Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
2020-07-06XiaoNingWuYunyuLiAihong
Xiao Ning, Wu Yunyu, Li Aihong, 3
Review
Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
Xiao Ning1, 2, 3, 4, Wu Yunyu1, 2, Li Aihong1, 2, 3
(, Yangzhou 225007, China; Jiangsu Collaborative Innovation Center for Modern Crop Production, Nanjing 210095, China; Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding, Yangzhou University, Yangzhou 225009, China; State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China)
Rice blast is one of the most destructive diseases affecting rice production worldwide. The development and rational use of resistant varieties has been the most effective and economical measure to control blast. In this review, we summarized the cloning and utilization of rice blast resistance genes, such as,,,,and. We concluded that three main problems in the current breeding of rice blast resistance are:availability of few(resistance) genes that confer resistance to both seedling and panicle blast, the resistance effect of pyramided lines is not the result of a simple accumulation of resistance spectrum, and only a fewgenes have been successfully used for molecular breeding. Therefore, novel utilization strategies for rice blastgenes in molecular breeding were proposed, such as accurately understanding the utilization ofgenes in main modern rice varieties, creating a core resistant germplasm with excellent comprehensive traits, screening and utilizing broad-spectrum and durable resistance gene combinations. Lastly, the trends and possible development direction of blast resistance improvement were also discussed, including new genes regulating resistance identified via GWAS (genome-wide association study) and improving rice blast resistance using genetic editing.
rice blast; resistance gene; molecular breeding strategy
Rice blast, caused byB.C. Couch, is one of the most destructive diseases encountered in rice production. Once rice is attacked by, pattern recognition receptors (PRRs) on the cell surface can specifically recognize pathogen-associated molecule patterns (PAMPs), and activate defense response by cell wall modification, callose deposition, and via expression of defense-related proteins in host cells, which is termed as PAMP-triggered immunity (PTI) (Jones and Dangl, 2006). However, PTI is a weak and non-specific resistance mechanism (Bernoux et al, 2011; Segonzac and Zipfel, 2011). In many cases,can secrete certain effectors to inhibit PAMP-induced PTI and can break resistance responses (Jones and Dangl, 2006; Birch et al, 2009; Block and Alfano, 2011; Mentlak et al, 2012). At the same time, rice has acquired more specific resistance proteins that directly or indirectly recognize pathogen effector proteins. This recognition mechanism activates a second layer of the defense response in rice, known as effector-triggered immunity (ETI), which results in the production of ion (Ca2+, K+and H+) currents, superoxide, nitric oxide, and programmed cell death at the site of invasion (Dangl et al, 1996; Nurnberger et al, 2004). ETI is a highly specialized disease resistance mechanism in the host (Boller and He, 2009), which is activated in the gene-for-gene model upon recognition by an R (resistance) protein of the corresponding effector protein of. Effector proteins are often encoded by avirulence genes in.
Thegenes in rice correspond to the avirulence () genes inin a gene-for-gene manner (Flor, 1956), which ensures that the interaction between a specific R protein in rice and the corresponding AVR effector in the pathogen render resistant. To date, more than 40genes have been identified in, while12 of them have been cloned. The cloned genes are(Kang et al, 1995),(Sweigard et al, 1995),(Jia et al, 2000),(Böhnert et al, 2004),(Li et al, 2009),(Miki et al, 2009),(Yoshida et al, 2009),(Cesari et al, 2013),(Fujisaki et al, 2015),(Wu J et al, 2015),(Zhang et al, 2015) and(Ray et al, 2016). The R protein encoded bygenes interacts directly or indirectly with the effector protein, thus sensing pathogen invasion and inducing disease resistance. Among the clonedandgene pairs,/(Jia et al, 2000; Orbach et al, 2000),/(Yoshida et al, 2009; Kanzaki et al, 2012),/(Miki et al, 2009; Ortiz et al, 2017),/(Cesari et al, 2013) and/(Ray et al, 2016) can interact directly with each other. While/(Fujisaki et al, 2015; Singh et al, 2016) and/(Park et al, 2012, 2016; Wang et al, 2016; Tang et al, 2017) require other proteins to complete the interaction. Despite the deployment of resistant varieties, blast epidemics can still occur, due to a lapse in host resistance and the emergence of new virulent pathotypes (Chuma et al, 2011). Several genetic events, including point mutations, insertion of transposable elements, deletion of partial or entire genes, etc, lead the function loss ofgenesin(Li W T et al, 2019). Thus, the effectiveness ofgenes highly dependents on the respectivegene. Deployment of blast resistantvarieties requires regular monitoring of race dynamics, and current and future frequency ofgenes across different regions (Selisana et al, 2017).
Currently, the uses of chemicals and resistant varieties are the main ways of rice blast management. In addition to increased costs, chemical control also causes serious environmental pollution and poses food safety risks. The use ofgenes to breed resistantvarieties remains the top-most economical and effective method to control rice blast (Wu Y Y et al, 2015). This review provided a summary of the identification, cloning and utilization of rice blast resistance genes, key problems in molecular breeding of rice to blast resistance and molecular breeding strategies based on clonedgenes. In addition, the trends and possible future development direction of blast resistance improvementwere also discussed.
Identification and cloning of rice blast resistance gene
genes are the foundation for disease resistance research andresistance breeding. The genetic analysis, gene mapping and cloning of rice blast resistance have been intensively studied. Since the first report of independently inherited threegenes,andduring 1960s (Yamasaki and Kiyosawa, 1966), more than 100 resistance genes or loci have been identified to date (LiW T et al, 2019; Li et al, 2020).genes are distributed on 11 chromosomes of rice genome, except chromosome 3, and more than 64% are clustered in chromosomes 6, 11 and 12, representing 18%, 25% and 21%, respectively (Ashkani et al, 2016).
Since the cloning of firstgene,, in 1999 (Wang et al, 1999), 31genes have been successfully cloned (Table 1). Except for, which is a recessivegene, the remaining 30genes are dominant. Among them,all genes except,,,and-show complete resistance.encodes a B-lectin kinase domain protein (Chen et al, 2006), whileencodes a proline-rich protein with a heavy metal domain (Fukuoka et al, 2009), andencodes an atypical protein with an armadillo repeat (Zhao et al, 2018).Remaining 28genes encode nucleotide-binding site leucine-rich repeat (NBS-LRR) domain proteins.,,,,,,andcontain two NBS-LRR protein structural genes for blast resistance (Lee et al, 2009; Okuyama et al, 2011; Hua et al, 2012; Cesari et al, 2013).,,andgenes are induced by pathogen infection, while the remaining genes express constitutively. Majority of the clonedgenes induce resistance against leaf blast at the seedling stage, while only a fewgenes, such as,and, confer resistance to panicle blast (Hayashi et al, 2010; Chen et al, 2011; Ma et al, 2015; Cao et al, 2019). Involvement of such a high numbers and types ofgenes in rice blast resistance breeding applications indicates a complex genetics of this disease interaction.
Key problems in rice blast resistance breeding
Higher variability inpopulation and a frequent emergence of new virulent races causea high selection pressure, resulting the resistant varieties often ‘losing’ resistance within 3–5 years of cultivation and becoming susceptible. Therefore, integration of broad-spectrum and durable resistance has become a key issue among rice breeders (Wu et al, 2007). However, because of a higher number ofgenes in rice, their deployment and utilization become an important challenge in blast resistance breeding to achieve broad-spectrum and durable resistance. Generally, two main strategies are deployed, the use of broad-spectrum resistance genes and gene pyramiding. However, some problems with these strategies remain to be solved.

Table 1. List of cloned blast resistance genes.
NBS-LRR, Nucleotide-binding site leucine-rich repeat; CC-NBS-LRR, Coiled-coil-nucleotide-binding site leucine-rich repeat.#genes showing partial resistance.
FewR genes are effective to both seedling and panicle blast
Among the identified and cloned resistance genes,,,,,,,,,andare broad-spectrum resistance genes to leaf blast.in the LAC23 cultivar from West Africa shows resistance to 98% of 792isolates in China (Chen et al, 2001).shows resistance against 6 races from the Philippines, and 26 of 29 isolates from Korea (Jeon et al, 2003)., which interacts withgene, is located on the short arm of chromosome 8, and shows resistance to over 2000 isolates originating from 55 countries (Berruyer et al, 2003).identified in a highly resistant variety Tetep is also confirmed to have broad-spectrum resistance against predominant races foundin India (Thakur et al, 2015). Sixgenes,,,,,and,harbor alleles of thelocus located on the short arm near the centromere of rice chromosome 6 (Qu et al, 2006; Zhou et al, 2006; Jeung et al, 2007; Deng et al, 2017). Thegene cluster from the US cultivar Zenith shows resistant to five US races (IH-1, IG-1, IC-17, IE-1 and IE1k) (RoyChowdhury et al, 2012).confers a high level of resistance to 43 isolates collected from 13 countries (Qu et al, 2006).was cloned from a gene cluster composed of nine gene members (namedto) encoding proteins with an NBS-LRR structure (Zhou et al, 2006).Lines carryingconfer resistance to 455 isolates collected from different regions of the Philippines and most of the 792 isolates from the 13 major rice regions of China (Chen et al, 1996).strongly resembles toin sequence and structure having only eight amino-acid differences within the three leucine-rich repeats (Qu et al, 2006; Zhou et al, 2006).fromshows broad-spectrum resistance to rice blast races from South Korea (Suh et al, 2009)., a resistance gene from Gumei 4, a local variety in China, is resistant to 50 isolates from all over the world (Deng et al, 2017; Zeng et al, 2018)., cloned from a Chinese local variety Digu, is an atypical resistance gene that encodes a C2H2transcription factor protein, which exhibits similar phenotypic incomplete resistance to several races of rice blast (Li et al, 2017). However, it should be noted that the broad-spectrum resistance of the above-mentionedgenes was mostly evaluated in different genetic backgrounds, thus the resistant phenotypes might have been masked by othergenes.
Amongvarious disease symptomscaused by, leaf blast and panicle blast are the most common. However, resistance to leaf and panicle blast is often inconsistent, and many varieties with high resistance to leaf blast at the seedling stage show susceptibility to panicle blast at the heading stage (Puri et al, 2009; Ishihara et al, 2014). Transcriptomic analysis showed that distinct defense-related gene expression is induced by leaf blast and panicle blast, suggesting that the genetic mechanisms of leaf blast and panicle blast resistance might differ and are independently controlled by differentgenes (Liu et al, 2016). Disease evaluation via artificial inoculation at the seedling stage is a high throughput method with clear resistance/susceptibility phenotyping, which ultimately leads to most research focusing on leaf blastscreening. However, less research has been carried out on the genetics of panicle blast resistance because of the increased fieldwork, complex phenotype evaluation system and high influence of environmental conditions for artificial inoculation testing. Presently, the evaluation of panicle blast resistance is mainly performed in disease nurseries under natural conditions. The above-mentioned broad-spectrumgenes such as,,,,,,,,,andhave not been evaluated using different isolates ofby artificial inoculation at the heading stage. Therefore, it is not clear whether they exhibit broad-spectrum resistance to panicle blast. Recently, Wu et al (2016, 2017) constructed a set of near-isogenic lines (NILs) for six resistance alleles of thelocus (,,,,and) in the genetic background of therice Yangdao 6 andrice 07GY31. Using an improved method of artificial inoculation, the panicle blast resistance evaluation of these NILs was carried out with representative isolates ofcollected from different ecological regions of China. Onlyshow stable broad-spectrum resistance to leaf blast and panicle blast in the genetic background ofandrice (Wu et al, 2016, 2017). However,,,,andonly show specific resistance to the blast population in some ecological areas, and the resistance frequency of panicle blast is significantly lower than that of leaf blast (Fig. 1) (Wu et al, 2016, 2017). Therefore, there are few of broad-spectrumgenes that can effectively protect against both seedling and panicle blast.
Resistance gene pyramiding effects is not simple accumulation of resistance spectrum of certain target R genes
Gene pyramiding is generally considered an effective way to develop varieties with broad-spectrum and durable resistance. The rice variety Jefferson, with the gene combination, has remained resistant since its first application in 1997 (McClung et al, 1997; Fjellstrom et al, 2004). Chen et al (2001) showed that resistance frequencies of monogenic lines withandare 92.45% and 89.65%, respectively, and those of polygene pyramiding lines (PPLs) withandare as high as 98.04% against 715 isolates of. The additive effect of the monogenic lines broadens the resistance spectrum of the PPLs, resulting in an increase in the blast resistance. Similarly, pyramiding ofandenhances resistance compared toandmonogenic lines (Xiao et al, 2016). Yu et al (2013) confirmed pyramiding of two genes with different overlapping resistance spectra can improve the resistance of plants. However, the resistance effect of pyramiding lines does not comprise the simple accumulation of the resistance spectrum of targetgenes. There is a significant interaction among the pyramidedgenes, causing both positive and negative deviation (Tabien et al, 2000). Therefore, a random combination of two or moregenes can result a lower resistance effect of PPLs than the monogenic lines. International Rice Research Institute constructed a set of NILs carrying,and, as well as PPLs carrying 2–3genes in the background of CO39. The results showed that the resistance of PPLis lower than that of the lines harboring only(Hittalmani et al, 2000). Similarly, He et al (2001) found that the resistance of PPLis lower than that of the monogenic lines. In the background of07GY31, the resistance level of PPLis significantly higher than that of monogenic lines harboring a singlegene. However, the resistance level of PPLis significantly lower than that of monogenic lines harboring(Xiao et al, 2017). In thebackground of Yangdao 6, the resistance frequency of monogenic lines withis slightly higher (70.8%) than that of the combinationwithPPL(69.1%) (Xiao et al, 2018). Recently, Wu et al (2019) evaluated the resistance effects of different alleles oflocus (,,,and) combined with other broad-spectrumgenes (such as,and) systematically. They found that different gene combinations produce different interaction effects, in which most PPLs show no resistance comprising the simple accumulation of the resistance spectra of the targetgenes. Among them,,andare the most effective gene combination patterns, displaying stable broad-spectrum resistance under various conditions. Therefore, the combination ofgenes directly affects the resistance level of the PPLs. Thus, to achieve broad-spectrum resistance to both leaf blast and panicle blast, resistance gene combination patterns must be assessed first in gene pyramiding breeding.

Fig. 1. Comparison of leaf blast and panicle blast resistance among different alleles oflocus.
A, Resistance reaction to leaf blast and panicle blast of different alleles from thelocusrice 07GY31 against theisolate JSBY4-3. B, Resistance reaction to leaf blast and panicle blast of different alleles from thelocus in the background ofrice Yangdao 6 (YD6) against the isolate of(AH3-1). C, Resistance frequency (RF) to leaf blast and panicle blast of different alleles of thelocus in the background ofrice 07GY31. D, RF to leaf blast and panicle blast of different alleles of thelocus in the background ofrice Yangdao 6. SB, Seedling blast; PB, Panicle blast. Data are Mean ± SD (= 3). Blast resistant phenotypes were reproduced from Wu et al (2016, 2017).
Only a few R genes have been successfully used in molecular breeding practice
The development of molecular biology has brought rice breeding to the stage of combining biotechnology with conventional technology. In the last decade, molecular breeding technology, represented by molecular marker-assisted selection, has played an important role in the improvement of rice blast resistance in elite recipients. Many studies have been reported in this area, and some successful and representative examples are listed in Table 2. The donorgenes used in these molecular breeding studies are mainly alleles or tightly linkedgenes from three loci,,and, including,,,andof thelocus;andin thelocus, andfromlocus. Since there are so many identifiedgenes, why is molecular breeding practice only limited to fewgenes? The reasons might be as follows: Firstly, the alleles or tightly linkedgenes from these loci often exhibit relatively broad-spectrum resistance, especially in leaf blast resistance at the seedling stage. However, manygenes in other loci have a narrower resistance spectrum or less resistance effect, resulting in noobvious effect using othergenes in resistant improvement. Secondly, the cumbersome chain of linkage drag of somegenes produces negative effects on agronomic traits, which limits their utilization in breeding practice.
Utilization strategies of rice blast resistance genes in molecular breeding
Understanding utilization of rice blast R genes in main modern varieties
An important prerequisite for molecular breeding improvement of rice blast resistance is to understand whichgenes have been utilized in modern varieties and whether the resistance performance of thesegenes is effective. Currently, the analysis of resistance genotypes in modern improved varieties uses linked markers or functional markers for clonedgenes.For example, Xiang et al (2018) analyzed distribution and use of rice blast resistant genes in the main cultivated rice varieties from Heilongjiang Province, China. The distribution frequencies (DFs) of,andare higher than those of other genes, reaching 31.37%, 29.41% and 18.62%, followed by,andwith DFs of 9.80%, 1.96% and 1.96%, respectively. However, nois detected.,,,andare detected in the core rice germplasms in Ningxia Province, China (Li Y D et al, 2019). Ma et al (2018) identified relatively high DFs ofandin local varieties in Guizhou Province, China, at 32.35% and 30.86%, respectively. While the DFs ofandare relatively lower, at 2.56% and 2.47%, respectively. Wu Y Y et al (2015) analyzed the distribution of clonedgenes in 277 mainandparental lines and showed that,,,,,,,andhave relatively higher DFs (>15%),whereas,,,andhave relatively lower DFs (< 10%), and,,andhave DFs of less than 2% and are found in local varieties, related wild species, or improved intermediate materials. Further analysis showed that somegenes are specifically distributed in the genomes of rice sub-species, for example,,,andare mainly distributed in-type accessions, and,,,andare mainly harbored in-type accessions, while,,andare evenly distributed in both accessions. The above results provide us with a general understanding of thegenes used in the main rice parental lines in China. However, it should be noted that the judgment of the existence of the targetgenes in the above studies are based on closely linked molecular markers or functional markers. Therefore, detection using molecular markers has a certain degree of correlation withgenes, but is more of an inference than a certainty, and cannot accurately reflect the presence of the targetgene. With the advances in third generation genome sequencing technology and the reduction of sequencing costs, it has become easier to quickly obtain high quality whole genome sequences.
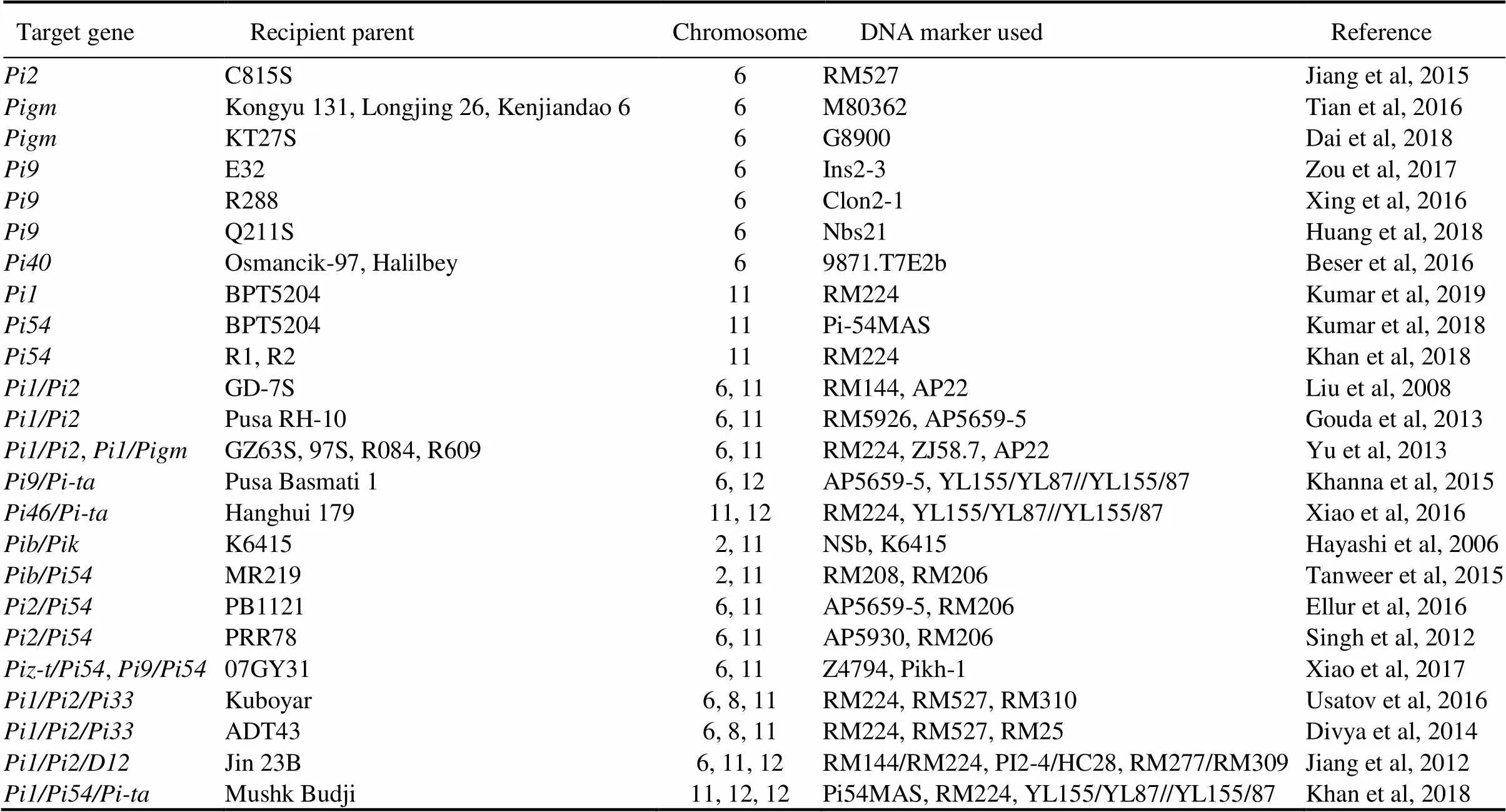
Table 2. Successful examples of application of broad-spectrum resistance genes in rice breeding practice.
Creation of core resistant germplasm with excellent comprehensive traits
The existence of a defined resistant germplasm with antarget gene is an important prerequisite for breeding applications. Apart from the above-mentioned clonedgenes and somegenes used in modern improved varieties, many broad-spectrum genes, including,,,,and, have not been utilized in modern varieties. Thesegenes are mainly distributed in local varieties or germplasms and might exhibit drag, causing poor agronomic traits and low yield. To promote the utilization of these genes in molecular breeding, it is necessary to overcome the linkage drag and create core resistant germplasms with excellent and comprehensive agronomic traits. Some explorations in this field have been made. For example,is a broad-spectrumgene against both leaf and panicle blast(Wu et al, 2016, 2017). However,also has the effect of reducing grain weight but increasing grain number (Deng et al, 2017). Although it can achieve yield balance, decrease in grain weight will lead to a reduction in rice yield and its marketability. Grain weight is a quantitative trait controlled by multiple genes, and therefore, it is affected by many other genes besides. In theory, individuals with no significant decrease in grain weight can be obtained by large-scale selection. Therefore, Wu et al (2016, 2017) used the elite restorer line Yangdao 6 (9311) as the recurrent parent, crossed it with thedonor Gumei 4, and backcrossed the progeny continuously. A series of NILs have been obtained through foreground selection of target genes and large-scale screening of agronomic traits. The results of agronomic trait investigation showed that although the grain weight of some NILs is decreased, there are also some lines with similar grain weight and other elite agronomic traits to the recurrent parents (Fig. 2-A and B). Finally, core resistance germplasm carryingwas selected and named as R9311. Using R9311 as the restorer line, two-line hybrid rice Yangliangyou 309 is bred. Moreover, using R9311 as thegene donor parent, two-line cytoplasmic male sterility (CMS) line Yangxian 6S and three-line CMS line Yangxian 9A are bred, followed two-line hybrid rice Yangliangyou 612 and three-line hybrid rice Yangxianyou 919 (Fig. 2-C). These hybrid rice combinations performed well in regional trials and production tests (Fig. 2-D), and havealso been approved by national certification.This serves as a successful example of the creation of core germplasm and molecular breeding for rice blast resistance.
Screening and utilization of broad-spectrum and durable resistance gene combinations
Gene pyramiding helps to develop varieties with broad-spectrum and durable resistance to. Many studies have shown that resistance is significantly associated with the number ofgenes, which means that the greater the number ofgenes found in the accessions, the higher their resistance against(Wu Y Y et al, 2015; Li et al, 2019). However, the number ofgenes is not the only factor affecting resistance. On one hand, as the number ofgenes increases, the improvement in the resistance level would gradually slowdown, which term as law of ‘diminishing returns’ between the number of pyramidedgenes and resistance (Xiao et al, 2018; Wu et al, 2019). On the other hand, as the number of pyramidedgenes increases, in addition to increasing the workload, the linkage drag with unacceptable traits might also increase. Therefore, how to employ a few of thesegenes to achieve broad-spectrum and durable resistance must be considered during rice blast resistance breeding (Yang et al, 2008). From the perspective of pyramidedgenes and their corresponding resistance, the combination pattern ofgenes is a key factor in determining the resistance level of different varieties against(Wu Y Y et al, 2015). Once differentgenes are pyramided, some gene combinations will show positive interactions, while other gene combinations might exhibit negative interactions (Hittalmani et al, 2000; Xiao et al, 2017; Wu et al, 2019). Therefore, it is essential to screen anddetermine the combination pattern ofgenes that exhibit broad-spectrum and durable resistance to promote the practical use of molecular breeding for blast resistance.
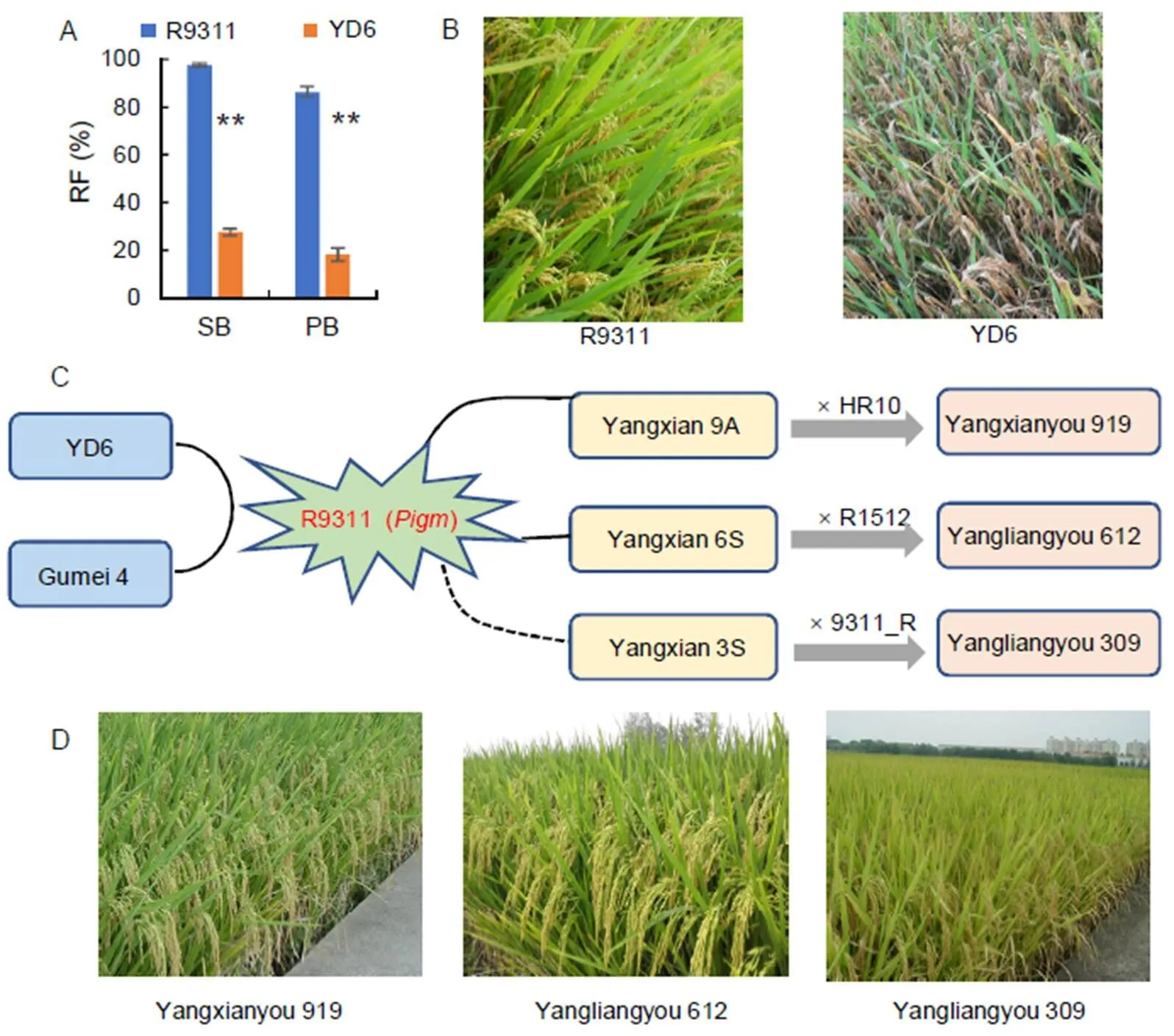
Fig. 2. Creation of core resistant germplasm R9311 and its breeding application.
A, Resistance comparison of leaf blast and panicle blast between R9311 and Yangdao 6 (YD6). Data are Mean ± SD (= 3). **,< 0.001. SB, Seedling blast; PB, Panicle blast; RF, Resistance frequency. B, Panicle blast resistance reaction of R9311 and YD6 in the natural blast nursery. C, Schematic diagram of the construction of the sterile lines and hybrids based on the core germplasm of R9311. D, Field performances of new breed hybrid rice.
Several successful molecular breeding studies (Table 2) have indicated that a singlegene, such as,,orfrom thelocus shows broad-spectrum resistance to leaf and specifically exhibits resistance to panicle blast in some ecological regions. However, onlyshows broad-spectrum resistance to both leaf and panicle blast, and has an important practical value in breeding. Combiningwithfrom thelocus is the most successful example of the application of gene pyramiding, and there have also been reports about pyramiding withand. Wu et al (2019) reported thatexhibits high resistance to leaf and panicle blast after pyramiding with,and, respectively. Moreover, they also found that thegenes of the alleles from thelocus exhibit excellent resistance after combining them with certain independently distributedgenes, such as(unpublished data) and(Xiao et al, 2018). Based on the distribution and utilization of cloned genes, we proposed that the broad-spectrumgenes of thelocus, especially, should be used as the backbone and core for gradual pyramiding of othergenes at three levels (Fig.3). Firstly, pyramiding withfrom thelocus,from thelocus, and the independently distributedgenesand. Thesegenes have higher DFs, less linkage drag of poor agronomic traits, and are relatively easy to integrate. Secondly, pyramiding withfrom thelocus,from thelocus, and the independently distributedgenesand. Thesegenes are generally distributed in local varieties or original germplasms and can be used in pyramiding breeding by creating core germplasms carrying targetgenes and showing elite agronomic traits. Thirdly, on the basis of further clarifying the resistance effect of gene combinations, pyramiding could be performed using certain partial resistance genes, such as,,and.
Conclusions and future perspectives
The development of genome sequencing technology has promoted the rapid identification and cloning of rice blastgenes andgenes, which has deepened understanding of the molecular mechanisms of riceblast fungus interaction and their co-evolution. It provides not only new genetic resources for rice blast resistance improvement, but also new technical ideas to regulate disease-resistance signaling pathways via genetic editing to achieve resistance improvement.
High-throughput whole genome sequence or targetgene sequencing in the elite rice cultivars or core resistant germplasm will provide useful technological means for breeding selection. Sincethe cloning of,and(Deng et al, 2017; Yang et al, 2019), functional single nucleotide polymorphism related to resistant genotypes can be used to design Kompetitive Allele-Specific PCR (KASP) markers and used in marker-assisted selection. Wang et al (2019) sequenced an important broad-spectrum blast resistant germplasm (Tetep) that is the donor of, and obtained a high-quality assembly, in which 455 nucleotide-binding site leucine-rich repeat (NLR) genes are annotated. Based on this, a few molecular markers have beendesigned to rapidly introduce clustered and paired NLRs in the Tetep genome to breed new resistant cultivars. With more donors ofgenes being sequenced, thegenes confering a resistant genotype on elite rice cultivars or core resistant germplasms could be identified.
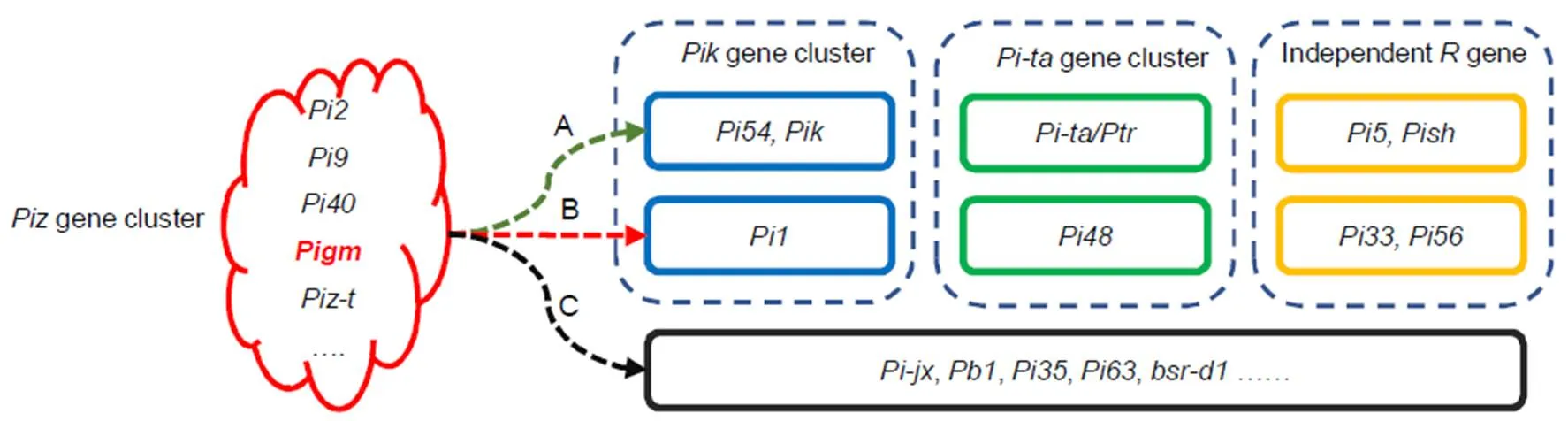
Fig. 3. Molecular breeding strategies using rice blast resistance genes.
A, Breeding strategies for improving blast resistance in the previous studies. B, Breeding strategies that have been used. C, Novel breeding strategies that can be utilized in further research.
With the increase in genetic research into blast resistance, understanding of the partial resistance of non-race specificity, controlled by multiple quantitative trait loci (QTLs), has became a hot topic. Partial resistance is generally regarded as a quantitative trait. It does not prevent infection bybut can reduce the proliferation of pathogens in the host and maintain a relatively low selection pressure on the population of, thus maintaining broad-spectrum and durable resistance (Niks et al, 2015). Since Wang et al (1994) first used restriction fragment length polymorphism markers to identify 19 QTLs controlling partial resistance in the durable resistance African upland rice cultivar Moroberekan, at least 500 QTLs for resistance to rice blast have been identified on the 12 chromosomes of rice to date (Li et al, 2019). In recent years, with the rapid development of molecular biology, a large number of partial resistance genes such as(Fukuoka et al, 2009),(Hayashi et al, 2010),(Fukuoka et al, 2014),(Xu et al, 2014),(Li et al, 2017),(Zhou et al, 2018) and(Inukai et al, 2019) have been cloned, suggesting that the rapid introduction of thesegenes into rice varieties through molecular breeding is feasible (Pilet-Nayel et al, 2017). Fukuoka et al (2015) first reported that the four partial resistance genes,,,and, can be combined to improve durable resistance of rice. However, because of the small resistant effect of a single partialgene, it must be combined with multiple partial resistance genes to obtain effective resistance. With the establishment of high-throughput molecular breeding methods, the creation of core germplasmsharboring target partial resistance without linkage drag will become an important step in rice blast resistance improvement in the future.
In recent years, a series of important advances have been made in understanding the molecular mechanism of rice blast resistance. Significant progress has been achieved in cloning and identifying a number of key PTI and ETI signal regulation genes (Liu et al, 2014; Nasir et al, 2018), downstream signaling pathway related genes (Choi et al, 2015), and R proteins, especially downstream signaling molecules directly regulated by NBS-LRR proteins. However, compared with those 30genes that have been cloned, there has been less research on the downstream signaling molecules ofgenes. Currently, three kinds of downstream signaling molecules that interact directly withgenes have been identified:signaling pathways downstream of,and(Chen et al, 2010; Kawano et al, 2010; Wang et al, 2018; Zhou et al, 2019); the ARM repeats ofand(t) downstream ofand, respectively (Jia and Martin, 2008; Wang et al, 2015; Zhao et al, 2018); and transcription factor signaling pathways ofand, which act downstream ofand, respectively (Inoue et al, 2013; Liu et al, 2017).encoding a guanylate triphosphatase (GTPase), a member of the RhoGTPase family, is a key regulator of rice resistance to pathogens and it can participate in both PTI (Akamatsu et al, 2013) and ETI (Kawano et al, 2010). Zhou et al (2019) suggested thatmight be a common regulatory factor downstream of rice NBS-LRR proteins. Therefore, strengthening research into the interaction betweenand othergenes, especially the study of different broad-spectrum alleles from theandloci, should be promoted. At the same time, further identification of new signaling genes and analysis of the molecular regulatory mechanism of broad-spectrum resistance againstare needed., an APETELA2/ethylene response factor (AP2/ERF) type transcription factor in rice, is rapidly and strongly induced by avirulent pathovars of. When the expression ofwas inhibited in RNAi transgenic lines, their blast resistance was enhanced (Liu et al, 2012). Similar research showed that mutant lines ofedited by CRISPR/Cas9show significantly higher resistance tocompared with the wildtype (Xu et al, 2019). The expression levels of defense-related genes involved in signaling pathways of salicylic acid, jasmonic acid and ethylene metabolisms are upregulated in the mutant lines after inoculation of the physiological races of. Otherwise, some QTLs identified as necessary loci are required for resistance to rice panicle blast. Inoue et al (2017) mapped four QTLs that contribute to-mediated panicle blast resistance. In addition, a genome-wide association study of blast resistant loci or genes was used to identify novelgenes, and newalleles,and, respectively (Wang et al, 2014; Li C G et al,2019). These identified genes not only provide new genetic resources for breeding broad-spectrum and durable rice cultivars, but also provide new strategies to improve resistance to rice blast.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFD0100400), the Key Studying and Developing Project of Jiangsu Province for Modern Agriculture (Grant No. BE2018351), the Major Project of Jiangsu Province for Significant New Varieties Development (Grant No. PZCZ201702), the Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding (Grant No. BM2018003), the National Natural Science Foundation of China (Grant No. 31971868), the National Modern Agricultural Industry Technology System Special Fund (Grant No. CARS-01-60), the ‘333’ Project of Jiangsu Province (Grant No. BRA2017163), the Key Studying and Developing Project of Yangzhou City for Modern Agriculture (Grant No. YZ2018048), and the Jiangsu Agricultural Science and Technology Innovation Fund [(Grant Nos. CX(18)1003) and CX(18)2022)], Open Research Fund of State Key Laboratory for Biology of Plant Diseases and Insect Pests (Grant No. SKLOF 201909), Opening Foundation of Key Laboratory of Plant Functional Genomics of the Ministry of Education (Grant No. ML201806), Fund of Institute of Agricultural Sciences for Lixiahe Region in Jiangsu (Grant No. SJ17201).
Akamatsu A, Wong H L, Fujiwara M, Okuda J, Nishide K, Uno K, Imai K, Umemura K, Kawasaki T, Kawano Y, Shimamoto K. 2013. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity., 13(4): 465–476.
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J Z, Matsumoto T, Ono K, Yano M. 2008. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer-specific rice blast resistance., 180(4): 2267–2276.
Ashkani S, Rafii M Y, Shabanimofrad M, Ghasemzadeh A, Ravanfar S A, Latif M A. 2016. Molecular progress on the mapping and cloning of functional genes for blast disease in rice (L.): Current status and future considerations., 36(2): 353–367.
Bernoux M, Ellis J G, Dodds P N. 2011. New insights in plant immunity signaling activation., 14(5): 512–518.
Berruyer R, Adreit H, Milazzo J, Gaillard S, Berger A, Dioh W, Lebrun M H, Tharreau D. 2003. Identification and fine mapping of, the rice resistance gene corresponding to theavirulence gene., 107(6): 1139–1147.
Beser N, Del Valle M M, Kim S M, Vinarao R B, Sürek H, Jena K K. 2016. Marker-assisted introgression of a broad-spectrum resistance gene,improved blast resistance of two elite rice (L.) cultivars of Turkey., 7(33): 1–15.
Birch P R J, Armstrong M, Bos J, Boevink P, Gilroy E M, Taylor R M, Wawra S, Pritchard L, Conti L, Ewan R, Whisson S C, van West P, Sadanandom A, Kamoun S. 2009. Towards understanding the virulence functions of RXLR effectors of the oomycete plant pathogen., 60(4): 1133–1140.
Block A, Alfano J R. 2011. Plant targets fortype III effectors: Virulence targets or guarded decoys?, 14(1): 39–46.
Böhnert H U, Fudal I, Dioh W, Tharreau D, Notteghem J L, Lebrun M H. 2004. A putative polyketide synthase/peptide synthetase fromsignals pathogen attack to resistant rice., 16(9): 2499–2513.
Boller T, He S Y. 2009. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens., 324: 742–744.
Bryan GT, Wu KS, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK,Tarchini R, Valent B. 2000. A single amino acid difference distinguishes resistant andsusceptible alleles of the rice blast resistance gene.,12:2033–2046.
Cao N, Chen Y, Ji Z J, Zeng Y X, Yang C D, Liang Y. 2019. Recent progress in molecular mechanism of rice blast resistance., 33(6): 489–498. (in Chinese with English abstract)
Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, Rivas S, Alaux L, Kanzaki H, Okuyama Y, Morel J B, Fournier E, Tharreau D, Terauchi R, Kroj T. 2013. The rice resistance protein pair RGA4/RGA5 recognizes theeffectors AVR-Pia and AVR1-CO39 by direct binding., 25(4): 1463–1481.
Chen D H, Zeigler R S, Ahn S W, Nelson R J. 1996. Phenotypic characterization of the rice blast resistance gene(t)., 80(1): 52–56.
Chen H L, Chen B T, Zhang D P, Xie Y F, Zhang Q F. 2001. Pathotypes ofin rice fields of central and southern China., 85(8): 843–850.
Chen J, Shi Y F, Liu W Z, Chai R Y, Fu Y P, Zhuang J Y, Wu J L. 2011. Aallele from rice cultivar Gumei2 confers resistance to., 38(5): 209–216.
Chen J, Peng P, Tian J S, He Y G, Zhang L P, Liu Z X, Yin D D, Zhang Z H. 2015., a rice blast resistance allele consisting of two adjacentgenes, was identified as a novel allele at thelocus., 35:117.
Chen L, Shiotani K, Togashi T, Miki D, Aoyama M, Wong H L, Kawasaki T, Shimamoto K. 2010. Analysis of the Rac/Rop small GTPase family in rice: Expression, subcellular localization and role in disease resistance., 51(4): 585–595.
Chen X W, Shang J J, Chen D X, Lei C L, Zou Y, Zhai W X, Liu G Z, Xu J C, Ling Z Z, Cao G, Ma B T, Wang Y P, Zhao X F, Li S G, Zhu L H. 2006. A B-lectin receptor kinase gene conferring rice blast resistance, 46(5): 794–804.
Choi C, Hwang S H, Fang I R, Kwon S I, Park S R, Ahn I, Kim J B, Hwang D J. 2015. Molecular characterization ofWRKY6, which binds to W-box-like element 1 of thepromoter and confers reduced susceptibility to pathogens., 208(3): 846–859.
Chuma I, Isobe C, Hotta Y, Ibaragi K, Futamata N, Kusaba M, Yoshida K, Terauchi R, Fujita Y, Nakayashiki H, Valent B, Tosa Y. 2011. Multiple translocation of theeffector gene among chromosomes of the rice blast fungusand related species.,7(7):e1002147.
Dai X J, He C, Zhou L, Liang M Z, Fu X C, Qin P, Yang Y Z, Chen L B. 2018. Identification of a specific molecular marker for the rice blast-resistant geneand molecular breeding of thermo-sensitive genic male sterile leaf-color marker lines., 38:72.
Dangl J L, Dietrich R A, Richberg M H. 1996. Death don’t have no mercy: Cell feath programs in plant-microbe interactions., 8(10): 1793–1807.
Divya B, Robin S, Rabindran R, Senthil S, Raveendran M, Joel A J. 2014. Marker assisted backcross breeding approach to improve blast resistance in Indian rice () variety ADT43., 200(1): 61–77.
Deng Y W, Zhai K R, Xie Z, Yang D Y, Zhu X D, Liu J Z, Wang X, Qin P, Yang Y Z, Zhang G M, Li Q, Zhang J F, Wu S Q, Milazzo J, Mao B Z, Wang E T, Xie H A, Tharreau D, He Z H. 2017. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance., 355: 962–965.
Ellur R K, Khanna A, Yadav A, Pathania S, Rajashekara H, Singh V K, Krishnan G S, Bhowmick P K, Nagarajan M, Vinod K K, Prakash G, Mondal K K, Singh N K, Prabhu K V, Singh A K. 2016. Improvement of Basmati rice varieties for resistance to blast and bacterial blight diseases using marker assisted backcross breeding., 242: 330–341.
Fjellstrom R, Conaway-Bormans C A, McClung A M, Marchetti M A, Shank A R, Park W D. 2004. Development of DNA markers suitable for marker assisted selection of three genes conferring resistance to multiple pathotypes., 44(5): 1790–1798.
Flor H H. 1971. Current status of the gene-for-gene concept., 9:275–296.
Fujisaki K, Abe Y, Ito A, Saitoh H, Yoshida K, Kanzaki H, Kanzaki E, Utsushi H, Yamashita T, Kamoun S, Terauchi R. 2015. Rice Exo70 interacts with a fungal effector, AVR-Pii, and is required for AVR-Pii-triggered immunity., 83(5): 875–887.
Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, Hayashi N, Takahashi A, Hirochika H, Okuno K, Yano M. 2009. Loss of function of a proline-containing protein confers durable disease resistance in rice., 325: 998–1001.
Fukuoka S, Yamamoto S I, Mizobuchi R, Yamanouchi U, Ono K, Kitazawa N, Yasuda N, Fujita Y, Nguyen T T T, Koizumi S, Sugimoto K, Matsumoto T, Yano M. 2014. Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast., 4: 1–7.
Fukuoka S, Saka N, Mizukami Y, Koga H, Yamanouchi U, Yoshioka Y, Hayashi N, Ebana K, Mizobuchi R, Yano M. 2015. Gene pyramiding enhances durable blast disease resistance in rice., 5: 7773.
Gouda P K, Saikumar S, Varma C MK, Nagesh K, Thippeswamy S, Shenoy V, Ramesha M S, Shashidhar H E. 2013. Marker-assisted breeding ofandgenes imparting resistance to rice blast in PRR78, restorer line of Pusa RH-10 Basmati rice hybrid., 132(1): 61–69.
Hayashi K, Yoshida H. 2009. Refunctionalization of the ancient rice blast disease resistance geneby the recruitment of a retrotransposon as a promoter., 57(3): 413–425.
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takatsuji H. 2010. Durable panicle blast-resistance geneencodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication., 64(3): 498–510.
He Y Q, Tang W H, Hei L, Zeigler R S. 2001. Identification of CO39 near-isogenic lines for rice blast., 27(6):838–841. (in Chinese with English abstract)
Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N. 2000. Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice., 100(7): 1121–1128.
Hua L X, Wu J Z, Chen C X, Wu W H, He X Y, Lin F, Wang L, Ashikawa I, Matsumoto T, Wang L, Pan Q H. 2012. The isolation of, an allele at thelocus which confers broad spectrum resistance to rice blast., 125(5): 1047–1055.
Huang Y L, Yan Z, Wang H, Shen G L, Zhang C H. 2018. Directed improvement of rice blast resistance of sterile line Q211S with molecular marker-assisted selection., 34(24):135–140. (in Chinese with English abstract)
Inoue H, Hayashi N, Matsushita A, Liu X Q, Nakayama A, Sugano S, Jiang C J, Takatsuji H. 2013. Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction., 110(23): 9577–9582.
Inoue H, Nakamura M, Mizubayashi T, Takahashi A, Sugano S, Fukuoka S, Hayashi N. 2017.() resistance is dependent on at least four QTLs in the rice genome., 10:36.
Inukai T, Nagashima S, Kato M. 2019.is a race-specific partial-resistance allele at theblast resistance locus in rice., 132(2): 395–404.
Ishihara T, Hayano-Saito Y, Oide S, Ebana K, La N T, Hayashi K, Ashizawa T, Suzuki F, Koizumi S. 2014. Quantitative trait locus analysis of resistance to panicle blast in the rice cultivar Miyazakimochi., 7(1): 2.
Jeon J S, Chen D, Yi G H, Wang G L, Ronald P C. 2003. Genetic and physical mapping of(t), a locus associated with broad-spectrum resistance to rice blast., 269(2): 280–289.
Jeung J U, Kim B R, Cho Y C, Han S S, Moon H P, Lee Y T, Jena K K. 2007. A novel gene,(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice., 115(8): 1163–1177.
Jia Y L, McAdams S A, Bryan G T, Hershey H P, Valent B. 2000. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance., 19(15): 4004–4014.
Jia Y L, Martin R. 2008. Identification of a new locus,(t), required for rice blast resistance gene-mediated resistance., 21(4): 396–403.
Jiang H C, Feng Y T, Bao L, Li X, Gao G J, Zhang Q L, Xiao J H, Xu C G, He Y Q. 2012. Improving blast resistance of Jin 23B and its hybrid rice by marker-assisted gene pyramiding., 30(4): 1679–1688.
Jiang J F, Mou T, Yu H H, Zhou F S. 2015. Molecular breeding of thermo-sensitive genic male sterile (TGMS) lines of rice for blast resistance usinggene., 8: 11.
Jones J D, Dangl J L. 2006. The plant immune system., 444: 323–329.
Kang S, Sweigard J A, Valent B. 1995. Thehost specificity gene family in the blast fungus., 8(6): 939–948.
Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, Alaux L, Fournier E, Tharreau D, Terauchi R. 2012. Arms race co-evolution ofand ricegenes driven by their physical interactions., 72(6): 894–907.
Kawano Y, Akamatsu A, Hayashi K, Housen Y, Okuda J, Yao A, Nakashima A, Takahashi H, Yoshida H, Wong H L, Kawasaki T, Shimamoto K. 2010. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity., 7(5): 362–375.
Khan G H, Shikari A B, Vaishnavi R, Najeeb S, Padder B A, Bhat Z A, Parray G A, Bhat M A, Kumar R, Singh N K. 2018. Marker-assisted introgression of three dominant blast resistance genes into an aromatic rice cultivar Mushk Budji., 8: 4091.
Khanna A, Sharma V, Ellur R K, Shikari A B, Krishnan G S, Singh U D, Prakash G, Sharma T R, Rathour R, Variar M, Prashanthi S K, Nagarajan M, Vinod K K, Bhowmick P K, Rajashekhara H, Singh N K, Prabhu K V, Singh A K. 2015. Marker assisted pyramiding of major blast resistance genesandin the genetic background of an elite Basmati rice variety, Pusa Basmati 1., 75(4): 417–425.
Kumar S V, Rambabu R, Bhaskar B, Madhavi K R, Srikanth S, Prakasam V, Sundaram R M, Madhav M S, Rao L V S, Prasad M S. 2018. Introgression of durable blast resistance geneintorice cv. Samba Mahsuri, through marker assisted backcross breeding., 9(2): 705–715.
Kumar S V, Srinivas Prasad M, Rambabu R, Madhavi K R, Bhaskar B, Abhilash Kumar V, Sundaram R M, Krishna Satya A, Sheshu Madhav M, Prakasam V. 2019. Marker-assisted introgression ofgene conferring resistance to rice blast pathogen pyricularia oryzae in the background of Samba Mahsuri., 8(1): 2133–2146.
Lee S K, Song M Y, Seo Y S, Kim H K, Ko S, Cao P J, Suh J P, Yi G, Roh J H, Lee S, An G, Hahn T R, Wang G L, Ronald P, Jeon J S. 2009. Rice-mediated resistance torequires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes., 181(4): 1627–1638.
Li C G, Wang D, Peng S S, Chen Y, Su P, Chen J B, Zheng L M, Tan X Q, Liu J L, Xiao Y H, Kang H X, Zhang D Y, Wang GL, Liu Y. 2019. Genome-wide association mapping of resistance against rice blast strains in South China and identification of a newallele., 12(1): 47.
Li W, Wang B H, Wu J, Lu G D, Hu Y J, Zhang X, Zhang Z G, Zhao Q, Feng Q, Zhang H Y, Wang Z Y, Wang G L, Han B, Wang Z H, Zhou B. 2009. Theavirulence geneencodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene., 22(4): 411–420.
Li W, Deng Y W, Ning Y S, Hu Z H, Wang G L. 2020. Exploiting broad-spectrum disease resistance in crops: From molecular dissection to breeding., 16: 1–25.
Li W T, Zhu Z W, Chern M, Yin J J, Yang C, Ran L, Cheng M P, He M, Wang K, Wang J, Zhou X G, Zhu X B, Chen Z X, Zhao W C, Ma B T, Qin P, Chen W L, Wang Y P, Liu J L, Wang W M, Wu X J, Li P, Wang J R, Zhu L H, Li S G, Chen X W. 2017. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance., 170(1): 114–126.
Li WT, Chern MS, Yin JJ, Wang J, Chen XW. 2019. Recent advances in broad-spectrum resistance to the rice blast disease., 50:114–120.
Li Y D, Li J J, Zhang M, Tian L, Yang S Q, Li P F, Zhang Y X. 2019. Analysis of blast resistance genes inrice core collection and progeny in Ningxia., 20(2):321–334. (in Chinese with English abstract)
Lin F, Chen S, Que Z Q, Wang L, Liu X Q, Pan Q H. 2007. The blast resistance geneencodes a nucleotide binding site leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1., 177(3): 1871–1880.
Liu DF, Chen XJ, Liu JQ, Ye JC, Guo ZJ. 2012. The rice ERF transcription factornegatively regulates resistance toand salt tolerance., 63(10): 3899–3911.
Liu H, Dong S Y, Gu F W, Liu W, Yang G L, Huang M, Xiao W M, Liu Y Z, Guo T, Wang H, Chen Z Q, Wang J F. 2017. NBS-LRR protein Pik-H4 interacts with OsBIHD1 to balance rice blast resistance and growth by coordinating ethylene-brassinosteroid pathway., 8: 127.
Liu Q, Yang J Y, Zhang S H, Zhao J L, Feng A Q, Yang T F, Wang X F, Mao X X, Dong J F, Zhu X Y, Leung H, Leach J E, Liu B. 2016.positively regulates panicle blast resistance but negatively regulates leaf blast resistance in rice., 29(1): 46–56.
Liu W D, Liu J L, Triplett L, Leach J E, Wang G L. 2014. Novel insights into rice innate immunity against bacterial and fungal pathogens., 52: 213–241.
Liu W G, Wang F, Jin S J, Zhu X Y, Li J H, Liu Z R, Liao Y L, Zhu M S, Huang H J, Fu F H, Liu Y B. 2008. Improvement of rice blast resistance in TGMS line by pyramiding ofandthrough molecular marker-assisted selection., 34(7): 1128–1136.
Liu X Q, Lin F, Wang L, Pan Q H. 2007. Themap-based cloning of, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus., 176(4): 2541–2549.
Ma J, Lei C L, Xu X T, Hao K, Wang J L, Cheng Z J, Ma X D, Ma J, Zhou K N, Zhang X, Guo X P, Wu F Q, Lin Q B, Wang C M, Zhai H Q, Wang H Y, Wan J M. 2015., encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice., 28(5): 558–568.
Ma J Q, Sun Y D, Yang Y, Li J B, Chen H C, Jiao A X, Tan J Y, Run R C, Xu M H. 2018. Distribution of rice blast resistance genes,,,in local rice varieties of Guizhou Province., 31(11): 2217–2222. (in Chinese with English abstract)
McClung A M, Marchetti M A, Webb B D, Bollich C N. 1997. Registration of ‘Jefferson’ rice., 37(2): 629–630.
Mentlak T A, Kombrink A, Shinya T, Ryder L S, Otomo I, Saitoh H, Terauchi R, Nishizawa Y, Shibuya N, Thomma B P H J, Talbot N J. 2012. Effector-mediated suppression of chitin-triggered immunity byis necessary for rice blast disease., 24(1): 322–335.
Miki S, Matsui K, Kito H, Otsuka K, Ashizawa T, Yasuda N, Fukiya S, Sato J, Hirayae K, Fujita Y, Nakajima T, Tomita F, Sone T. 2009. Molecular cloning and characterization of thelocus from a Japanese field isolate of., 10(3): 361–374.
Nasir F, Tian L, Chang C, Li X, Gao Y, Tran L P, Tian C. 2018. Current understanding of pattern-triggered immunity and hormone-mediated defense in rice () in response toinfection., 83: 95–105.
Niks R E, Qi X Q, Marcel T C. 2015. Quantitative resistance to biotrophic filamentous plant pathogens: Concepts, misconceptions, and mechanisms., 53: 445–470.
Nurnberger T, Brunner F, Kemmerling B, Piater L. 2004. Innate immunity in plants and animals: Striking similarities and obvious differences., 198(1): 249–266.
Okuyama Y, Kanzaki H, Abe A, Yoshida K, Tamiru M, Saitoh H, Fujibe T, Matsumura H, Shenton M, Galam D C, Undan J, Ito A, Sone T, Terauchi R. 2011. A multifaceted genomics approach allows the isolation of the rice-blast resistance gene consisting of two adjacent NBS-LRR protein genes., 66(3): 467–479.
Orbach M J, Farrall L, Sweigard J A, Chumley F G, Valent B. 2000. A telomeric avirulence gene determines efficacy for the rice blast resistance gene., 12(11): 2019–2032.
Ortiz D, de Guillen K, Cesari S, Chalvon V, Gracy J, Padilla A, Kroj T. 2017. Recognition of theeffector AVR-Pia by the decoy domain of the rice NLR immune receptor RGA5., 29(1): 156–168.
Park C H, Chen S B, Shirsekar G, Zhou B, Khang C H, Songkumarn P, Afzal A J, Ning Y, Wang R Y, Bellizzi M, Valent B, Wang G L. 2012. Theeffector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice., 24(11): 4748–4762.
Park C H, Shirsekar G, Bellizzi M, Chen S B, Songkumarn P, Xie X, Shi X T, Ning Y S, Zhou B, Suttiviriya P, Wang M, Umemura K, Wang G L. 2016. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice., 12(3): e1005529.
Pilet-Nayel M L, Moury B, Caffier V, Montarry J, Kerlan M C, Fournet S, Durel C E, Delourme R. 2017. Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection., 8: 1838.
Puri K D, Shrestha S M, Chhetri G B K, Joshi K D. 2009. Leaf and neck blast resistance reaction in tropical rice lines under greenhouse condition., 165:523–532.
Qu S H, Liu G F, Zhou B, Bellizzi M, Zeng L R, Dai L Y, Han B, Wang G L. 2006. The broad-spectrum blast resistance geneencodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice., 172(3): 1901–1914.
Ray S, Singh P K, Gupta D K, Mahato A K, Sarkar C, Rathour R, Singh N K, Sharma T R. 2016. Analysis ofgenome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene,., 7: 1140.
RoyChowdhury M, Jia Y L, Jackson A, Jia M H, Fjellstrom R, Cartwright R D. 2012. Analysis of rice blast resistance genein rice germplasm using pathogenicity assays and DNA markers., 184(1): 35–46.
Segonzac C, Zipfel C. 2011. Activation of plant pattern-recognition receptors by bacteria., 14(1): 54–61.
Selisana SM, Yanoria MJ, Quime B, Chaipanya C, Lu G, Opulencia R, Wang GL, Mitchell T, Correll J, Talbot NJ, Leung H, Zhou B. 2017. Avirulence () gene-based diagnosis complements existing pathogen surveillance tools for effective deployment of resistance () genes against rice blast disease., 107(6): 711–720.
Shang J J, Tao Y, Chen X W, Zou Y, Lei C L, Wang J, Li X B, Zhao X F, Zhang M J, Lu Z K, Xu J C, Cheng Z K, Wan J M, Zhu L H. 2009. Identification of a new rice blast resistance gene,, by genomewide comparison of paired nucleotide-binding site-leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes., 182(4): 1303–1311.
Sharma T R, Rai A K, Gupta S K, Singh N K. 2010. Broad- spectrum blast resistance genecloned from rice line Tetep designated as., 19(1): 87–89.
Singh R, Dangol S, Chen Y F, Choi J, Cho Y S, Lee J E, Choi M O, Jwa N S. 2016.effector AVR-Pii helps to establish compatibility by inhibition of the rice NADP-malic enzyme resulting in disruption of oxidative burst and host innate immunity., 39(5): 426–438.
Singh V K, Singh A, Singh S P, Ellur R K, Choudhary V, Sarkel S, Singh D, Krishnan S G, Nagarajan M, Vinod K K, Singh U D, Rathore R, Prashanthi S K, Agrawal P K, Bhatt J C, Mohapatra T, Prabhu K V, Singh A K. 2012. Incorporation of blast resistance into ‘PRR78’, an elite Basmati rice restorer line, through marker assisted backcross breeding., 128: 8–16.
Su J, Wang W J, Han J L, Chen S, Wang C Y, Zeng L X, Feng A Q, Yang J Y, Zhou B, Zhu X Y. 2015. Functional divergence of duplicated genes results in a novel blast resistance geneat thelocus., 128(11): 2213–2225.
Suh J P, Roh J H, Cho Y C, Han S S, Kim Y G, Jena K K. 2009. Thegene for durable resistance to rice blast and molecular analysis of-advanced backcross breeding lines., 99(3): 243–250.
Sweigard J A, Carroll A M, Kang S, Farrall L, Chumley F G, Valent B. 1995. Identification, cloning, and characterization of, a gene for host species specificity in the rice blast fungus., 7(8): 1221–1233.
Tabien R E, Li Z, Paterson A H, Marchetti M A, Stansel J W, Pinson S R M, Park W D. 2000. Mapping of four major rice blast resistance genes from ‘Lemont’and ‘Teqing’and evaluation of their combinatorial effect for field resistance., 101(8): 1215–1225.
Takagi H, Uemura A, Yaegashi H, Tamiru M, Abe A, Mitsuoka C, Utsushi H, Natsume S, Kanzaki H, Matsumura H, Saitoh H, Yoshida K, Cano L M, Kamoun S, Terauchi R. 2013. MutMap-Gap: Whole-genome resequencing of mutant F2progeny bulk combined withassembly of gap regions identifies the rice blast resistance gene., 200(1): 276–283.
Takahashi A, Hayashi N, Miyao A, Hirochika H. 2010. Unique features of the rice blast resistancelocus revealed by large scale retrotransposon-tagging., 10: 175.
Tang M Z, Ning Y S, Shu X L, Dong B, Zhang H Y, Wu D X, Wang H, Wang G L, Zhou B. 2017. The Nup98 homolog APIP12 targeted by the effector AvrPiz-t is involved in rice basal resistance against., 10(1): 5.
Tanweer F A, Rafii M Y, Sijam K, Rahim H A, Ahmed F, Ashkani S, Latif M A. 2015. Introgression of blast resistance genes (putativeand) into elite rice cultivar MR219 through marker-assisted selection., 6: 1002.
Thakur S, Singh P K, Das A, Rathour R, Variar M, Prashanthi S K, Singh A K, Singh U D, Chand D, Singh N K, Sharma T R. 2015. Extensive sequence variation in rice blast resistance genemakes it broad spectrum in nature., 6: 345.
Tian H G, Cheng H Q, Hu J, Lei C L, Zhu X D, Qian Q. 2016. Effect of introgressedgene on rice blast resistance and yield traits of japonica rice in cold area., 47(5): 520–526. (in Chinese with English abstract)
Usatov A V, Kostylev P I, Azarin K V, Markin N V, Makarenko M S, Khachumov V A, Bibov Y M. 2016. Introgression of the rice blast resistance genes,andinto Russian rice varieties by marker-assisted selection., 76(1): 18–23.
Wang CH, Yang YL, Yuan XP, Xu Q, Feng Y, Yu HY, Wang YP, Wei XH. 2014. Genome-wide association study of blast resistance inrice., 14:311.
Wang G L, Mackill D J, Bonman J M, McCouch S R, Champoux M C, Nelson R J. 1994. RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar., 136(4): 1421–1434.
Wang J, Qu B Y, Dou S J, Li L Y, Yin D D, Pang Z Q, Zhou Z Z, Tian M M, Liu G Zn, Xie Q, Tang D Z, Chen X W, Zhu L H. 2015. The E3 ligase OsPUB15 interacts with the receptor-like kinase PID2 and regulates plant cell death and innate immunity., 15: 49.
Wang L, Zhao LN, Zhang XH, Zhang QJ, Jia YX, Wang G, Li SM, Tian DC, Li WH, Yang SH. 2019. Large-scale identification and functional analysis ofgenes in blast resistance in the Tetep rice genome sequence., 116(37): 18479–18487.
Wang Q, Li Y Y, Ishikawa K, Kosami K I, Uno K, Nagawa S, Tan L, Du J M, Shimamoto K, Kawano Y. 2018. Resistance protein Pit interacts with the GEF OsSPK1 to activate OsRac1 and trigger rice immunity., 115(49): 11551–11560.
Wang R Y, Ning Y S, Shi X T, He F, Zhang C Y, Fan J B, Jiang N, Zhang Y, Zhang T, Hu Y J, Bellizzi M, Wang G L. 2016. Immunity to rice blast disease by suppression of effector-triggered necrosis., 26(18): 2399–2411.
Wang Z X, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T. 1999. Thegene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes., 19(1): 55–64.
Wu J, Liu X L, Dai L Y, Wang G L. 2007. Advances on the identification and characterization of broad-spectrum blast resistance genes in rice., 19(2): 233–238. (in Chinese with English abstract)
Wu J, Kou Y J, Bao J D, Li Y, Tang M Z, Zhu X L, Ponaya A, Xiao G, Li J B, Li C Y, Song M Y, Cumagun C J R, Deng Q Y, Lu G D, Jeon J S, Naqvi N I, Zhou B. 2015. Comparative genomics identifies theavirulence effector AvrPi9 that triggers-mediated blast resistance in rice., 206(4): 1463–1475.
Wu Y Y, Xiao N, Yu L, Pan C H, Li Y H, Zhang X X, Liu G Q, Dai Z Y, Pan X B, Li A H. 2015. Combination patterns of majorgenes determine the level of resistance to thein rice (L.)., 10(6): e0126130.
Wu Y Y, Yu L, Pan C H, Dai Z Y, Li Y H, Xiao N, Zhang X X, Ji H J, Huang N S, Zhao B H, Zhou C H, Liu G Q, Liu X J, Pan X B, Liang C Z, Li A H. 2016. Development of near-isogenic lines with different alleles oflocus and analysis of their breeding effect under Yangdao 6 background., 36(2): 1–12.
Wu Y Y, Chen Y, Pan C H, Xiao N, Yu L, Li Y H, Zhang X X, Pan X B, Chen X J, Liang C Z, Dai Z Y, Li A H. 2017. Development and evaluation of near-isogenic lines with different blast resistance alleles at thelocus inrice from the lower region of the Yangtze River, China., 101(7): 1283–1291.
Wu Y Y, Xiao N, Chen Y, Yu L, Pan C H, Li Y H, Zhang X X, Huang N S, Ji H J, Dai Z Y, Chen X J, Li A H. 2019. Comprehensive evaluation of resistance effects of pyramiding lines with different broad-spectrum resistance genes againstin rice (L.)., 12(1): 11.
Xiang Y C, Wang L L, Xu F, Ma D R. 2018. Study on the distribution of rice blast resistant genes in rice resources of Heilongjiang Province., 16(23): 7705–7717. (in Chinese with English abstract)
Xiao N, Wu Y Y, Pan C H, Yu L, Chen Y, Liu G Q, Li Y H, Zhang X X, Wang Z P, Dai Z Y, Liang C Z, Li A H. 2017. Improving of rice blast resistances inby pyramiding majorgenes., 7: 1918.
Xiao N, Wu Y Y, Wang Z P, Li Y H, Pan C H, Zhang X X, Yu L, Liu G Q, Zhou C H, Ji H J, Huang N S, Jiang M, Dai Z Y, Li A H. 2018. Improvement of seedling and panicle blast resistance inrice varieties followingintrogression., 38:142.
Xiao W M, Luo L X, Wang H, Guo T, Liu Y Z, Zhou J Y, Zhu X Y,Yang Q Y, Chen Z Q. 2016. Pyramiding ofandto improve blast resistance and to evaluate the resistance effect of the twogenes., 15(10): 2290–2298.
Xing X, Liu X L, Chen H L, Yang F Y, Li Y C, Liao H, You L, Liu J L, Dai L Y, Wang G L. 2016. Improving blast resistance of rice restorer R288 by molecular marker-assisted selection ofgene.,30(5):487–491.
Xu P, Wang H, Tu RR, Liu QN, Wu WX, Fu XM, Cao LV, Shen XH. 2019. Orientation improvement of blast resistance in rice via CRISPR/Cas9 system., 33(4):313–322.(in Chinese with English abstract)
Xu X, Hayashi N, Wang C T, Fukuoka S, Kawasaki S, Takatsuji H, Jiang C J. 2014. Rice blast resistance gene(t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein., 34(2): 691–700.
Yamasaki Y, Kiyosawa S. 1966. Studies on inheritance of resistance of rice varieties to blast: 1. Inheritance of resistance of Japanese varieties to several strains of the fungus., 14: 39–69.
Yang G L, Chen S P, Chen L K, Sun K, Huang C H, Zhou D H, Huang Y T, Wang J F, Liu Y Z, Wang H, Chen Z Q, Guo T. 2019. Development of a core SNP arrays based on the KASP method for molecular breeding of rice., 12(1): 21.
Yang J Y, Chen S, Zeng L X, Li Y L, Chen Z, Zhu X Y. 2008. Evaluation on resistance of major rice blast resistance genes toisolates collected fromrice in Guangdong Province, China., 22(2): 190–196. (in Chinese with English abstract)
Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, Tosa Y, Chuma I, Takano Y, Win J, Kamoun S, Terauchi R. 2009. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen., 21(5): 1573–1591.
Yu M M, Dai Z Y, Pan C H, Chen X J, Yu L, Zhang X X, Li Y H, Xiao N, Gong H B, Sheng S L, Pan X B, Zhang H X, Li A H. 2013. Resistance spectrum difference between two broad-spectrum blast resistance genes,and, and their interaction effect on., 39(11):1927–1934. (in Chinese with English abstract)
Yuan B, Zhai C, Wang W J, Zeng X S, Xu X K, Hu H Q, Lin F, Wang L, Pan Q H. 2011. Theresistance toin rice is mediated by a pair of closely linked CC-NBS-LRR genes., 122(5): 1017–1028.
Zeng S Y, Li C, Du C C, Sun L T, Jing D D, Lin T Z, Yu B, QIAN H F, Yao W C, Zhou Y W, Gong H B. 2018. Development of specific markers forin marker-assisted breeding of panicle blast resistantrice., 32(5): 453–461. (in Chinese with English abstract)
Zhai C, Lin F, Dong Z Q, He X Y, Yuan B, Zeng X S, Wang L, Pan Q H. 2011. The isolation and characterization of, a rice blast resistance gene which emerged after rice domestication., 189(1): 321–334.
Zhang S L, Wang L, Wu W H, He L Y, Yang X F, Pan Q H. 2015. Function and evolution ofavirulence generesponding to the rice blast resistance gene., 5: 11642.
Zhao H J, Wang X Y, Jia Y L, Minkenberg B, Wheatley M, Fan J B, Jia M H, Famoso A, Edwards J D, Wamishe Y, Valent B, Wang G L, Yang Y N. 2018. The rice blast resistance geneencodes an atypical protein required for broad-spectrum disease resistance., 9(1): 2039.
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang G L. 2006. The eight amino-acid differences within three leucine-rich repeats betweenandresistance proteins determine the resistance specificity to., 19(11): 1216–1228.
Zhou X G, Liao H C, Chern M, Yin J J, Chen Y F, Wang J P, Zhu X B, Chen Z X, Yuan C, Zhao W, Li W T, He M, Ma B T, Wang J C, Qin P, Chen W L, Wang Y P, Liu J L, Qian Y W, Wang W M, Wu X J, Li P, Zhu L H, Li S G, Ronald P C, Chen X W. 2018. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance., 115(12): 3174–3179.
Zhou Z Z, Pang Z Q, Zhao S L, Zhang L L, Lv Q M, Yin D D, Li D Y, Liu X, Zhao X F, Li X B, Wang W M, Zhu L H. 2019. Importance of OsRac1 and RAI1 in signalling of nucleotide- binding site leucine-rich repeat protein-mediated resistance to rice blast disease., 223(2): 828–838.
Zou J F, Li Y C, Liu X L, L iu J L, Chen H L, Yang F Y, Huang J, Liao H. 2017. Improving blast reisistance of rice restorer ‘E32’ and its hybrid through molecular marker-assisted selection., 31(1):11–14.
5 August 2019;
4 December 2019
LI Aihong (yzlah@126.com)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.003
(Managing Editor: Li Guan)
杂志排行
Rice Science的其它文章
- Genetic Variation Dissection of Rice Blast Resistance Using an Indica Population
- OsPS6 Plays Important Role in Anther Development and Microspore Formation
- Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
- RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
