RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
2020-07-06AritraKarmakarSanandaBhattacharyaShinjiniSenguptaNusratAliSailendraNathSarkarKarabiDattaSwapanDatta
Aritra Karmakar, Sananda Bhattacharya, Shinjini Sengupta, Nusrat Ali,, Sailendra Nath Sarkar, Karabi Datta, Swapan K. Datta
Research Paper
RNAi-Mediated Silencing ofGene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
Aritra Karmakar1, Sananda Bhattacharya1, Shinjini Sengupta1, Nusrat Ali1,2, Sailendra Nath Sarkar1, Karabi Datta1, Swapan K. Datta1
()
Phytic acid is the principal storage form of phosphorus in plant seeds and an essential signallingmolecule in several regulatory processes of plant development. However, it is known as an anti-nutrient compound owing to its potent chelating property. Thus, reducing the phytic acid content in crops is desirable. Studies involving regulation ofandgenes to generate low phytate rice have been reported earlier. However, the functional significance ofand the effect of its down-regulation on phytic acid content and the associated pleiotropic effects on rice have not yet been investigated. In this study, tissue specific RNAinterference (RNAi)-mediated down-regulation of a majorhomolog () resulted in 46.2% decrease in phytic acid content of T2transgenic seeds with a subsequent3-fold enhancement in the inorganic phosphorus content. Silencing ofaltered the transcript levels of essential phytic acid pathway genes, without significantly affecting the transcript levels of otherhomologs. Furthermore, the mapping of elements through X-ray microfluorescence analysis revealed significant changes in the spatial distribution pattern and translocation of elements in low phytate seeds. Additionally, low phytate polished seeds exhibited 1.3-fold and 1.6-fold enhancement in iron and zinc content in the grain endosperm, respectively. Silencing of-also altered the amino acid and-inositol content of the transgenic seeds. Our results successfully established that RNAi-mediated silencing ofgene significantly reduced the phytate levels in seeds without hampering the germination potential of seeds and plant growth. The present study provided an insight into the mechanism of phytic acid biosynthesis pathway.
inositol triphosphate kinase-1;phytic acid;mineral content; RNAinterference silencing;X-ray microfluorescence
Phytic acid (-inositol-1,2,3,4,6-hexakisphosphate; InsP6) is the most abundant phosphorus-containing compound that accounts for up to 75% of total seed phosphorus. Moreover, it acts as the storehouse of several elements in seeds and plays an important role in several developmental and signaling processes (Raboy, 2009). Besides sequestering the inorganic phosphate (Pi), phytic acid is a potent chelator of mineral cations,which attributes to the presence of six highly negatively charged phosphate groups in it. Phytic acid binds to mineral cations, such as iron (Fe2+), zinc (Zn2+), calcium(Ca2+) and magnesium (Mg2+), to generate mixed salts referred to as phytates. The mineral-containing phytates are deposited as globoids in the microvacuoles commonly referred to as protein storage vacuoles (PSVs) which predominantly accumulate in the aleurone layer and the embryo of mature seeds. During germination, the stored phosphorus and elements are released from the-inositol ring by the action of enzyme known as phytase (InsP6phosphohydrolase). This step is essential and beneficial for the seedling growth and development owing to the inability of the seedling to absorb nutrients from environment (Loewus and Murthy, 2000; Raboy, 2003). Monogastric animals such as human beings lack phytase are unable to digest phytates and absorb ions like Fe2+, Zn2+and Ca2+, resulting in mineral deficiency in humans and cattle populations that primarily rely on whole grains and legume-based products as staple foods (Raboy, 2000; Bregitzer and Raboy, 2006).
To counter this problem, supplements of mineral phosphates were started. However, the use of these supplements in cattle feed results in high phosphorus accumulation and pollution in water bodies. Therefore, attempts have been made to generate transgenic crops, such as maize, wheat, soybeanand rice, expressing microbial phytases. However, these crops require laborious processing of grounding transgenic crops to powder and incubating this in water to activate the heat-labile enzymes before feeding animals (Lucca et al, 2001; Drakakaki et al, 2005). Another alternative strategy is the use of ‘low phytic acid’ () mutants. Severalmutant lines have been developed in maize (Raboy et al, 2000; Pilu et al, 2003; Shi et al, 2005), barley and rice (Larson et al, 2000; Liu et al, 2007). However, poor agronomic properties such as decreased germination rate and reduced seedling emergence have been reported in these mutants. Hence,a different approach such as down-regulation of essentialgenes involved in the phytic acid biosynthesis pathway has emerged as a possible strategy to mitigate such potential limitations. Recent reports in rice have successfully demonstrated the development of low phytate rice by targeting the enzymes involved in early and late stages of phytic acid biosynthetic pathway-inositol-3-phosphate synthase() and inositol 1,3,4,5,6-pentakisphosphate 2-kinase () under constitutive () and-inositol-3-phosphate synthase ()promoter (Feng and Yoshida, 2004), seed-specific promoters such as() (Kuwano et al, 2006), and aleurone- and embryo-specific promoters such as() (Kuwano et al, 2009; Ali et al, 2013a,b).
Plants employ two pathways for the synthesis of phytic acid, lipid-dependent and lipid-independent pathway. The lipid-dependent pathway operates in all plant tissues, whereas the lipid-independent pathway is the major route for phytic acid synthesis in seeds. The intermediate steps of lipid-independent pathway are catalyzed by a family of multi-functional inositol (1,3,4) P35/6-kinases or inositol triphosphate kinases (ITPKs) that convert inositol triphosphate (InsP3) to inositol pentaphosphate (InsP5) via sequential pho- sphorylation (Raboy, 2009). The literature survey till date provides no reports showing the down-regulation ofgene to develop low phytate rice and to study its effects on plants. In this study, we generated transgenic rice plants by RNAinterference (RNAi)-mediated down-regulation of a major ITPK homolog, i.e., driven by aleurone- and embryo-specific early maturing () wheat promoter to determine the functional significance ofgene in the phytic acid biosynthesis. This study also exploredhow the down-regulation ofgene affected phytic acid content in seeds, the expression of phytic acid biosynthetic pathway-related genes, the-inositol and amino acid contents, and finally how reduced phytic acid levels affected the spatial distribution and content of important elements in rice seeds. Additionally, the seed germination parameters and the different phenotypic characteristics were also studied compared with non-transgenic (NT) rice plants.
Materials and methods
Rice materials and growth conditions
L. subsp.cv. Khitish procured from the Chinsurah Rice Research Station, Hooghly, West Bengal, India was used for both cloning and genetic transformation. The seeds were surface-sterilized using sodium hypochlorite (4% available chlorine) and Tween 20, and germinated on filter paper soaked with distilled water in plant growth chamber at 30ºC and 75% relative humidity (FLI-2000, Eyela, Japan). Low phytate transgenic and respective NT control plants were grown inpotsunder greenhouse conditions at 32ºC/25ºC for 16 h/ 8 h respectivelywith relative humidity of 70%‒80% throughout the experiment.
Cloning of wheat promoter
The aleurone- and embryo-specific early maturing () promoter (Genbank Accession No: X52103) was isolated from genomic DNA (gDNA) of mature wheat seed by Genomic DNA (gDNA) PCR using Hotstar Hi Fiedelity DNA polymerase (Qiagen, Hilden, Germany). The 705 bp purified PCR product was cloned into pTZ57r/t vector (InsTA Clone PCR Cloning kit, Thermo Fischer Scientific, USA) atdIII/II (ThermoScientific, USA) restriction sites and sequenced. Subsequently, the promoter was subcloned at theI site of RNAi pIPKb006 binary vector, and the orientation was checked by digestion ofpromoter withI-I restriction enzymes (ThermoScientific, USA).
Cloning of rice ITP5/6K-1 gene and RNAi vector construction
A fragment (438 bp) of ricegene sequence (Genbank Accession No: AK 106544;LOC_Os10g01480; 198‒636 nt) was selected to generate RNAi vector. Total RNA was isolated from mature seeds of Khitish using RNeasy Plant Mini Kit following the manufacturer’sprotocol (Qiagen,Germany), and the first strand cDNA was synthesized using iScriptTMcDNA Synthesis Kit (BioRad, USA). Using gene specific primers (ITP5/6K-1F:5′-CACCGCCAGGAAGAAGAGCATTC-3′and ITP5/6K-1R: 5′-CTGGAGAACCAGAGGAGGATCAAGC-3′), the 438 bp fragmentofgene was amplified, and the purified fragment was cloned into a pENTR-D-TOPO entry vector (Invitrogen,USA) and sequencedsubsequently. The 438 bpgene fragment was finally cloned into a binary RNAi destination vector pIPKb006 mediated by LR clonase based on recombinationreaction following the manufacturer’s protocol (Invitrogen, USA), and the complete RNAi construct (pEm-ITP5/6K-1-Nos) was transformed intoEHA105 strain, which was used for rice transformation experiments. The transgenic lines ofRNAi construct were abbreviated as IEIT1.
Tissue culture of rice plants and Agrobacterium- mediated genetic transformation
The tissue culture and genetic transformation of the Khitish mature seeds were performedfollowing the protocols as described earlier (Datta et al, 2000; Karmakar et al, 2016). The regenerated putative transformed plants were transferred to soilrite for gradual hardening. The hardened plants were then transferred to pot containing soil in the greenhouse and allowed to grow to maturity.
PCR-based screening of transgenic plants
gDNA was extracted from the leaves of transgenic and NT rice plants as described earlier (Huang et al, 1997). A total of 100 ng gDNA was used as template, and putative T0transgenic lines, progenies of T1and T2transgenic lines were screened for the presence of both wheatpromoter (EmF:5′-CAAGCTTGGACCAGAAACCCAATTTGC-3′ and EmR: 5′-GAAGATCTTCCCTTCAGCGAG-3′) andintron (RGA2F:5′-GCTGCAGCTTCAGCTTTCTTGTACAAAGTGG-3′ and RGA2R: 5′-CGGATCCGTGGGGGATCCTCAGAAAAG-3′).
Total RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR) expression analysis
Total RNA was isolated from 100 mg mature seeds using Nucleospin® RNA Mini Kit (MN, Germany) following the manufacturer’s protocol. The purified RNA was treated with DNase (Fermentas, USA), and the first strand cDNA was synthesised from 1 µg of total RNA using iScriptTMcDNA Synthesis Kit (BioRad, USA). qRT-PCR was performed in a CFX 96 Real-Time system (BioRad, USA) following earlier described protocol (Molla et al, 2013).The optimized cycle was as follows: 95ºC for 3 min, 95ºCfor 30 s, annealing temperature (Tm, ºC) for 30 s, 72ºC for 30 s. Ricegene was used as the internal control to normalize all the data. The relative expression levels were determined using the 2-ΔΔCTmethod (Livak and Schmittgen, 2001). The primers used for qRT-PCR analysis are listed in Supplemental Table 1.
Southern blot analysis
gDNA was isolated from the leaves ofNT and PCR positive T2transgenic plants following the modified cetyltrimethyl ammonium bromide(CTAB) method (Stein et al, 2001). The DNA was quantified using a Nanodrop spectrophotometer (Thermo Fischer, USA). gDNA (15 µg) was digested withI restriction endonuclease (Fermentas, USA). The digested DNA samples were separated by 1% agarose gel and immobilized on a nylon membrane (Hybond N+, Amersham, GE Healthcare, USA) by capillary transfer mechanism. DNA was transferred to the membrane, and prehybridization, hybridization and washing were performed according to the manufacturer’s protocol (DIG DNA Labelling and Detection Kit, Roche, Germany). The 705 bppromoter, digested with the restriction enzymeI and labelled with DIG-11-dUTP, was used as the probe for hybridization. Immunological detection was carried out according to the manufacturer’s protocol (Roche, Germany).
Colorimetric estimation of phytic acid by Wade reagent
Phytic acid was extracted from 50 mg ground mature seed powder using 0.2 mol/L HCl for 1 h. The clear supernatant was collected, and 50 µL of the extract was mixed with 1 mL Wade reagent (0.03% FeCl3∙6H2O solution containing 0.3% sulfosalicylic acid in water) and 2.9 mL water (Raj Bhandari and Kawabata, 2006). The absorbance was measured spectrophotometrically at 500 nm. The concentration of phytic acid was expressed as mg/g using phytic acid standard curve prepared with phytic acid standard (Sigma Aldrich, USA).
Estimation of phytic acid content by high performance liquid chromatography(HPLC)
Phytic acid levels in transgenic and NT plant seeds were analyzed by the reversed-phase HPLC analysis.
Sample preparation and HPLC procedure was followed the modified protocol (Coelho et al, 2005). Seed sample (100 mg) was ground to powder and extracted with 1 mL of 2.4 mol/L HCl at room temperature (25ºC) for 16 h on a thermal block with continuous shaking at500 r/min for 20 min. The extract was centrifuged at 11000 r/min for 20 min. The supernatant was passed through 0.22 µm Millipore filter (Millipore, France). The filtrate was vacuum-dried in a round bottom flask, and the residue was finally redissolved in 100 µL MilliQ water (Millipore, France).
The mobile phase consisted of the mixture of formic acid (35 mmol/L): methanol (44:56) containing 10 mL/L TBA-OH (tetra-butyl ammonium hydroxide 40% in water). The pH was adjusted to 4.3 with 9 mol/L sulphuric acid. The mobile phase was filtered through a nylon filter (0.45 µm) under vacuum and degassed for 15 min. Spherisorb®S5 SAX analytical column (4.0 mm × 250 mm) (Waters,USA) was used, and phytic acid was detected by a Waters 2414 Refractive Index Detector(Sigma Aldrich,USA). The flow rate was kept at 0.5 mL/min. The calibration curve was prepared with phytic acid standard. The retention time of standard and samples were compared, and accordingly phytic acid contents in transgenic and NT samples were quantified.
Determination of total phosphorus (TP)and inorganic phosphorus
The TP content in seeds was determined by the alkaline peroxodisulfate digestion method using 50 mg mature seeds crushed to powder with mortar and pestle (Woo et al, 1995). The spectrophotometric assay was carried out at 800 nm (Chen et al, 1956).
Pi level in seeds was determined using 50 mg mature seeds ground to powder witha mortar and pestle as described by Lott et al (1995).The resulting colour of phosphomolybdate complex was measured spectro-photometrically at 800 nm (Chen et al, 1956). The samples were prepared in triplicate, and the calibration curves for TP and Pi were prepared with phosphorus standard solution (0.65 mmol/Lphosphorus, Sigma Aldrich, USA).
Determination of elemental distribution based on X-ray microfluorescence (µ-XRF)
Dehusked rice seeds were longitudinally cut into 300 µm sections with a scalpel and exposed to 50-keV monochromatized X-ray beam (beam current was set at 0.6 mA) for energy dispersive X-ray imaging using a XGT-7200 µ-XRF spectrometer (Horiba Scientific, France) and subsequent elemental maps were obtained. The measurements were carried out in vacuum environment and no filters were used between source and sample for optimum detection of elements. When the samples were exposed to the microbeam, fluorescent X-rays were emitted and detected using liquid nitrogen cooled Silicon-Lithium semiconductor detector. The X-ray fluorescence intensity in µ-XRF analysis is directly proportional to the element concentration. The analysis was repeated thrice using three independent mature seeds of the same NT and transgenic plant.
Estimation of metal content
Mature transgenic and NT seeds were milled in a rice miller (Satake, Japan) for 1 min. The milled seeds (1 g)were digested following the modified dry ashing digestion (Jiang et al, 2007). The acidic ash solution was filtered through Whatman filter (No. 42), and final volume was made upto 15 mL. The contents of Fe, Mg, manganese (Mn) and Zn were analyzed using an atomic absorption spectrophotometer (AAS, Aanalyst 200, Perkin Elmer, USA) with respective hollow cathode lamps (HCL, Perkin Elmer, USA). The calibration curves were prepared with respective standard metal solutions (Merck, Germany).
Histochemical localization of Zn and Fe
Rice seeds were sectioned longitudinally, and the histochemical localization of Zn (Duarte et al, 2016) and Fe (Sivaprakash et al, 2006) in the sections were observed under a microscope (Carl Zeiss Stereo microscope, Stemi 2000-C, Germany).
Amino acid analysis
Rice seed powder (20 mg) crushed witha mortar and pestle was extracted according to Ali et al (2013b) and derivatized using AccQ-Fluor Reagent Kit (WAT 052880-Waters Corporation, Milton, Massachusetts, USA) following the manufacturer’s protocol. The samples were prepared in triplicate and the amino acid content was analyzed following the manufacturer’s protocol (Waters, USA). The calibration curve was prepared with Amino Acid Standard H (ThermoScientific, USA).
Estimation of myo-inositol content
A total of 100 mg seeds were ground to powder with a mortar and pestle and extracted using 1 ml of 50% aqueous ethanol, and the derivatization procedure of-inositol was carried out following Ali et al (2013b). Triplicate samples were prepared and the-inositol content was identified as hexatrimethylsilyl ether derivative using a gas chromatography-mass spectrometer (GC-MS) coupled with column (30 m ×0.25 mm ×0.25 μm) HP-5MSI-19091-433I (6890N Network GC system with 5973 Inert Mass Selective Detector, Agilent Technologies, USA). The-inositol hexatrimethylsilyl ether was identified in database library NBS75K by comparing the mass fragmentation pattern.-inositol standard (Sigma-Aldrich, USA) was prepared in aqueous solution, which was dried, derivatized and analyzed in similar way.
α-amylase and β-amylase activity assay during seed germination
Seeds were germinated on filter paper at 37ºC. At regular time intervals of 0, 12, 24, 36, 48, 60, 72, 84 and 96 h, the seeds were collected and stored frozen at -80ºC. α-amylase and β-amylase activity assays were determined as previously described (Ali et al, 2013b). Total protein was determined according to Bradford (1976) using bovine serum albumin (Sigma-Aldrich, USA) as standard. The reducing sugar was estimated by adding 1 mL dinitrosalicylic acid reagent (1% of 3,5-dinitrosalicylic acid, 0.4 mol/L NaOH and 1 mol/Lpotassium sodium tartarate). The samples were prepared in triplicate and the absorbance was measured at 540 nm. A standard curve using maltose monohydrate solution(Sigma-Aldrich, USA) was prepared in similar manner as previously reported studies (Bernfeld, 1955; Miller, 1959).
Morphological analysis of transgenic plants
The seeds were surface-sterilized with Bavistin for 10‒12 min, thoroughly washed with distilled water for 4‒5 times and allowed to germinate in dark on filter paper soaked with distilled water at 37ºC. The morphology of transgenic seeds was recorded at regular intervals (24, 48, 72 and 96 h), and the result was compared with NT seeds. To study stress tolerance, the NT and transgenic seeds were treated with 5 µmol/L abscisic acid (ABA) (SRL Pvt. Ltd, India), 200 mmol/L sodium chloride (NaCl) (Merck, Germany) and 15% polyethylene glycol-8000 (PEG-8000) (HiMedia Laboratories, India) solutions, and the germination rate was recorded after 48 h. The experiment was repeated three times to confirm the observations. Germination rate (%) = (Number of seeds germinated / Total seeds) × 100.
Different agronomic traits of transgenic plants growing under greenhouse conditions were noted and compared with NT plants. Several agronomic parameters such as plant height, number of tillers per plant, number of panicles per plant, panicle length, 1000-grain weight, seed length and width, and length/width ratio were considered. Ten plants of each NT and transgenic line were grown in greenhouse, and five randomly chosen plants from each transgenic line were considered for agronomic performance study.
Thin hand sections were prepared from embryo structure at different developmental stages, viz., 7, 14, 21 and 28 d after pollination (DAP). The sections were stained in 0.1% toluidine blue solution (SRL Pvt. Ltd, India) for 5 min at room temperature, and the histological structure of three randomly chosen embryos were studied under a microscope (CarlZeiss Stereo microscope, Stemi 2000-C, Germany) (Kuwano et al, 2009).
Statistical analysis
All the statistical analysis was performed using the Graph Pad Prism 5 software. The experimental data are presented as mean ± standard error (SE) based on three replications. One-way and two-way analyses of variance (ANOVA) were used to compare differences between NT and transgenic plants, and significant difference between group means were calculated following the Bonferroni post-Hoc tests.
Results
Determination of expression levels of different OsITPK homologs and identification of major transcript
Six homologs ofgenes have been identified in rice (Suzuki et al, 2007). The expression levels of these sixhomologs in rice seeds were determined by qRT-PCR, and the highest transcript level was observed forandin Khitish mature seedsThe transcript expression levels ofhomologs presented in the order of>>>=(Supplemental Figs. 1-A and 2)
Generation and screening of transgenic rice lines
A total of 20 putative-resistant transgenic plants (T0) were obtained through-mediated transformation and subsequent tissue culture. The putative transgenic plants (T0) generated were screened by PCR for the presence of exogenouspromoter (705 bp) and wheatintron (350 bp). Out of 20 plants, 14 plants showed the desired amplification of thepromoter andintron. No amplification was observed in NT plants.
These PCR-positive transgenic plants (T0) were further screened by biochemical analysis for the phytic acid content in T1seeds. Six T0transgenic lines (IEIT1-2, -6, -11, -12, -13 and -14) exhibited a significant decrease in the phytic acid content (7.25, 10.34, 10.28, 8.38, 9.11 and 9.64 mg/g, respectively) than NT (13.08mg/g) (Supplemental Fig. 3-A). Based on these results, the segregation analysis of transgenic seeds (T1to T3) was performed by germinating the transgenic seeds in aselection medium (Murashige and Skoog medium containing 50 mg/L). T1progenies were screened by PCR analysis of gDNA for thepromoter andintron (Supplemental Fig. 3-B and -C). Among the PCR-positive T1plants, seeds of T1(i.e., T2seeds), IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2, exhibited a significant reduction in the phytic acid content as confirmed by HPLC analysis. Therefore, these four lines were selected for subsequent biochemical and molecular analyses.
Expression analysis of T2 transgenic lines by qRT-PCR
To determine the down-regulation ofgene in transgenic plants at the transcript level, qRT-PCR analysis was performed using gene-specific primers. Results exhibited a significant down- regulation ofgene in IEIT1-2-5 (2.17-fold), IEIT1-2-13 (1.96-fold), IEIT1-12-1 (2.63-fold) and IEIT1-12-2 (2.27-fold) lines (< 0.05) with respect to NT, indicating successful silencing of thegene. In addition, we checked the transcript levels of three other genes of phytic acid biosynthesis pathway, namely,and. The down-regulation of thegene resulted in enhanced transcript levels ofandgenes with respect to NT (< 0.05). However, thegene that catalyzes the final step in the phytic acid biosynthesis, i.e., from Ins (1, 3, 4, 5, 6) P5to InsP6, exhibited a significant down-regulation in its transcript levels with respect to NT (< 0.05) (Fig. 1). To further assess the effect of down-regulation ofon other homologs of the ITPK gene family, the expression levels of other five homologs (to) were verified, and all five homologs showed no significant alteration in their transcript levels with respect to NT (> 0.05) (Supplemental Fig. 4).
Southern blot analysis of transgenic plants
To determine the integration of the transgene cassette into the plant genome, PCR-positive plants were selected for Southern blot analysis. Digestion of the gDNA withI restriction enzyme revealed a stable integration of the transgene cassette into the genome of transgenic rice plants (T2). Single copy integration was observed in lines including IEIT1-2-5, IEIT1-12-2 and IEIT1-12-14. Two bands were observed in lines IEIT1-2-13 and IEIT1-12-1, whereas four bands were observed in line IEIT1-2-10. However, no hybridization signal was detected in the non-transgenicDNA sample (Supplemental Fig. 5).
Estimation of phytic acid and seed phosphorus contents
The HPLC analysis revealed the mean phytic acid contents in transgenic lines IEIT1-2-5, IEIT1-2-13, IEIT1-12-1, IEIT1-12-2 and NT to be 5.69, 6.36, 6.59, 5.71and 10.58 mg/g, respectively. Chromatograms obtained from the HPLC/RI method revealed a significant decrease in the phytic acid content as compared to NT, with the maximum decrease of 46.2% observed in IEIT1-2-5 (< 0.001) (Fig. 2-A and Supplemental Fig. 6).
In order to determine the effect of down-regulation ofgene on the phosphorus content of seeds, the Pi and TP contents of transgenic and NT seeds were determined. The average Pi contents of IEIT1-2-5 (0.25 mg/g), IEIT1-2-13 (0.24 mg/g), IEIT1-12-1 (0.29 mg/g) and IEIT1-12-2 (0.28 mg/g) transgenic were found to be significantly elevated in comparison to NT seeds (0.09 mg/g) (Fig. 2-B). A maximum of 3.2-fold enhancement was observed in the transgenic line IEIT1-12-1 (< 0.05),whereas no significant differences were found between the average TP contents in transgenic and NT seeds (> 0.05) (Fig. 2-C).
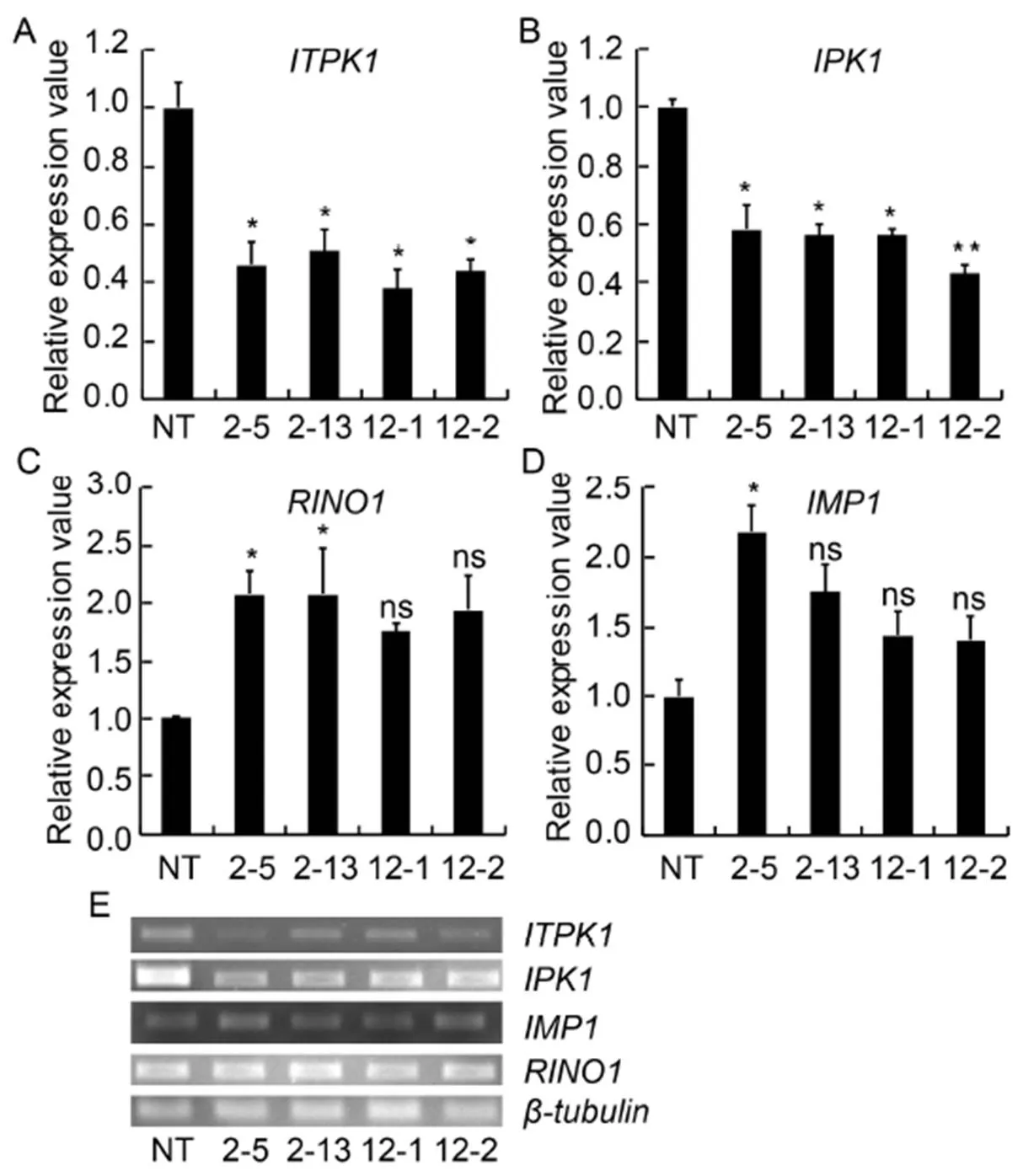
Fig. 1. Expression analysisof different genes of phytic acid biosynthesis pathwayin selected RNAi transgenic as compared to non-transgenic (NT)control.
All values were normalized usingas the reference gene.
2-5, 2-13, 12-1 and 12-2 are positive T2plants IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2, respectively.
Values are presented as Mean ± SE (=3). * and ** indicate significant differences at the 0.05 and 0.01 levels, respectively, and ns indicates non-significant difference at the 0.05 level.
Determination of elemental distribution in rice seeds by µ-XRF analysis
We next determined the localization of elements, such as P, S, Ca and K, in transgenic and NT mature seeds using the µ-XRF analysis. In Fig. 3, the elemental maps depict that in NT seeds, P, S and K were localized predominantly to the embryo and aleurone layer, and the lesser concentration was observed in the endosperm region. However, Ca was predominantly accumulated around the embryonic region than in the aleurone and endosperm. In contrast, the distribution pattern of these elements in transgenic seeds was found to be altered with respect to NT seeds. The X-ray imaging analysis clearly depicted that P, S and Ca were spread deeper into the endosperm region, and a broader distribution of these elements was noted in the endosperm of transgenic seeds than NT seeds,whileK was found to be the most abundantly present in the embryo and endosperm region of transgenic seeds.
Quantification of metal content in seeds
Since essential elements such as Fe, Zn, Mg and Mn cannot be detected in the µ-XRF analysis, the concentration of their ions (Fe2+, Mg2+, Mn2+and Zn2+)in transgenic polished seeds (T2) was determined by atomic absorption spectroscopy. The enhanced levels of these ions were found in low-phytate transgenic seeds as compared to NT seeds. In the IEIT1-2-5 transgenic (T2) line, a 1.3-fold enhancement in Fe2+content in transgenic seeds was noted. Other metal ions such as Mg2+, Mn2+and Zn2+reported a maximum increase of 1.4-fold in IEIT1-12-2, 1.7-fold in IEIT1-2-13 and 1.6-fold in IEIT1-2-5, respectively, as compared to NT (Fig. 4).
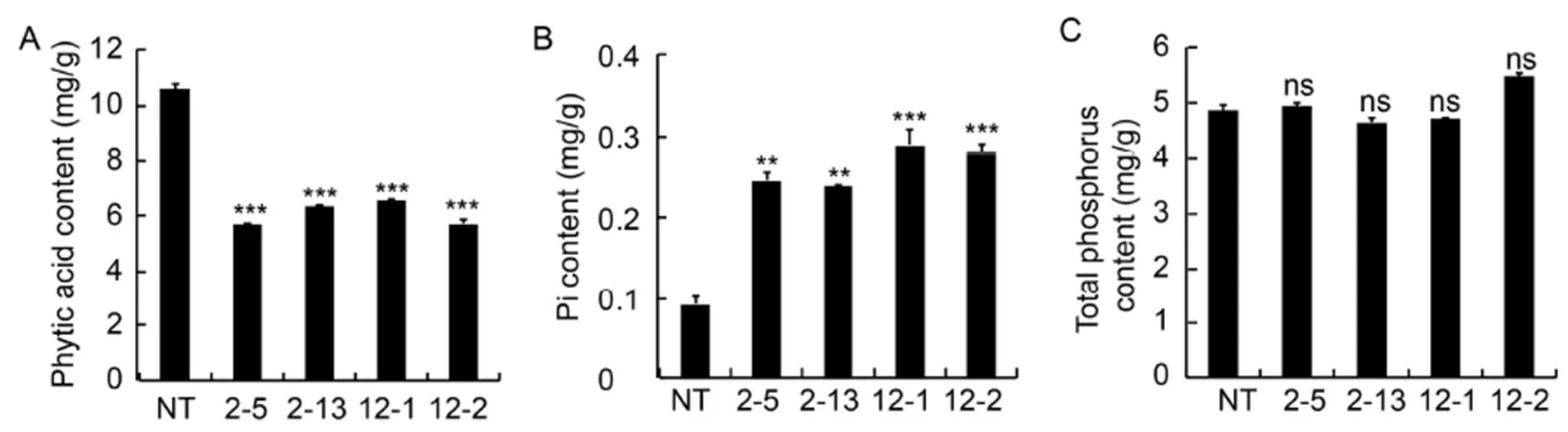
Fig. 2. Analysis of phytic acid (A), inorganic phosphorus (Pi) (B) and total phosphorus (TP) content (C).
2-5, 2-13, 12-1 and 12-2 are positive T2plants IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2, respectively.
Values are presented as Mean ± SE (=3). ** and *** indicate significant differences at the 0.05 and 0.001 levels, respectively, and ns indicates non-significant difference atthe0.05 level.
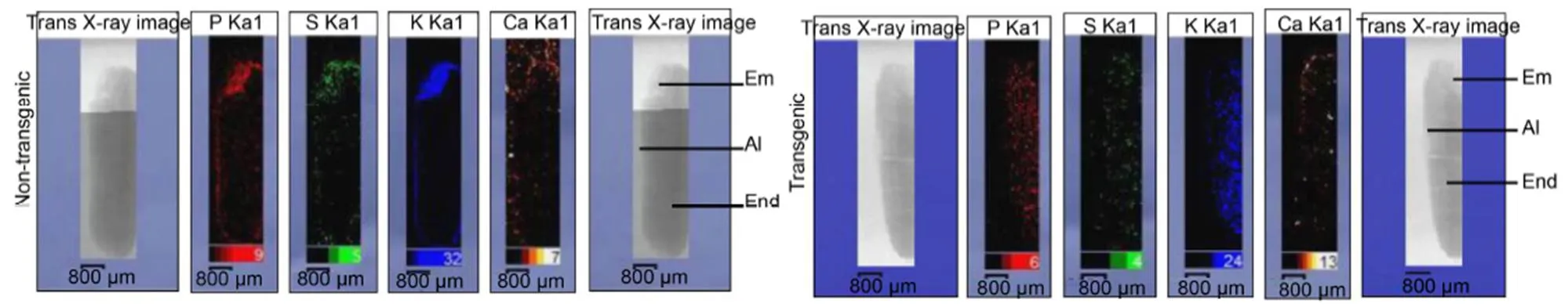
Fig. 3. Comparison of elemental maps of P, S, K and Ca in longitudinal section of entire mature transgenic and non-transgenic seeds of Khitish.
Em, Embryo; Al, Aleurone layer; End, Endosperm.
Histochemical localization of Zn and Fe
NT and transgenic rice grains were stained using dithizone and Perl’s Prussian blue to assess the histochemical localization of Zn and Fe. Variation in the intensity was observed among seed regions (embryo, endosperm and aleurone). Within the seed, the staining intensity followed the order of embryo > aleurone > endosperm. However, the presence of Zn and Fe in the endosperm cells of transgenic rice grains was indicated by significant variation in color intensity as compared to NT. The endosperm of NT showedno significant colored reaction, whereas transgenic rice grains revealed higher color intensity, indicating a greater accumulation of Zn and Fe in the endosperm region (Fig. 5).
Amino acid analysis and myo-inositol content of seeds
The amino acid contents of transgenic and NT seeds were assessed by the HPLC method, which clearly indicated that silencing ofdid not lead to any deleterious effect on the amino acid content of transgenic seeds (Table 1 and Supplemental Fig. 7).-inositol plays an essential role in plant metabolism and during the growth and developmental process. Hence, the effect of silencinggene on the-inositol content of seeds was examined by the GC-MS and the results revealed a significantly elevated-inositol content in the transgenic seeds in comparison to NT seeds (< 0.05) (Fig. 6).
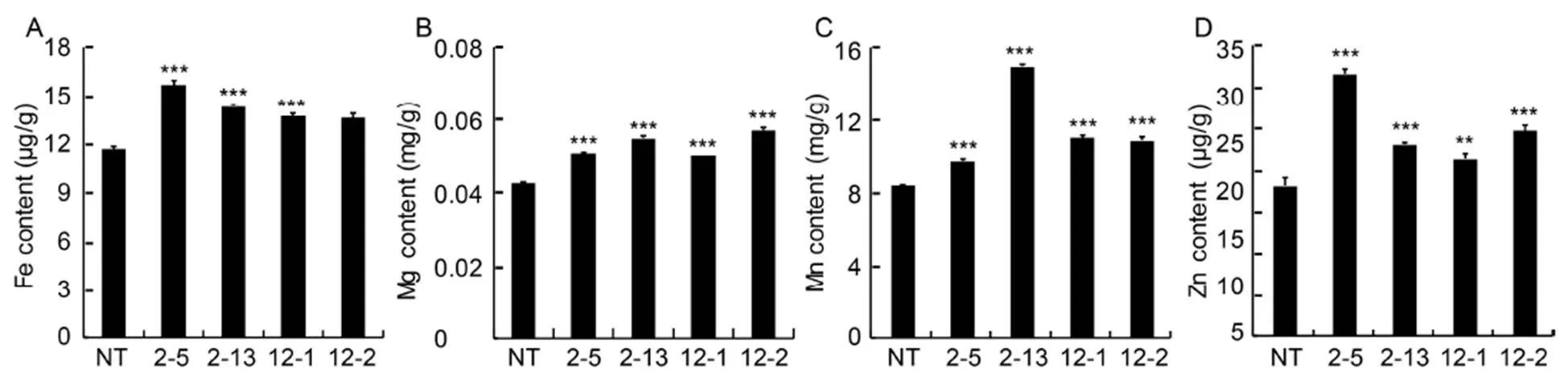
Fig. 4.Analysis of iron (Fe2+) (A), magnesium (Mg2+) (B), manganese (Mn2+) (C) and zinc (Zn2+) (D) content in milled seeds of non-transgenic (NT) and transgenic (T2) seeds by atomic absorption spectroscopy.
2-5, 2-13, 12-1 and 12-2 are positive T2plants IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2, respectively.
Values are presented as Mean ± SE (=3). ** and*** indicate significant differences at the 0.01 and0.001 levels, respectively.
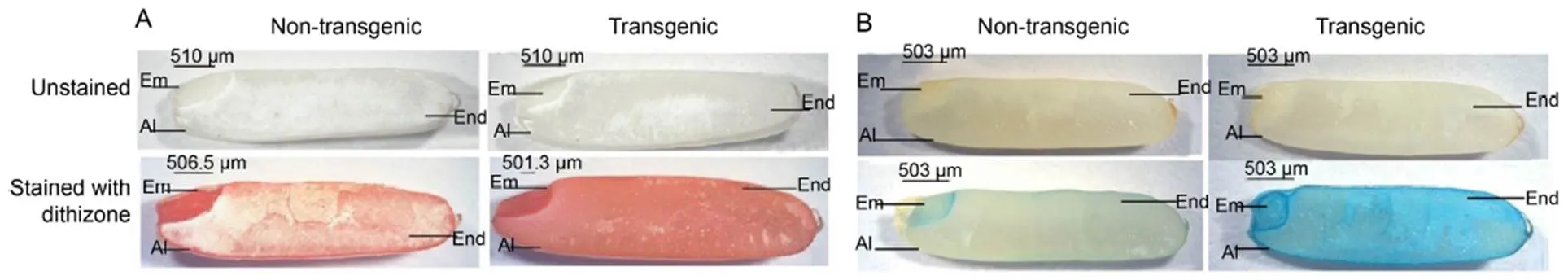
Fig. 5. Histochemical localization of zinc (A) and iron (B) in rice seeds.
Em, Embryo; Al, Aleurone layer; End, Endosperm.
Values are Mean ± SE (=3) (<0.05). The same lower case letters in the same row indicate significant difference at the 0.05 level.
Seed germination analysis
The effect of reduced phytic acid content on seed germination is examined via performing seed germination experiment under normal and stress conditions. The transgenic and NT seeds exhibited a normal germination rate in absence of stress(Supplemental Fig. 8-A and -B). However, under 5 µmol/L ABA stress treatment, the transgenic seeds showed decreased germination rate (20.0%) compared to NT seeds (58.3%) due to reduction in phytic acid content. However, in response to 200 mmol/L NaCl and 15% PEG, the transgenic seeds showed enhanced germination rate (32.6% and 65.0%) compared to NT (20.0% and 51.7%), respectively (Supplemental Fig. 8-C). Moreover, the activities of essential starch-degrading enzymes such as α-amylase and β-amylase of transgenic (T2) seeds also showed similar activities compared to the NT seeds during the course of germination (Supplemental Fig. 9-A and -B).
Internal morphology of seeds and agronomic traits of transgenic plants
The morphology and internal structure of the embryo of transgenic and NT seeds at different development stages (7, 14, 21 and 28 DAP) were studied, and no abnormality was observed (Supplemental Fig. 9-C).
Different agronomic traits of transgenic plants (T2) were compared and no significant difference in plant height, number of tillers per plant, panicle length, and growth pattern was observed with respect to NT. Other morphological traits such as seed length, seed width and 1000-grain weight were also almost identical in transgenic and NT seeds (Table 2).
Discussion
In this study, we aimed to decipher the functional significance ofin the phytic acid biosynthesis pathway. Previous studies have demonstrated successful silencing of,andgenes using tissue-specific promoters to develop ‘’rice (Kuwano et al, 2009; Ali et al, 2013a, b). The importance of ITPK genes inwas previously studied by developing the knockout mutants of,andgenes, which exhibited a decrease in seed InsP6content ranging from 40% to 60% (Kim and Tai, 2011). In rice, six homologs ofgene have been previously identified (Suzuki et al, 2007), however, the actual effect of silencing ofgenes on lowering the phytic acid content of rice remained unknown.
Fig. 6.-inositol content of seed by gas chromatography-mass spectrometer analysis.
A and B, Mass-fragmentation pattern of non-transgenic and transgenic seeds as observed in GC-MS analysis.-inositol was estimated as hexatrimethylsilyl ether derivative and identified by comparing the mass fragmentation pattern in the database library NBS75K. C,-inositol content of transgenic seeds (T2) as compared to non-transgenic (NT) seeds.
2-5, 2-13, 12-1 and 12-2 are positive T2plants IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2, respectively.
Values are presented as Mean ± SE (=3). ** indicates significant difference at the 0.01 level.
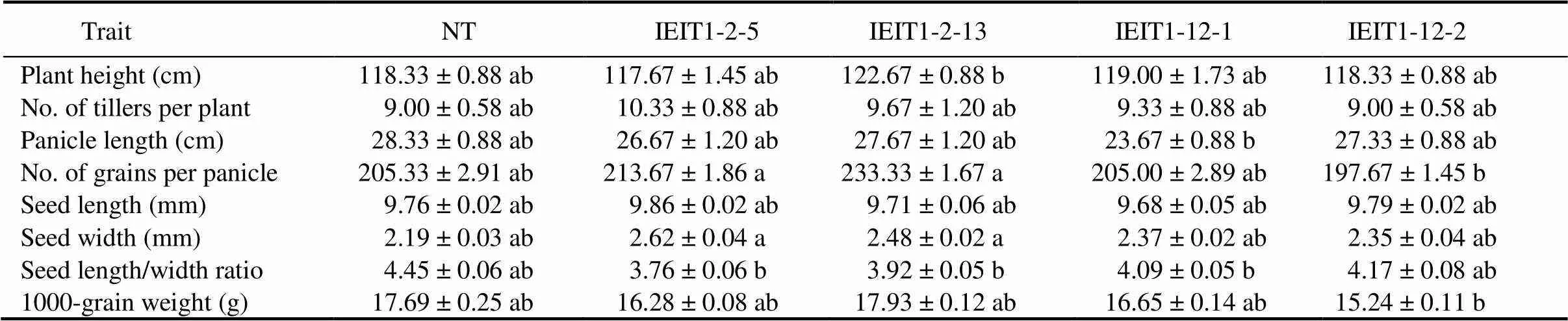
Table 2. Phenotypic evaluation of low phytate transgenic (T1) and non-transgenic (NT)plants under greenhouse conditions.
Values are Mean ± SE (=5). The same lowercase letters in the same row indicate no significant difference at the 0.05 level.
Silencing of OsITP5/6K-1 gene expression significantly decreased phytic acid content and affected the expression of essential phytic acid biosynthetic genes
Different studies have indicated that a large amount (80%) of phytic acid accumulated in the aleurone layer and embryo regions in rice during seed development (Bohn et al, 2008; Sparvoli and Cominelli, 2015), hence we strategically down-regulated thegene in a seed-specific manner using the aleurone- and embryo-specificpromoter. An earlier report demonstrated wheatpromoter to drive green fluorescent protein (gfp)-tagged expression in the embryo and aleurone tissues of transgenic barley and rice (Furtado and Henry, 2005). Initially, we studied differential expression patterns of sixhomologs using qRT-PCR analysis and determined that among the sixhomologs, the expression levels ofandwere significantly high (Supplemental Fig. 1-A). Hence, RNAi vectors were constructed to target thegene (RNAi vector was also constructed using thehomolog that resulted in the successful silencing of phytic acid; unpublished data). To check homology, we alignedRNAi sequence (438 bp) with otherhomologues using a multiple sequence alignment tool clustalW (www.genome.jp/tools/clustalw/) and no significant homology was found with other homologues (Supplemental Fig. 2). Our study demonstrated that transgenic plants generated by silencingcan successfully reduce the phytic acid content by integrating the transgene cassette into the genome. These transgenic varieties also exhibited normal growth and similar agronomic characteristics as compared with NT plants (Table 2 and Supplemental Fig. 8).
Based on the decreased phytic acid content of T1seeds, six promising transgenic lines, namely IEIT1-2, IEIT1-6, IEIT1-11, IEIT1-12, IEIT1-13 and IEIT1-14 were selected for the next-generation study. Among T2transgenic seeds, IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2 exhibited significant down-regulation of phytic acid. Thus, IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2 were selected for further molecular and biochemical analyses. IEIT1-2-5 exhibited 46.2% down-regulation in the phytic acid content compared to the NT control as evident from the HPLC analysis (Fig. 2-A). The reduction in the phytic acid levels resulted in an increment of 2- to 3-fold in the Pi levels among all the transgenic lines (Fig. 2-B). Decreased phytic acid and concomitant elevated Pi content substantiated the strict co-relation between phytic acid content and Pi levels.Previously, RNAi-mediated silencing ofandgenes has demonstrated to cause 58% and 69% reduction in phytic acid, consequently resulting in a simultaneous enhancement in the Pi content in ‘’ rice (Ali et al, 2013a,b). The reduction in phytic acid did not alter the total phosphorus content in transgenic seeds (Fig. 2-C), suggesting a balance mechanism of transgenic plants to support the P-related mechanisms in seed.
The qRT-PCR analysis revealed a 2.63-fold down- regulation in the expression ofgene at the transcript level. Moreover, thegene, which is present downstream of thegene, exhibited a substantial down-regulation (2.32-fold) in its expression. These results clearly indicated that silencing of thegene down-regulated thegene. On the contrary, other phytic acid biosynthetic enzymes such asandshowed enhanced expression in their transcript levels (Fig. 1-A to -D). It can be speculated that a large fraction of Ins3 (P) remained unutilized due to down-regulation of thegene and the accumulation of Ins3 (P) resulted in the pathway shifting toward the reversible steps catalyzed by theandgene to synthesize enhanced-inositol (Fig. 7-A). However, RNAi-mediated seed-specific silencing ofgene did not significantly alter the expression ofandgenes in an earlier study (Ali et al, 2013a). From real-time quantitative expression analysis of phytic acid pathway genes, it can be inferred that down-regulation of bothandgenes resulted in the lowering of phytate content in transgenic seeds. Additionally, qRT-PCR of otherhomologs (to) clearly signified the specificity of silencing of thegene and did not perturb the expression of other ITPK homologs (Supplemental Fig. 4).
Lowering of phytic acid content resulted in change in spatial distribution of minerals, amino acid profile and myo-inositol content
It was reported that a reduction in the phytic acid content would enhance the availability of free elements in the seed and therefore may affect the normal distribution patterns of elements (Sakai et al, 2015). Hence, we studied the spatial distribution pattern of elements (P, S, K and Ca) in entire mature seeds using the µ-XRF imaging analysis, which revealed the precise distribution of these elements in the aleurone, embryo and endosperm regions of NT andseeds. In NT seeds, P, S and K were found to be accumulated in higher concentrations in the embryo, aleurone and sub-aleurone layer, whereas relatively weak signals for P, S and K were found in the endosperm region. However, inseeds, strong signals for P, Sand K were obtained in the endosperm region, and the accumulation of P, S and K in the aleurone layer and embryo ofseeds were considerably less than in the NT seeds. In NT seeds, Ca was predominantly concentrated around the embryo region as well as in and around the aleurone layer. Inseeds, although Ca was abundantly present around the aleurone layer, relatively higher concentration was noted in the endosperm region as compared to NT seeds (Fig. 3). The plausible reason could be that a reduction in phytate globoids in the aleurone layer ofseeds inhibits the formation of phytate salt from metal ions, gradually extruding them from the aleurone layer to the endosperm region (Liu et al, 2004). The elements like K, S and P that failed to form phytate complexes diffused readily from the aleurone layer and the embryo to the endosperm region in low-phytate seeds. In the case of Ca, since it is a divalent cation, it perhaps remained bound to phytate globoids in the aleurone layer and got concentrated in the aleurone and sub-aleurone regions, while the unbound Ca ions produced more signals in the endosperm region as compared to NT seeds owing to low phytate content ofseedsThis result is in accordance with the previous report of Sakai et al (2015), who determined that reduced InsP6changes distribution pattern of elements such as P, K, Zn and Fe in wild type andseeds using synchrotron-based µ-XRF. However, we failed to obtain any signal for Fe and Zn since these ions were present in trace amounts in a single matured seed and beyond the detection limit of µ-XRF. Hence, the silencing ofgene and low phytate content caused translocation of elements from the embryo and aleurone layer to the endosperm region, consequently enriching these elements in the endosperm region (Fig. 7-B).
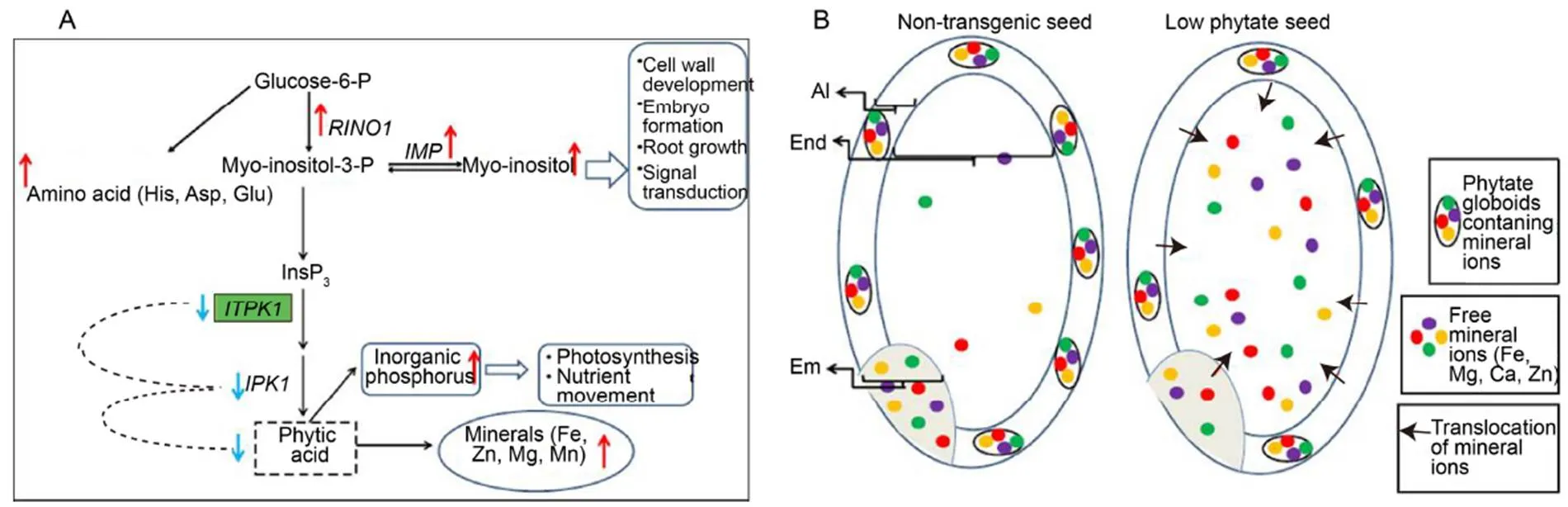
Fig. 7. Effect of silencingon phytic acid biosynthesis pathway and distribution of mineral ions in seed.
A,Hypothetical model depicting effect of down-regulation ofgene on different phytic acid pathway enzymes and metabolites. Red arrowindicates up-regulation, Blue arrowindicates down-regulation. B, Diagram depicting mechanism in translocation of mineral ions in low phytate transgenic seeds.Em, Embryo; Al, Aleurone layer; End, Endosperm.
During commercial milling of rice, both the aleurone layer and the embryo are removed, rendering the final product mineral-deficient. Hence, we further studied the concentration of essential elements (Fe, Mg, Mn and Zn) in matured polished seeds using atomic absorption spectroscopy. Fe content showed 1.3-fold increase compared to NT, and Mg, Mn and Zn exhibited a concomitant increase of 1.4-fold, 1.7-fold and 1.6-fold, respectively (Fig. 4). We further determined the histochemical localization of Zn and Fe using dithizone and Perl’s Prussian blue staining, which validated the presence of higher concentrations of Fe and Zn in the endosperm region of transgenic rice compared to the NT seeds (Fig. 5). Higher abundance of Fe and Zn in the endosperm ofseeds indicated the possibility of translocation of free Fe and Zn ions from the aleurone and embryo to the endosperm region owing to low phytate content inseeds. In a previous study, RNAi-mediated down-regulation ofandgenes leads to 1.8-fold increase in Fe and 2.2-fold increase in Zn levels in polished seeds (Ali et al, 2013a,b; Aggarwal et al, 2018).
Further, we analyzed the amino acid profile of transgenic seeds. Overall, the results confirmed that seed-specific silencing ofgene did not affect the amino acid content of rice grains, and the amino acid content of transgenic seeds was found to be elevated compared to NT (Supplemental Fig. 7 and Table 1). Amino acids such as histidine, leucine, methionine and phenylalanine showed 2- to 3-fold increase, whereas alanine, arginine, aspartic acid, glutamic acid, glycine, isoleucine, lysine, serine, tyrosine and valine showed 1.5- to 2.0-fold enhancement in transgenic seeds. Proline showed 1.4-fold increase in its content in transgenic seeds. However, the content of cysteine declined to 44% in transgenic samples, the reason for which can not be ascertained. A plausible reason for the increase in amino acid content in transgenic seeds could be the elevated levels of molecules such as glucose-6-phosphate, which acts as a precursor metabolite for amino acid biosynthesis. The increase in the amino acid content in low phytate transgenic seeds is not reported in the previous study (Ali et al, 2013a).
Did enhanced myo-inositol play a role in overcoming abiotic stress?
-inositol is an essential metabolite necessary for plant growth and development. GC-MS analysis of T2seeds exhibited a significant increase in the-inositol content indown-regulated lines (Fig. 6). This result also validates the increase in theandtranscript levels via qRT-PCR, asandcatalyze the synthesis of-inositol in seeds. However, the RNAi-mediated down-regulation ofgene did not alter the-inositol levels in T4transgenic seeds as compared to NT seeds (Ali et al, 2013a).
A number of earlier studies have reportedmutants to exhibit altered seed germination with impaired morphology (Kuwano et al, 2009). However, we observed that the germination rate and seed morphology of transgenic seeds were almost identical to that of NT seeds (Supplemental Fig. 8-A and -B). Two important starch-degrading enzymes, namely α- amylase and β-amylase, which are considered as indicators for assessing the germination potential (Galani et al, 2011) in cereals, exhibited similar activities, further demonstrating normal germination behavior of both transgenic and NT seeds (Supplemental Fig. 9-B and -C). An earlier report demonstrated that low phytate and high Pi content prove detrimental to embryogenesis, leading to impaired germination (Kuwano et al, 2009). Keeping this in view, the embryos of transgenic seeds were collected at different stages of development, sectioned, stained, and analyzed microscopically. No major alteration was observed in the morphology and the internal structure of embryos in transgenic samples (Supplemental Fig. 9-C). To study the response under stress, when the transgenic seeds were treated with 5 µmol/L ABA solution, the germination rate decreased compared to NT, due to decline in phytic acid content. However, the transgenic seeds showed enhanced stress response under 200 mmol/L NaCl and 15% PEG, which might be due to significant increase in-inositol content (Fig. 6 and Supplemental Fig. 8-C). This result suggested that the phytic acid may not be the absolute requirement for efficient germination and seedling emergence (Shi et al, 2007) and-inositol plays an important role to overcome the abiotic stress (Loewus and Murthy, 2000; Kaur et al, 2013).
In conclusion, the findings of this study demonstrated that tissue-specific RNAi mediated downregulation of thegene led to significant reduction in seed phytate level and we speculated that the transgenic plants attempted to compensate the effect of silencing by enhancing the transcript levels of,as wellas-inositol and amino acid content. Furthermore, lowering of phytate resulted in the translocation of elements from the aleurone layer and the embryo to the endosperm region in transgenic seeds. The present study also provided an insight into the mechanism of phytic acid biosynthesis pathway. While whole metabolite and proteomic analysis ofseeds would provide a better understanding of the interplay of various metabolites of phytic acid biosynthesis pathway.
Supplemental DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Primers used for quantitative real-time PCR.
Supplemental Fig. 1. Expression pattern of OsITPK homologues and RNAi vector map.
Supplemental Fig. 2. Multiple sequence alignment of six rice ITPK homologues and ITP5/6K-1 RNAi sequence using ClustalW.
Supplemental Fig. 3. Screening of transgenic plants based on PCR and biochemical analysis.
Supplemental Fig. 4. Expression analysisof ITPK homologue genes in selected RNAi transgenic as compared to non-transgenic control.
Supplemental Fig. 5. Southern blot analysis of transgenic lines IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2.
Supplemental Fig. 6.HPLC chromatogram of phytic acid extracts from non-transgenic seeds (NT) compared to transgenic lines IEIT1-2-5, IEIT1-2-13, IEIT1-12-1 and IEIT1-12-2.
Supplemental Fig. 7. Amino acid content in non- transgenic and transgenic (T2) seeds by high performance liquid chromatography.
Supplemental Fig. 8. Seed germination analysis.
Supplemental Fig. 9. Internal structure of seed and enzyme activity analysis during germination.
Ali N, Paul S, Gayen D, Sarkar S N, Datta K, Datta S K. 2013a. RNAi mediated down regulation of-inositol-3-phosphate synthase to generate low phytate rice.,6: 12.
Ali N, Paul S, Gayen D, Sarkar S N, Datta K, Datta S K. 2013b. Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene ().,8: e68161.
Aggarwal S, Kumar A, Bhati K K, Kaur G, Shukla V, Tiwari S, Pandey A K. 2018. RNAi-mediated downregulation of inositol pentakisphosphate kinase (IPK1) in wheat grains decreases phytic acid levels and increases Fe and Zn accumulation.,9: 259.
Bernfeld P. 1955. Amylases α and β.: Colowick SP, Kalpan NO. Methods in Enzymology. New York: Academic Press: 149–158.
Bohn L, Meyer A S, Rasmussen S K. 2008. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding.,9(3): 165–191.
Bradford M M.1976. A rapid and sensitive method for thequantitation of microgram quantities of protein utilizing theprinciple of protein-dye binding., 72: 248–254.
Bregitzer P, Raboy V. 2006. Effects of four independent low-phytate mutations in barley (L.) on seed phosphorus characteristics and malting quality.,83(5): 460–464.
Chen P S, Toribara T Y, Warner H. 1956. Microdetermination of phosphorus.,28: 1756–1758.
Coelho C M M, Tsai S M, Vitorello V A. 2005. Dynamics of inositol phosphate pools (tris-, tetrakis- and pentakisphosphate) in relation to the rate of phytate synthesis during seed developmentin common bean ().,162(1): 1–9.
Datta K, Koukolikova-Nicola Z, Baisakh N, Oliva N, Datta S K. 2000.-mediated engineering for sheath blight resistance ofrice cultivars from different ecosystems.,100(6): 832–839.
Drakakaki G, Marcel S, Glahn R P, Lund E K, Pariagh S, Fischer R, Christou P, Stoger E. 2005. Endosperm-specific co-expression of recombinant soybean ferritin andphytase in maize results in significant increases in the levels of bioavailable iron.,59(6): 869–880.
Duarte R F, Prom-u-thai C, Amaral D C, Faquin V, Guilherme L R G, Reis A R, Alves E. 2016. Determination of zinc in rice grains using DTZ staining and ImageJ software.,68: 53–58.
Feng X G, Yoshida K T. 2004. Molecular approaches for producing low-phytic-acid grains in rice.,21(3): 183–189.
Furtado A, Henry R J. 2005. The wheatpromoter drives reporter gene expression in embryo and aleurone tissue of transgenic barley and rice.,3(4): 421–434.
Galani S, Aman A, Qader S A U. 2011. Germination potential index of Sindh rice cultivars on biochemical basis, using amylase as an indicator.,10: 18334–18338.
Huang N, Angeles E R, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennet J, Khush G S. 1997. Pyramiding of bacterial blight resistance genes in rice: Marker-assisted selection using RFLP and PCR.,95(3): 313–320.
Karmakar S, Molla K A, Chanda P K, Sarkar S N, Datta S K, Datta K. 2016. Green tissue-specific co-expression of chitinase and oxalate oxidase 4 genes in rice for enhanced resistance against sheath blight.,243(1): 115–130.
Kaur H, Verma P, Petla B P, Rao V, Saxena S C, Majee M. 2013. Ectopic expression of the ABA-inducible dehydration-responsive chickpea-myo-inositol 1-phosphate synthase 2 (CaMIPS2) inenhances tolerance to salinity and dehydration stress.,237(1): 321–335.
Kim S, Tai T. 2011. Identification of genes necessary for wild-type levels of seed phytic acid inusing a reverse genetics approach., 286(2): 119–133.
Kuwano M, Ohyama A, Tanaka Y, Mimura T, Takaiwa F, Yoshida K T. 2006. Molecular breeding for transgenic rice with low- phytic-acid phenotype through manipulating-inositol 3-phosphate synthase gene.,18(3): 263–272.
Kuwano M, Mimura T, Takaiwa F, Yoshida K T. 2009. Generation of stable ‘low phytic acid’ transgenic rice through antisense repression of the 1D--inositol 3-phosphate synthase gene () using the 18-kDa oleosin promoter.,7(1): 96–105.
Larson S R, Rutger J N, Young K A, Raboy V. 2000. Isolation and genetic mapping of a non-lethal rice (L.) low phytic acid 1 mutation.,40: 1397–1405.
Liu J C, Ockenden I, Truax M, Lott J N A. 2004. Phytic acid-phosphorus and other nutritionally important mineral nutrient elements in grains of wild-type and low phytic acid (lpa1-1) rice.,14(2): 109–116.
Liu Q L, Xu X H, Ren X L, Fu H W, Wu D X, Shu Q Y. 2007. Generation and characterization of low phytic acid germplasm in rice (L.).,114(5): 803–814.
Livak K J, Schmitteng T D. 2001. Analysis of relative gene expression data using a real-time quantitative PCR and2-ΔΔCTmethod., 25(4): 402–408.
Loewus F A, Murthy P P N. 2000.-Inositol metabolism in plants.,150: 1–19.
Lott J N A, Greenwood J S, Batten G D. 1995. Mechanisms and regulation of mineral nutrient storage during seed development.: Kigel J, Galili G. Seed Development and Germination. New York: Marcel Dekker: 215–235.
Lucca P, Hurrell R, Potrykus I. 2001. Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains.,102: 392–397.
Miller G L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar.,31: 426–428.
Molla K A, Karmakar S, Chanda P K, Ghosh S, Sarkar S N, Datta S K, Datta K. 2013. Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen) in transgenic rice.,14(9): 910–922.
Pilu R, Panzeri D, Gavazzi G, Rasmussen S K, Consonni G, Nielsen E. 2003. Phenotypic, genetic and molecular characterization of a maize low phytic acid mutant ().,107(6): 980–987.
Raboy V, Gerbasi P F, Young K A, Stoneberg S D, Pickett S G, Bauman A T, Murthy P P N, Sheridan W F, Ertl D S. 2000. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1.,124(1): 355–368.
Raboy V. 2003.-Inositol-1,2,3,4,5,6-hexakisphosphate.,64(6): 1033–1043.
Raboy V. 2009. Approaches and challenges to engineering seed phytate and total phosphorus.,177(4): 281–296.
Raj Bhandari M, Kawabata J. 2006. Cooking effects on oxalate, phytate, trypsin and alpha amylase inhibitors of wild type yam tubers of Nepal.,19: 524–530.
Sakai H, Iwai T, Matsubara C, Usui Y, Okamura M, Yatou O, Tarada Y, Aoki N, Nishida S, Yoshida K T. 2015. A decrease in phytic acid content substantially affects the distribution of mineral elements within rice seeds., 238: 170–177.
Shi J R, Wang H Y, Hazebroek J, Ertl D S, Harp T. 2005. The maizeencodes a-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds.,42(5): 708–719.
Shi J R, Wang H Y, Schellin K, Li B L, Faller M, Stoop J M, Meeley R B, Ertl D S, Ranch J P, Glassman K. 2007. Embryo- specific silencing of a transporter reduces phytic acid content of maize and soybean seeds.,25: 930–937.
Sivaprakash K R, Krishnan S, Datta S K, Parida A K. 2006. Tissue-specific histochemical localization of iron and ferritin gene expression in transgenic indica rice Pusa Basmati (L.).,85(2): 157–160.
Sparvoli F, Cominelli E. 2015. Seed biofortification and phytic acid reduction: A conflict of interest for the plant?,4(4): 728–755.
Stein N, Herren G, Keller B. 2001. A new DNA extraction method for high-throughput marker analysis in a large-genome species such as.,120(4): 354–356.
Suzuki M, Tanaka K, Kuwano M, Yoshida K T. 2007. Expression pattern of inositol phosphate-related enzymes in rice (L.): Implications for the phytic acid biosynthetic pathway.,405: 55–64.
Woo L, Maher W. 1995. Determination of phosphorus in turbid waters using alkaline potassium peroxodisulphate digestion.,315(1): 123–135.
11 March 2019;
18 June 2019
Swapan K. Datta(swpndatta@yahoo.com)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.007
(Managing Editor: Wang Caihong)
杂志排行
Rice Science的其它文章
- Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
- Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases
- Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
- RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
