits are still affected (Wang S H et al, 2015; Liao et al, 2018; Song et al, 2018). (3) More interestingly, overexpression ofpromotes chlorophyll b accumulation, leadingto dark-green-colored leaves, enhanced disease resistance without cell death, and accelerated differentiation of callus (Wang et al, 2005).
Several studies ofhave gained some insights into molecular mechanisms governing the balance between immunity and growth. The E3 ubiquitin ligase SPL11 negatively regulates programmed cell death and disease resistance (Liu J L et al, 2012). And, SPL11 interacts with mono-ubiquitinatesSPIN1, a flowering time regulator involved in() pathway, tocause delayed flowering inmutant under long-day conditions (Vega-Sanchez et al, 2008). While over accumulated in leaves,increases chlorophyll b content and disease resistance without visible cell death (Wang et al, 2005). Moreover, OsLSD1 can interact with OsbZIP58 in seed to activate gibberellin biosynthesis geneand to downregulate the SOD1-encoding gene to modulate seed germination (Wu et al, 2014). In order to facilitate utilization ofgenes, attentions should be paid to the roles ofgenes on regulation of growth in the future.
Prospects
Several genes such as,,and thepairs have been characterized to play important roles in balancing yield and resistance in rice (Deng et al, 2017; Li W T et al, 2017; Wang et al, 2018; Zhou et al, 2018). These discoveries provide precious gene resources which are useful for crop breeding. Unlike the resistance provided by these genes, mutations ingenes always result in sustained and overactive activation of immune responses which causes HR-like cell death and adversely impacts plant growth. Thus, it is difficult to directly apply thosegenes to breeding application, especially for the recessive genes. However,the studies on theseprovide insights to understand the crosstalk between immunity and growth. For examples, in-depth study ongenehas strengthened our understanding on how a E3 ligase regulates plant immunity and flowering (Vega-Sanchez et al, 2008; Liu et al, 2015; Fan et al, 2018). Due to activation of cell death, the photosynthetic capacity of someis reduced which might inhibit plant development (Jiao et al, 2012; Sun et al, 2017; Xiao et al, 2018).
It is common that enhanced resistance obtained is often associated with substantial penalties to fitness. It is not only true togenes, but also to other resistance genes. However, previous studies showed that we can overcome this problem by driving these defense genes under control of pathogen specific, inducible promoters. TBF1 is identified as an important transcription factor controlling the growth-to-defense switch upon immune induction. Translation of TBF1 is normally inhibited by two upstream open reading frames within the 5′ leader sequence (Pajerowska-Mukhtar et al, 2012). Using the-cassette as a promoter to drivehas been shown to enhance rice broad-spectrum disease resistance with minimal adverse effects on plant growth and development (Xu et al, 2017). The promoter ofcontains a binding site of TAL9, which is a transcriptional activator- like effector secreted by the type III secretion system (Moscou and Bogdanove, 2009). The expression ofis specifically induced by pathogens containing TAL9a. Using thepromoter to drivegene has achieved high yield and enhanced resistance in rice (Liu et al, 2019). Promoters, like the- cassette and thepromoter, are ubiquitous in plant genomes. Identification and isolation of more such promoters would provide useful tools.
acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31701779 and 31922066), the Applied Basic Research Programs of Science and Technology Department from Sichuan Province (Grant No. 2019YJ0432) and China Postdoctoral Science Foundation (Grant No. 2017M612984).
Supplemental data
The following material is available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Summary of 23 clonedgenes.
Bae H, Kim W T. 2013. The N-terminal tetra-peptide (IPDE) short extension of the U-box motif in rice SPL11 E3 is essential for the interaction with E2 and ubiquitin-ligase activity., 433(2): 266–271.
Cao N, Chen Y, Ji Z J, Zeng Y X, Yang C D, Liang Y. 2019. Recent progress in molecular mechanism of rice blast resistance., 33(6): 489–498. (in Chinese with English abstract)
Chen X F, Pan J W, Cheng J, Jiang G H, Jin Y, Gu Z M, Qian Q, Zhai W X, Ma B J. 2009. Fine genetic mapping and physical delimitation of the lesion mimic gene() in rice (L.)., 24(4): 387.
Chen X F, Hao L, Pan J W, Zheng X X, Jiang G H, Jin Y, Gu Z M, Qian Q, Zhai W X, Ma B J. 2012., a cell death and defense- related gene, encodes a putative splicing factor 3b subunit 3 (SF3b3) in rice., 30(2): 939–949.
Chern M, Canlas P E, Fitzgerald H A, Ronald P C. 2005a. Rice NRR, a negative regulator of disease resistance, interacts withNPR1 and rice NH1., 43(5): 623–635.
Chern M, Fitzgerald H A, Canlas P E, Navarre D A, Ronald P C. 2005b. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light., 18(6): 511–520.
Chern M, Xu Q F, Bart R S, Bai W, Ruan D L, Sze-To W H, Canlas P E, Jain R, Chen X W, Ronald P C. 2016. A genetic screen identifies a requirement for cysteine-rich-receptor-like kinases in rice NH1 (OsNPR1)-mediated immunity., 12(5): e1006049.
Coll N S, Epple P, Dangl J L. 2011. Programmed cell death in the plant immune system., 18(8): 1247–1256.
Cui Y, Shen J B, Gao C J, Zhuang X H, Wang J Q, Jiang L W. 2016. Biogenesis of plant prevacuolar multivesicular bodies., 9(6): 774–786.
Deng Y W, Zhai K R, Xie Z, Yang D Y, Zhu X D, Liu J Z, Wang X, Qin P, Yang Y Z, Zhang G M, Li Q, Zhang J F, Wu S Q, Milazzo J, Mao B Z, Wang E T, Xie H A, Tharreau D, He Z H. 2017. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance., 355: 962–965.
Du D, Liu M M, Xing Y D, Chen X L, Zhang Y Y, Zhu M D, Lu X, Zhang Q L, Ling Y H, Sang X C, Li Y F, Zhang C W, He G H. 2019. Semi-dominant mutation in the cysteine-rich receptor-like kinase gene,, conducts constitutive defence response in rice.(), 21(1): 25–34.
Fan J B, Bai P F, Ning Y S, Wang J Y, Shi X T, Xiong Y H, Zhang K, He F, Zhang C Y, Wang R Y, Meng X Z, Zhou J G, Wang M, Shirsekar G, Park C H, Bellizzi M, Liu W D, Jeon J S, Xia Y, Shan L B, Wang G L. 2018. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice., 23(4): 498–510.
Fujiwara T, Maisonneuve S, Isshiki M, Mizutani M, Chen L T, Wong H L, Kawasaki T, Shimamoto K. 2010. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice., 285(15): 11308–11313.
Ge C W, E Z G, Pan J J, Jiang H, Zhang X Q, Zeng D L, Dong G J, Hu J, Xue D W. 2015. Map-based cloning of a spotted-leaf mutantgeneinrice., 75(3): 595–603.
Gomez-Navarro N, Miller E A. 2016. COP-coated vesicles., 26(2): R54–R57.
Green D R, Galluzzi L, Kroemer G. 2014. Metabolic control of cell death., 345: 1250256.
Henry E, Yadeta K A, Coaker G. 2013. Recognition of bacterial plant pathogens: Local, systemic and transgenerational immunity., 199(4): 908–915.
Hoeberichts F A, Woltering E J. 2003. Multiple mediators of plant programmed cell death: Interplay of conserved cell death mechanisms and plant-specific regulators., 25(1): 47–57.
Hoisington D A, Neuffer M G, Walbot V. 1982. Disease lesion mimicsin maize: I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1., 93(2): 381–388.
Huang Q N, Yang Y, Shi Y F, Chen J, Wu J L. 2010. Spotted-leaf mutants of rice ()., 17(4): 247–256.
Jiang C J, Shimono M, Maeda S, Inoue H, Mori M, Hasegawa M, SuganoS, Takatsuji H. 2009. Suppression of the rice fatty-acid desaturase geneenhances resistance to blast and leaf blight diseases in rice., 22(7): 820–829.
Jiao B B, Wang J J, Zhu X D, Zeng L J, Li Q, He Z H. 2012. A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice., 5(1): 205–217.
Jin B, Zhou X R, Jiang B L, Gu Z M, Zhang P H, Qian Q, Chen X F, Ma B J. 2015. Transcriptome profiling of themutant reveals thathas a negative role in the biosynthesis of serotonin for rice disease resistance., 8: 18.
Ke S W, Liu S C, Luan X, Xie X M, Hsieh T F, Zhang X Q. 2019. Mutation in a putative glycosyltransferase-like gene causes programmed cell death and early leaf senescence in rice., 12(1): 7.
Kim J A, Cho K, Singh R, Jung Y H, Jeong S H, Kim S H, Lee J E, Cho Y S, Agrawal G K, Rakwal R, Tamogami S, Kersten B, Jeon J S, An G, Jwa N S. 2009. Rice() is a potential positive regulator of fungal disease resistance., 28(5): 431–439.
Kojo K, Yaeno T, Kusumi K, Matsumura H, Fujisawa S, Terauchi R, Iba K. 2006. Regulatory mechanisms of ROI generation are affected by ricemutations., 47(8): 1035–1044.
Lee D, Lee G, Kim B, Jang S, Lee Y, Yu Y, Seo J, Kim S, Lee Y H, Lee J, Kim S, Koh H J. 2018. Identification of a spotted leaf sheath gene involved in early senescence and defense response in rice., 9: 1274.
Li W T, Zhu Z W, Chern M, Yin J J, Yang C, Ran L, Cheng M P, He M, Wang K, Wang J, Zhou X G, Zhu X B, Chen Z X, Wang J C, Zhao W, Ma B T, Qin P, Chen W L, Wang Y P, Liu J L, Wang W J, Wu X J, Li P, Wang J R, Zhu L H, Li S G, Chen X W. 2017. A natural allele of a transcription factor in rice confers broad- spectrum blast resistance., 170(1): 114–126.
Li Z Q, Ding B, Zhou X P, Wang G L. 2017. The rice dynamin- related protein OsDRP1E negatively regulates programmed cell death by controlling the release of cytochrome c from., 13(1): e1006157.
Liao Y X, Bai Q, Xu P Z, Wu T K, Guo D M, Peng Y B, Zhang H Y, Deng X S, Chen X Q, Luo M, Ali A, Wang W M, Wu X J. 2018. Mutation in rice abscisic acid2 results in cell death, enhanced disease-resistance, altered seed dormancy and development., 9: 405.
Liu J L, Li W, Ning Y S, Shirsekar G, Cai Y H, Wang X L, Dai L Y, Wang Z L, Liu W D, Wang G L. 2012. The U-Box E3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants., 160(1): 28–37.
Liu J L, Park C H, He F, Nagano M, Wang M, Bellizzi M, Zhang K, Zeng X S, Liu W D, Ning Y S, Kawano Y, Wang G L. 2015. The RhoGAP SPIN6 associates with SPL11 and OsRac1 and negatively regulates programmed cell death and innate immunity in rice., 11(2): e1004629.
Liu M M, Shi Z Y, Zhang X H, Wang M X, Zhang L, Zheng K Z, Liu J Y, Hu X M, Di C R, Qian Q, He Z H, Yang D L. 2019. Inducible overexpression ofimproves both yield and disease resistance in rice., 5(4): 389–400.
Liu Q E, Ning Y S, Zhang Y X, Yu N, Zhao C D, Zhan X D, Wu W X, Chen D B, Wei X J, Wang G L, Cheng S H, Cao L Y. 2017.negatively regulates cell death and immunity by degrading OsNPR1 in rice., 29(2): 345–359.
Liu X Q, Li F, Tang J Y, Wang W H, Zhang F X, Wang G D, Chu J F, Yan C Y, Wang T Q, Chu C C, Li C Y. 2012. Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice., 7(11): e50089.
Lorrain S, Vailleau F, Balague C, Roby D. 2003. Lesion mimic mutants: Keys for deciphering cell death and defense pathways in plants?, 8(6): 263–271.
Ma J, Chen J, Wang M, Ren Y L, Wang S, Lei C L, Cheng Z J, Sodmergen. 2018. Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice., 69(5): 1051–1064.
Ma J, Wang Y F, Ma X D, Meng L Z, Jing R N, Wang F, Wang S, Cheng Z J, Zhang X, Jiang L, Wang J L, Wang J, Zhao Z C, Guo X P, Lin Q B, Wu F Q, Zhu S S, Wu C Y, Ren Y L, Lei C L, Zhai H Q, Wan J M. 2019. Disruption of gene, encoding a novel CUE domain-containing protein, leads to cell death and enhanced disease response in rice., 17(8): 1679–1693.
Matsui H, Takahashi A, Hirochika H. 2015. Rice immune regulator, OsPti1a, is specifically phosphorylated at the plasma membrane., 10(3): e991569.
McMahon H T, Mills I G. 2004. COP and clathrin-coated vesicle budding: Different pathways, common approaches., 16(4): 379–391.
Mei K R, Guo W. 2018. The exocyst complex., 28(17): R922–R925.
Merchante C, Stepanova A N, Alonso J M. 2017. Translation regulation in plants: An interesting past, an exciting present and a promising future., 90(4): 628–653.
Mori M, Tomita C, Sugimoto K, Hasegawa M, Hayashi N, Dubouzet J G, Ochiai H, Sekimoto H, Hirochika H, Kikuchi S. 2007. Isolation and molecular characterization of amutant by modified activation-tagging in rice., 63(6): 847–860.
Moscou M J, Bogdanove A J. 2009. A simple cipher governs DNA recognition by TAL effectors., 326: 1501.
Mur L A, Kenton P, Lloyd A J, Ougham H, Prats E. 2008. The hypersensitive response; the centenary is upon us but how much do we know?, 59(3): 501–520.
Nurnberger T, Brunner F, Kemmerling B, Piater L. 2004. Innate immunity in plants and animals: Striking similarities and obvious differences., 198: 249–266.
Pajerowska-Mukhtar K M, Emerine D K, Mukhtar M S. 2013. Tell me more: Roles of NPRs in plant immunity., 18(7): 402–411.
Pajerowska-Mukhtar K M, Wang W, Tada Y, Oka N, Tucker C L, Fonseca J P, Dong X N. 2012. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition., 22(2): 103–112.
Paul P, Simm S, Mirus O, Scharf K D, Fragkostefanakis S, Schleiff E. 2014. The complexity of vesicle transport factors in plants examined by orthology search., 9(5): e97745.
Qiao Y L, Jiang W Z, Lee J, Park B, Choi M S, Piao R H, Woo M O, Roh J H, Han L Z, Paek N C, Seo H S, Koh H J. 2010. SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice ()., 185(1): 258–274.
Qin P, Fan S J, Deng L C, Zhong G R, Zhang S W, Li M, Chen W L, Wang G L, Tu B, Wang Y P, Chen X W, Ma B T, Li S G. 2018. LML1, encoding a conserved eukaryotic release factor 1 protein, regulates cell death and pathogen resistance by forming a conserved complex with SPL33 in rice., 59(5): 887–902.
Ruan B P, Hua Z H, Zhao J, Zhang B, Ren D Y, Liu C L, Yang S L, Zhang A P, Jiang H Z, Yu H P, Hu J, Zhu L, Chen G, Shen L, Dong G J, Zhang G H, Zeng D L, Guo L B, Qian Q, Gao Z Y. 2018. OsACL-A2 negatively regulates cell death and disease resistance in rice., 17(7): 1344–1356.
Sekiguchi Y, Furuta T. 1965. On a rice mutant showing particular reaction to some spotting disease. Preliminary report., 30: 71–72. (in Japanese)
Shen X L, Liu H B, Yuan B, Li X H, Xu C G, Wang S P. 2011. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis., 34(2): 179–191.
Song G, Kwon C T, Kim S H, Shim Y, Lim C, Koh H J, An G, Kang K, Paek N C. 2018. The rice() encodes a plant spastin that inhibits ROS accumulation in leaf development and functions in leaf senescence., 9: 1925.
Sun C H, Liu L C, Tang J Y, Lin A H, Zhang F T, Fang J, Zhang G F, Chu C C. 2011., encoding a putative coproporphyrinogen III oxidase, is involved in lesion initiation in rice., 38(1): 29–37.
Sun L T, Wang Y H, Liu L L, Wang C M, Gan T, Zhang Z Y, Wang Y L, Wang D, Niu M, Long W H, Li X H, Zheng M, Jiang L, Wan J M. 2017. Isolation and characterization of a spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice., 7: 41846.
Takahashi A, Agrawal G K, Yamazaki M, Onosato K, Miyao A, Kawasaki T, Shimamoto K, Hirochika H. 2007. Ricenegatively regulates RAR1-dependent defense responses., 19(9): 2940–2951.
Tang J Y, Zhu X D, Wang Y Q, Liu L C, Xu B, Li F, Fang J, Chu C C. 2011. Semi-dominant mutations in the CC-NB-LRR-type R gene,, lead to constitutive activation of defense responses in rice., 66(6): 996–1007.
Tong X H, Qi J F, Zhu X D, Mao B Z, Zeng L J, Wang B H, Li Q, Zhou G X, Xu X J, Lou Y G, He Z H. 2012. The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway., 71(5): 763–775.
Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity., 13(4): 459–465.
Tu B, Hu L, Chen W L, Li T, Hu B H, Zheng L, Lv Z, You S J, Wang Y P, Ma B T, Chen X W, Qin P, Li S G. 2015. Disruption ofcauses irregular vascular bundles and perturbs mineral nutrient assimilation in rice., 5: 18609.
Undan J R, Tamiru M, Abe A, Yoshida K, Kosugi S, Takagi H, Yoshida K, Kanzaki H, Saitoh H, Fekih R, Sharma S, Undan J, Yano M, Terauchi R. 2012. Mutation in, a gene encoding a protein with two double-stranded RNA binding motifs, causes lesion mimic phenotype and early senescence in rice (L.)., 87(3): 169–179.
van der Biezen E A, Jones J D. 1998. The NB-ARC domain: A novel signalling motif shared by plant resistance gene products and regulators of cell death in animals., 8(7): R226–R228.
Vega-Sanchez M E, Zeng L R, Chen S B, Leung H, Wang G L. 2008. SPIN1, a K homology domain protein negatively regulated and ubiquitinated by the E3 ubiquitin ligase SPL11, is involved in flowering time control in rice., 20(6): 1456–1469.
Wang J, Ye B Q, Yin J J, Yuan C, Zhou X G, Li W T, He M, Wang J C, Chen W L, Qin P, Ma B T, Wang Y P, Li S G, Chen X W. 2015.Characterization and fine mapping of a light-dependent leaf lesion mimic mutant 1 in rice., 97: 44–51.
Wang J, Zhou L, Shi H, Chern M, Yu H, Yi H, He M, Yin J J, Zhu X B, Li Y, Li W T, Liu J L, Wang J C, Chen X Q, Qing H, Wang Y P, Liu G F, Wang W M, Li P, Wu X J, Zhu L H, Zhou J M, Ronald P C, Li S G, Li J Y, Chen X W. 2018. A single transcription factor promotes both yield and immunity in rice., 361: 1026–1028.
Wang L J, Pei Z Y, Tian Y C, He C Z. 2005. OsLSD1, a rice zinc finger protein, regulates programmed cell death and callus differentiation., 18(5): 375–384.
Wang S H, Lim J H, Kim S S, Cho S H, Yoo S C, Koh H J, Sakuraba Y, Paek N C. 2015. Mutation of SPOTTED LEAF3 (SPL3) impairs abscisic acid-responsive signalling and delays leaf senescence in rice., 66(22): 7045–7059.
Wang S, Lei C L, Wang J L, Ma J, Tang S, Wang C L, Zhao K J, Tian P, Zhang H, Qi C Y, Cheng Z J, Zhang X, Guo X P, Liu L L, Wu C Y, Wan J M. 2017., encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice., 68(5): 899–913.
Wang Z H, Wang Y, Hong X, Hu D H, Liu C X, Yang J, Li Y, Huang Y Q, Feng Y Q, Gong H Y, Li Y, Fang G, Tang H R, Li Y S. 2015. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice., 66(3): 973–987.
Wu J H, Zhu C F, Pang J H, Zhang X R, Yang C L, Xia G X, Tian Y C, He C Z. 2014. OsLOL1, a C2C2-type zinc finger protein, interacts with OsbZIP58 to promote seed germination through the modulation of gibberellin biosynthesis in., 80(6): 1118–1130.
Xiao G Q, Zhou J H, Lu X Y, Huang R F, Zhang H W. 2018. Excessive UDPG resulting from the mutation of UAP1 causes programmed cell death by triggering reactive oxygen species accumulation and caspase-like activity in rice., 217(1): 332–343.
Xu G Y, Yuan M, Ai C R, Liu L J, Zhuang E, Karapetyan S, Wang S P, Dong X N. 2017. uORF-mediated translation allows engineered plant disease resistance without fitness costs., 545: 491–494.
Yamanouchi U, Yano M, Lin H X, Ashikari M, Yamada K. 2002. A rice spotted leaf gene,, encodes a heat stress transcription factor protein., 99(11): 7530–7535.
Yin J J, Yi H, Chen X W, Wang J. 2019. Post-translational modifications of proteins have versatile roles in regulating plant immune responses., 20(11): 2807.
You Q Y, Zhai K R, Yang D L, Yang W B, Wu J N, Liu J Z, Pan W B, Wang J J, Zhu X D, Jian Y K, Liu J Y, Zhang Y Y, Deng Y W, Li Q, Lou Y G, Xie Q, He Z H. 2016. An E3 ubiquitin ligase- BAG protein module controls plant innate immunity and broad- spectrum disease resistance., 20(6): 758–769.
Yuan Y X, Zhong S H, Li Q, Zhu Z R, Lou Y G, Wang L Y, Wang J J, Wang M Y, Li Q L, Yang D L, He Z H. 2007. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility., 5(2): 313–324.
Zeng L R, Qu S H, Bordeos A, Yang C W, Baraoidan M, Yan H Y, Xie Q, Nahm B H, Leung H, Wang G L. 2004. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity., 16(10): 2795–2808.
Zhang X B, Feng B H, Wang H M, Xu X, Shi Y F, He Y, Chen Z, Sathe A P, Shi L, Wu J L. 2018. A substitution mutation in OsPELOTA confers bacterial blight resistance by activating the salicylic acid pathway., 60(2): 160–172.
Zhao J Y, Liu P C, Li C R, Wang Y Y, Guo L Q, Jiang G H, Zhai W X. 2017. LMM5.1 and LMM5.4, two eukaryotic translation elongation factor 1A-like gene family members, negatively affect cell death and disease resistance in rice., 44(2): 107–118.
Zhou Q, Zhang Z F, Liu T T, Gao B D, Xiong X Y. 2017. Identification and map-based cloning of the light-induced lesion mimic mutant 1 () gene in rice., 8: 2122.
Zhou X G, Liao H C, Chern M S, Yin J J, Chen Y F, Wang J P, Zhu X B, Chen Z X, Yuan C, Zhao W, Wang J, Li W T, He M, Ma B T, Wang J C, Qin P, Chen W L, Wang Y P, Liu J L, Qian Y W, Wang W M, Wu X J, Li P, Zhu L H, Li S G, Ronald P C, Chen X W. 2018. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance., 115(12): 3174–3179.
Zhu X B, Yin J J, Liang S H, Liang R H, Zhou X G, Chen Z X, Zhao W, Wang J, Li W T, He M, Yuan C, Miyamoto K, Ma B T, Wang J C, Qin P, Chen W L, Wang Y P, Wang W M, Wu X J, Yamane H, Zhu L H, Li S G, Chen X W. 2016. The multivesicular bodies (MVBs)-localized AAA ATPase LRD6-6 inhibits immunity and cell death likely through regulating MVBs-mediated vesicular trafficking in rice., 12(9): e1006311.
14 August 2019;
9 December 2019
WANG Jing (jingwang406@sicau.edu.cn)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.004
(Managing Editor: Fang Hongmin)
杂志排行
Rice Science的其它文章
- Genetic Variation Dissection of Rice Blast Resistance Using an Indica Population
- OsPS6 Plays Important Role in Anther Development and Microspore Formation
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
- RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
- Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
