RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
2020-07-06HeYongqiZhaoJiaFengDefengHuangZhiboLiangJiamingZhengYufeiChengJinpingYingJifengWangZhoufei
He Yongqi, Zhao Jia, Feng Defeng, Huang Zhibo, Liang Jiaming, Zheng Yufei, Cheng Jinping, Ying Jifeng, Wang Zhoufei
Research Paper
RNA-Seq Study Reveals AP2-Domain-Containing Signalling Regulators Involved in Initial Imbibition of Seed Germination in Rice
He Yongqi1, #, Zhao Jia1, #, Feng Defeng1, Huang Zhibo1, Liang Jiaming1, Zheng Yufei1, Cheng Jinping2, Ying Jifeng3, Wang Zhoufei1
(Laboratory of Seed Science and Technology /Guangdong Key Laboratory of Plant Molecular Breeding / State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou 510642, China; Laboratory of Seed Science and Technology, State Key Laboratory of Crop Genetics and Germplasm Enhancement, Jiangsu Collaborative Innovation Center for Modern Crop Production, Nanjing Agricultural University, Nanjing 210095, China; State Key Laboratory of Rice Biology, China National Rice Research Institute, Hangzhou 310006, China; These authors contributed equally to this work)
A number of internal signals are required for seed germination. However, the precise signalling responses in the initial imbibition of seed germination are not yet fully understood in rice. In this study, the RNA sequencing (RNA-Seq) approach was conducted in 8 h imbibed seeds to understand the signalling responses in the initial imbibition of rice seed germination. A total of 563 differentially expressed genes (DEGs) with at least 4-fold change were identified in 8 h imbibed seeds compared to dry seeds. MapMan analysis revealed that the majority of signalling response-related DEGs were hormone- and transcription factor-related genes, in which the largest number of DEGs belong to the AP2-domain-containing regulators, and their expressions were significantly induced in the initial imbibition of seed germination in rice. Moreover, at least five AP2-domain-containing transcription factor OsDREBs were identified in the initial imbibition of rice seed germination, and the expressions of 251 DEGs were putatively regulated by OsDREBs through the dehydration-responsive element (DRE)-element assay. It suggested that the OsDREBs might play important roles in the regulation of initial seed imbibition in rice. The identified genes provide a valuable resource to study the signalling regulation of seed germination in the future.
rice; seed imbibition; signalling response; plant hormone; transcription factor; RNA-sequencing
Rice (L.) is an important food crop in the world. Recently, the approach of direct seeding is becoming popular in rice, which requires seeds behaving good germination characteristics (Mahender et al, 2015). Seed germination starts with the imbibition of dry seed and terminates with the radicle protrusion through seed coat. Seed imbibition process can be divided into three phases including the rapid phase (Phase I), the plateau phase (Phase II), and the again rapid phase of water uptake (Phase III) (Wang et al, 2011). Previous genome-wide gene expression analyses of seed germination are mainly focused on Phase II when seed cells switch from a quiescent state to a metabolically active state in rice (Dametto et al, 2015; He et al, 2015; Wei et al, 2015; Narsai et al, 2017). Seed germination is regulated by a variety of internal and external signals (Bewley, 1997; Zhang et al, 2018). How the signalling responses occur at Phase I of seed germination is largely unknown in rice.
Gibberellin (GA) and abscisic acid (ABA) are the key internal signals for seed germination in plants. GA releases seed dormancy and promotes seed germination, while ABA behaves the opposite functions (Graeber et al, 2012; Shu et al, 2016; Nee et al, 2017). For example, the GA-deficient mutants (and) and the plants with constitutively expressing the ABA biosynthesis gene show strong seed dormancy (Lee et al, 2002; Shu et al, 2013; Nonogaki et al, 2014), while the GA2-oxidases mutants () and ABA-deficient mutants behave speedy germination (Yamauchi et al, 2007; Frey et al, 2012). In addition to GA and ABA, auxin, cytokinins (CK) and jasmonate (JA) can inhibit seed germination, while ethylene (ET) and brassinosteroid (BR) can promote seed germination (Kucera et al, 2005; Preston et al, 2009; Dave et al, 2011; Linkies and Leubner-Metzger, 2012). For example, the high levels of auxin will release the expressions of auxin- responsive transcription factors (TFs) ARF10 and ARF16 to activate the transcription of ABI3 for dormancy maintenance (Liu et al, 2013). ET promotes seed germination via counteracting the inhibitory effects of ABA (Corbineau et al, 2014). Meanwhile, seed germination under various environmental conditions is also regulated by plant hormones.
TFs, such as WRKY, MYB and AP2/EREBP (APETALA2/ethylene-responsive element binding proteins), are important signalling regulators in seed germination. For example,AtWRKY6, AtWRKY18, AtWRKY40, AtWRKY41, AtWRKY60, AtWRKY2 and AtWRKY63 regulate seed germination through the ABA-dependent or ABA-independent pathways (Rushton et al, 2010; Ding et al, 2014; Huang et al, 2016). SixMYB members, AtMYB2, AtMYB7, AtMYB30, AtMYB33, AtMYB101and AtMYB96, mediate seed germination and seedlinggrowth through the ABA-signalling pathway (Abe et al, 2003; Reyes and Chua, 2007; Zheng et al, 2012; Kim et al, 2015; Lee et al, 2015). Meanwhile,AP2/ERFBP members ABI4 and RAV1 involve in seed germination and early seedling development via the ABA-signalling pathway (Feng et al, 2014), while AP2 transcription factor LEP promotes seed germination through the GA-biosynthesis or signalling pathways (Ward et al, 2006). However, the precise signalling responses in the initial imbibition of seed germination are not yet fully understood in rice.
In this study, RNA sequencing (RNA-Seq) was conducted in 8 h imbibed seeds (Phase I) to reveal the signalling responses that occurred at the initial imbibition stage of seed germination in rice. Here, the hormones and TF regulators associated with signalling responses were focused on. Interestingly, a larger number of AP2-domain-containing regulators were identified at the initial imbibition stage of rice seed germination. The identified genes can be further studied to reveal the signalling regulation of seed germination in the future.
MATERIALS AND METHODS
Plant materials and germination assay
rice Nipponbare was used for seed germination assay according to He et al (2019). Fifty seeds per replicate were germinated in Petri dishes (diameter = 9 cm) with 10 mL distilled water under 30 ºC ± 1 ºC for 10 d in a growth chamber. Seeds after 0 h (dry seeds) and 8 h imbibition were harvested for RNA sequencing. Meanwhile, seeds after 4, 8, 12, 18, 24, 36, 48, 60 and 72 h imbibition were harvested for the following quantitative real-time PCR (qRT-PCR) assay. Three biological replications were performed.
Water content assay
Fifty dry seeds per replicate were firstly weighed (W1), and then seeds after 2, 6, 8, 12, 18, 24, 36, 42, 48, 60 and 72 h imbibition were harvested and weighed (W2), respectively. The water content was defined as the weight of water uptake per one gram of dry seed. Thus, the water content (g/g) = (2–1) /1. Three biological replications were performed.
RNA sequencing approach
Approximately 80–100 mg powder of seeds were used for the total RNA extraction using the TransZol Plant kit (Transgen, Beijing, China) according to the manufacturer’s protocol. Construction of the cDNA libraries and HiSeq2500 sequencing were performed at Novogene Biotechnology Co., Ltd., Beijing, China.
Analysis of differentially expressed genes
The clean reads with 21 to 49 bp length were mapped onto the Nipponbare reference genome (MSU Rice Genome Annotation Project Release 7) using Tophat version 2.0.12 (Kim et al, 2013). Expression levels of genes were quantified in terms of FPKM (fragments per kilo base of exon per million) using RSEM version 1.1.11 (Li and Dewey, 2011). The log2-fold changes of gene FPKM were calculated between 0 and 8 h imbibed seeds. The differentially expressed genes (DEGs) with at least 4-fold change were selected for further pathway analyses. A metabolic pathway overview was depicted by MapMan version 3.6.0RC1 (http:// mapman.gabipd.org) and shown using color intensity (Usadel et al, 2009).
Gene expression analysis
The gene expression at various germination stages of rice was analyzed using the publicly available data on Genevestigator (https://www.genevestigator.com/gv/plant. jsp). Total RNA was extracted from 80–100 mg powder of each sample using the Plant kit (Transgen, Beijing, China) according to the manufacturer’s protocol. qRT-PCR was performed according to He et al (2019). A total volume of 10 μL containing 1 μL cDNA, 0.8 μL gene-specific primers (10 μmol/L), 5 μL SYBR Green Mix and 3.2 μL RNase free ddH2O was used. The PCR conditions were as follows: 95 ºC for 5 min, followed by 40 cycles of 95 ºC for 15 s and 60 ºC for 30 s. The ricewas used as an internal control. Primers used for qRT-PCR are listed in Supplemental Table 1. Normalized transcript levels were calculated using the comparative CT method (Livak and Schmittgen, 2001). Three biological replications were performed.
RESULTS
Differentially expressed genes in initial imbibition of seed germination
To determine the time points for transcriptional analyses, the process of rice seed germination was analyzed. Based on seed imbibition, the first 12 h of imbibition was associated with the rapid water uptake (Phase I), and the 36 h of imbibition was the germination time point when the radicle protrudes through seed coat (Phase III, Fig. 1). To reveal the DEGs in the initial imbibition of seed germination in rice, the dry (0 h) and imbibed (8 h) seeds (at Phase I) were collected for RNA sequencing. By comparison, the data of RNA sequencing were high consistent (Pearson correlation > 0.991) between samples, and then the mean FPKM of three biological repeats was used for further analysis.
A total of 492 and 71 DEGs with at least 4-fold change were up-regulated and down-regulated respectively in 8 h imbibed seeds compared to dry seeds in rice (Fig. 2-A and Supplemental Table 2). MapMan analysis revealed that these DEGs mainly included transcription factor (81), large enzyme family (63), signalling (42), protein (35), stress (31), transport (29), hormone (26) and development (20) (Fig. 2-B). Further pathway investigation showed that 193 DEGs (removing redundant genes) were involved in the process of seed germination (Supplemental Table 3). In which, the hormone- and transcription- related DEGs involved in the process of seed germination were focused for further analysis below (Fig. 2-C).
Hormone-related DEGs involved in initial imbibition of seed germination in rice
A total of 25 hormone-related DEGs, including auxin-, ET-, JA-, ABA- and GA-related genes, were identified in the initial imbibition of seed germination in rice (Table 1). In which, 4 and 21 DEGs were down- regulated and up-regulated in 8 h imbibed seeds compared to dry seeds, respectively. By comparison, the majority of DEGs were ET-related genes (12) followed by JA- (6) and auxin-related genes (4). Interestingly, half of ET-related genes were AP2-domain- containing genes (6) that were significantly induced after 8 h imbibition in rice. Meanwhile, five 12- oxophytodienoate reductase involved in JA biosynthesis and two auxin-responsivegenes were significantly induced after 8 h imbibition in rice.
The expression patterns of these DEGs in embryo and endosperm during seed germination were further conducted using Genevestigator (Fig. 3). The similar expression patterns of 18 hormone-related DEGs (seven genes not available) were observed in embryo and endosperm during 24 h imbibition stage in rice. By comparison, the continuously high expressions of LOC_Os08g35110, LOC_Os06g51320 and LOC_ Os06g11210, corresponding to auxin-responsive gene, GA-regulated protein GASR8 and 12-oxophytodienoate reductase, respectively, were observed during 24 h imbibition stage. However, the expressions of LOC_Os06g44970, LOC_Os03g08490 and LOC_Os08g39850, encoding auxin efflux carrier component and two AP2 domain proteins, respectively, were gradually up-regulated with the increase of seed imbibition. The expressions of remaining DEGs (except LOC_Os03g18030), e.g. LOC_Os03g08860 and LOC_Os09g35010, were firstly up-regulated and then down-regulated during 24 h imbibition stage.

Fig. 1.Dynamic changes of seed germination in Nipponbare.
A, Changes of water content during seed germination. B, Germination state. Scale bar, 1 cm.

Fig. 2. Differentially expressed genes (DEGs) between dry seeds (0 h) and imbibed seeds (8 h) in rice.
A, Total, up- and down-regulated DEGs with at least 4-fold change. B, Functional classification of DEGs using MapMan analysis. C, Hormone- and transcription factor-regulated DEGs involved in seed germination pathway. Red, Up-regulation; Gray, No change; Blue, Down-regulation. Values represent the log2fold changes of gene. ABA, Abscisic acid; GA, Gibberellin.

Table 1. Hormone-related differentially expressed genes involved in the initial imbibition of seed germination in rice.
Transcription factor-related DEGs involved in initial imbibition of seed germination in rice
A total of 37 TF-related DEGs, including AP2/ERFBP-, HB-, MYB-, WRKY-, HSF-, MADS- and RNA processing-related genes, were identified in the initial imbibition of seed germination in rice (Table 2). In which, the majority of DEGs were AP2/ERFBP- related genes (16) followed by HB- (6), MYB- (5) and WRKY-related genes (3). By comparison, the majority of DEGs were up-regulated at the early germination stage, while 4 and 33 DEGs were down-regulated and up-regulated, respectively. Interestingly, all the AP2/ ERFBP- and MYB-related genes were significantly up-regulated after 8 h imbibition in rice. However, the expression of LOC_Os01g53260 corresponding to WRKY23 was most significantly down-regulated at the early germination stage in rice.
The expression patterns of these genes in embryo and endosperm during seed germination were further explored using Genevestigator (Fig. 4). The higher expressions of AP2/ERFBP-related TFs were observed compared to the other types of TFs during 24 h imbibition stage in rice. Meanwhile, the expressions of majority AP2/ERFBP-related TFs (except LOC_ Os03g08490 and LOC_Os06g36000) were significantly induced in both embryo and endosperm of rice. Particularly, the continuously higher expressions in embryo were observed in three AP2/ERFBP-related TFs (LOC_Os07g47790, LOC_Os03g08460 and LOC_ Os05g03040). The similar expression patterns were observed in other six AP2/ERFBP-related TFs (LOC_ Os09g35010, LOC_Os09g35030, LOC_Os08g43210, LOC_Os08g43200, LOC_Os04g34970 and LOC_ Os02g32590), which were obviously induced at 4 h imbibition and then decreased gradually with the time of seed imbibition. These expressed genes might play important roles in signalling responses during the early germination in rice.

Fig. 3. Expression pattern of hormone-related differentially expressed genes in embryo and endosperm during 24 h imbibition stage in rice.
Red, Up-regulation; Gray, No change; Blue, Down-regulation. Values represent the log2-fold changes of gene.

Table 2. Transcription factor-related differentially expressed genes involved in the initial imbibition of seed germination in rice.
Expression and bioinformatics analysis of AP2- domain-containing TF OsDREBs during seed germination
Based on above results, the AP2-domain-containing- related genes are the important signalling regulators in the initial imbibition of seed germination in rice. To validate RNA sequencing results and to explore the expression of AP2/ERFBP TFs at the initial imbibition stage, qRT-PCR analyses were firstly conducted in 10 randomly selected AP2/ERFBP TFs. By comparison, the consistent change trends of expression were observed in the majority of AP2/ERFBP TFs between RNA-Seq and qRT-PCR approaches in rice (Supplemental Fig. 1).
Interestingly, at least five AP2-domain-containing TFOsDREBs, including OsDREB1A (LOC_Os09g35030),OsDREB1B (LOC_Os09g35010), OsDREB1G/OsDREB1(LOC_Os08g43210), OsDREB1H (LOC_Os09g35020) and OsDREB1J (LOC_Os08g43200), were identified in the initial imbibition of rice seed germination. To further explore the expressions of OsDREBs during seed germination, qRT-PCR analyses were conducted at three imbibition stages in rice (Fig. 5-A). The similar expression patterns were observed that OsDREBs were significantly induced at Phase I (0 to 12 h) and Phase II (12 h to 36 h) compared with dry seeds, while not at Phase III (36 to 72 h). The obviously peak expressions of these genes were observed at the 18 h imbibition stage, and after that, their expressions were decreased gradually with seed imbibition.
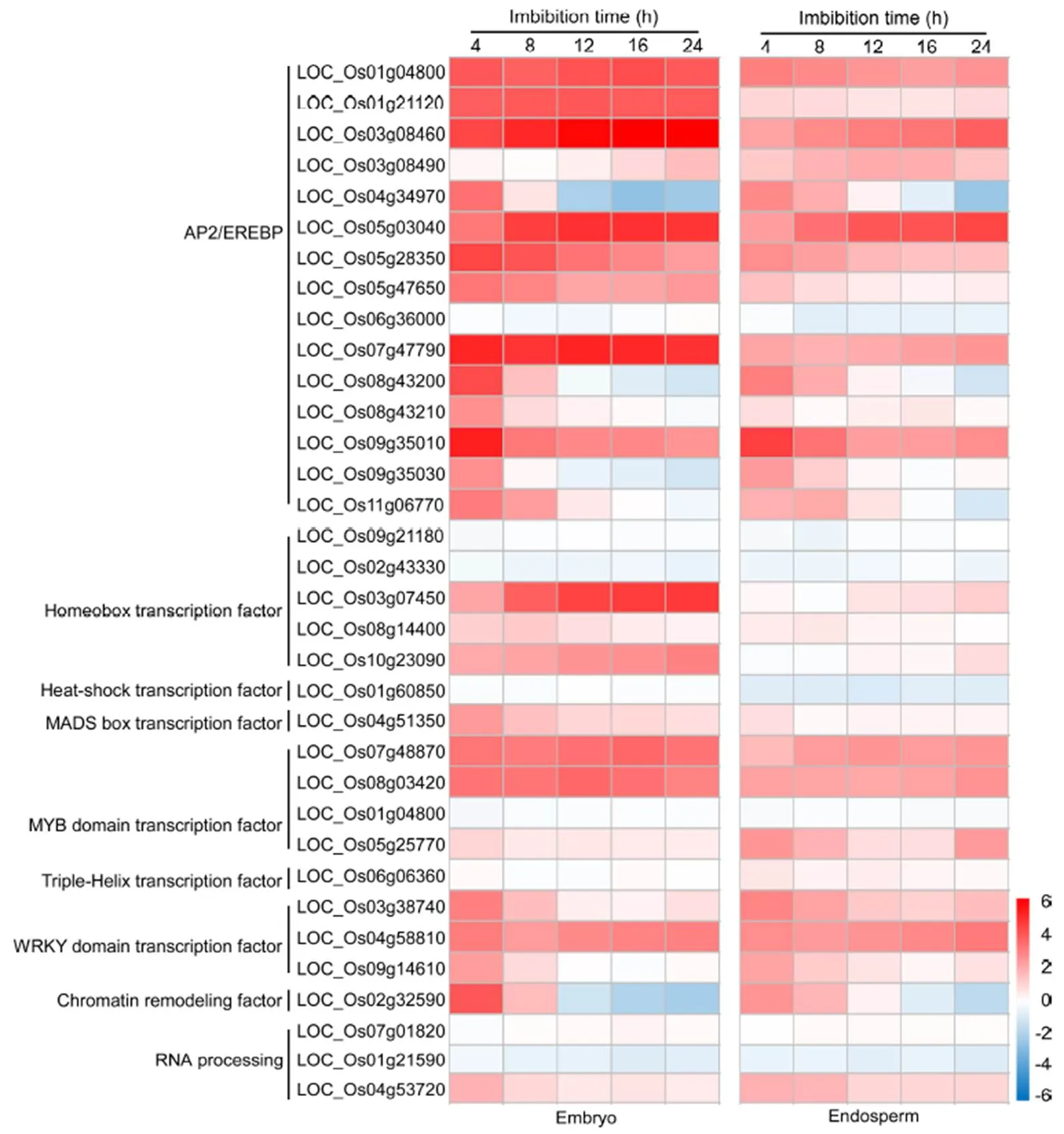
Fig. 4. Expression pattern of transcription factor-related differentially expressed genes in embryo and endosperm during 24 h imbibition stage in rice using Genevestigator.
Red, Up-regulation; Gray, No change; Blue, Down-regulation. Values represent the log2-fold changes of gene.
To reveal which DEGs are to be putatively regulated by OsDREBs during the early seed germination, the sequence analysis of DEG promoter was conducted. Presences of dehydration-responsive element (DRE)-elements including GCCGAC and/or ACCGAC were observed in 251 DEGs (Fig. 5-B and Supplemental Table 4), suggesting the expressions of these genes might be regulated by OsDREBs during the early seed germination in rice. Further analysis indicated that the majority of 251 DEGs mainly included RNA processing transcription (26), large enzyme family (22), signalling(16) and stress responses (15) (Fig. 5-C and Supplemental Table 5). All these results indicated that OsDREBs might play important roles in the regulation of the initial imbibition in rice seed germination.
Expression patterns of DEGs putatively regulated by OsDREBs during seed germination
To further explore the regulatory roles of OsDREBs on seed germination, the expressions of above mentioned large enzyme family- and stress response-related DEGswere focused on analyses during seed germination in rice.
Majority of large enzyme family- and stress response- related DEGs (except LOC_Os11g25990, LOC_ Os07g11739, LOC_Os04g41680 and LOC_Os02g06250) were significantly induced during seed germination in rice (Fig. 6). By comparison, the expressions of LOC_ Os07g09630, LOC_Os10g09110, LOC_Os03g02920 and LOC_Os12g02080 were obviously higher in embryo than in endosperm during 24 h imbibition in rice. However, the similar expression patterns were observed in the majority of large enzyme family- and stress response-related DEGs (except LOC_Os02g03200) between in embryo and in endosperm during seed germination. For example, the expressions of LOC_ Os07g44140 and LOC_Os07g23570 were obviously induced at 4 h imbibition stage and then gradually decreased in embryo and endosperm during seed germination. The expressions of LOC_Os11g47610 and LOC_Os11g47590 were gradually induced in embryo and endosperm during seed germination. These expressed large enzyme family- and stress response-related DEGs might be regulated by OsDREBs during seed germination in rice.

Fig. 5.Differentially expressed genes (DEGs) putatively regulated byduring seed germination in rice.
A, Expression of five OsDREBs during three stages of seed germination in rice. The transcript levels were examined by quantitative real-time PCR, and the transcript levels were calculated relative to the genes at 0 h germination stage. Values indicate Mean ± SD (= 3). B, Number of DEGs with the dehydration-responsive-elements in the promoter sequences of DEGs. C, Functional classification of DEGs with DRE-elements by MapMan analysis.
DISCUSSION
Seed germination inhibited by ABA and promoted by GA that is well investigated at the molecular level. RNA-Seq study of rice embryo at 0, 12 and 48 h imbibition stages indicated that ABA, GA and BR were involved in seed germination (Wei et al, 2015). In this study, RNA-Seq study of whole grain was conducted at the early stage (0–8 h imbibition) to reveal the signalling responses in the initial imbibition of seed germination in rice. Similar with Wei et al (2015), we observed that the expression of ABA biosynthesis geneencoding 9-- epoxycarotenoid dioxygenase 1 was significantly down-regulated in the initial imbibition process of seed germination in rice. However, the expression profiles of auxin-, ET- and JA-related genes indicated that auxin, ET and JA are involved in the signalling responses during the initial imbibition of rice seed germination in this study. For example,, encoding a member of the auxin efflux carrier proteins, was identified, which can regulate the auxin distribution in rice (Inahashi et al, 2018; Wang et al, 2018). Meanwhile, two auxin-responsive genesandwere identified in this study. Previous studies have shown that SAUR proteins promote auxin-induced cell expansion via an acid growth mechanism in, in which SAUR inhibits PP2C-D phosphatases and then activates plasma membrane H+-ATPases to promote cell expansion (Spartz et al, 2014; Ren et al, 2018; Stortenbeker and Bemer, 2019). The roles of auxin-related genes, e.g.,and, on the early signalling responses of seed germination need to be further investigated in rice.
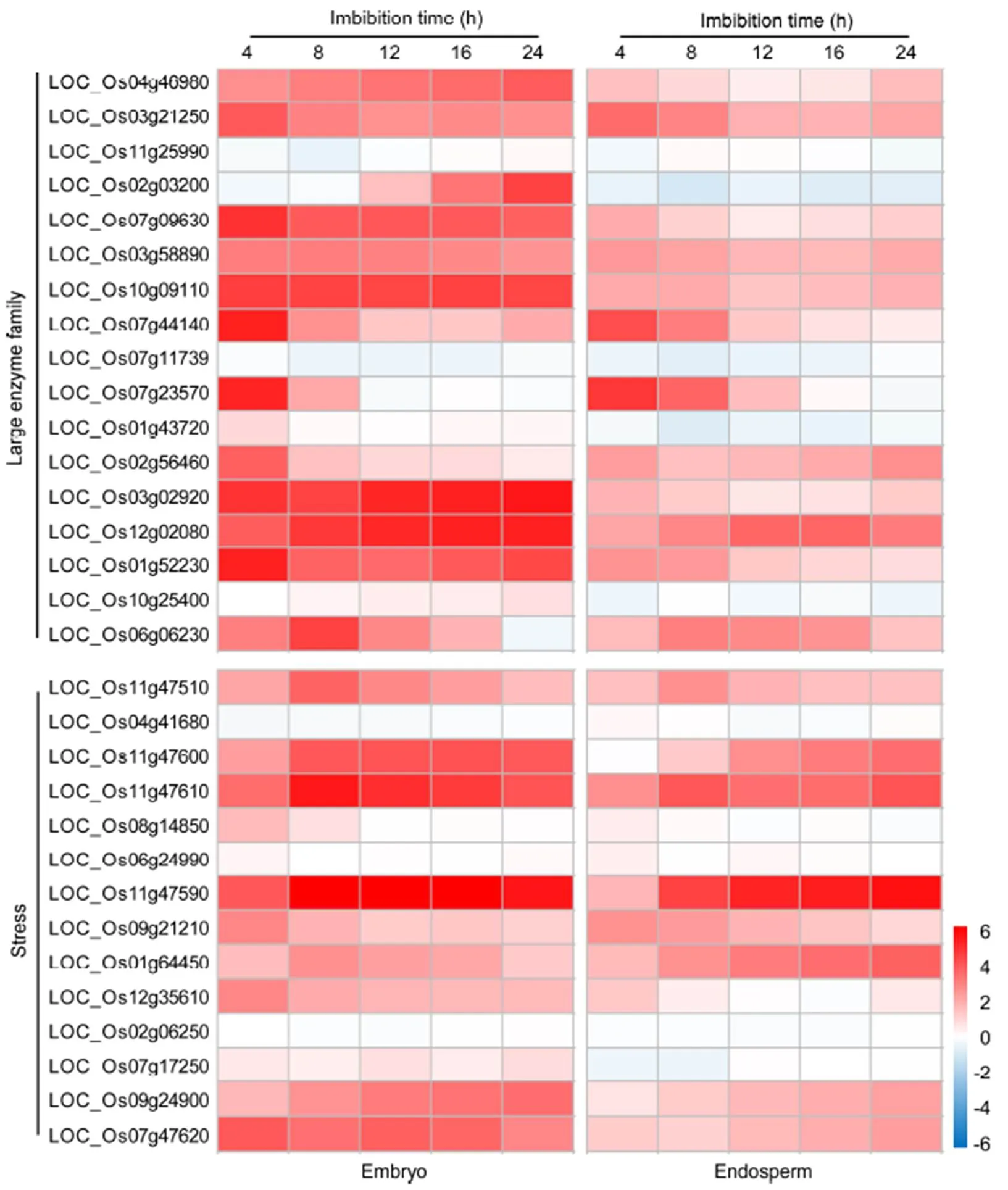
Fig. 6. Expression pattern of large enzyme family- and stress response-related differentially expressed genes putatively regulated byOsDREBs in embryo and endosperm during 24 h imbibition in rice using Genevestigator.
Red, Up-regulation; Gray, No change; Blue, Down-regulation. Values represent the log2-fold changes of gene.
Interestingly, a large more ET-related genes, half of which belong to AP2-domain-containing regulators, were identified compared to GA- and ABA-related genes in the initial imbibition of seed germination in this study. ET synthesis can induce the expression of GA biosynthetic enzymes (Chiwocha et al, 2005; Matilla and Matilla-Vázquez, 2008), and ET can reduce the synthesis of ABA, and enhance its degradation or alter sensitivity to its action (Beaudoin et al, 2000; Ghassemian et al, 2000; Chiwocha et al, 2005; Subbiah and Reddy, 2010). ET may promote seed germination indirectly through the interactions with GA and ABA. In this study, we observed that several ET-related genes e.g. ET receptorwere involved in the initial imbibition of seed germination in rice. Previously, five ET receptor homologs, including,,,and, have been identified in rice (Yau et al, 2004). Similar withand(Chen et al, 2002),is found to be located in the endoplasmic reticulum in rice (Yu et al, 2017). Overexpression ofcan lead to accumulation of starch in stem internodes of rice (Wuriyanghan et al, 2009), which may also help rice to survive after submergence (Yu et al, 2017). The loss of ETR1 can lead to accelerated germination in, while loss ofcan delay seed germination under salt stress (Wilson et al, 2014). The regulatory roles ofin the early seed germination need further investigation.
RNA-Seq study of rice embryo indicated that TFs ERF, bHLH and HD-ZIP families are connected with functional alterations at the early germination stage, while WRKY, AUX/IAA, TCP, Tify and NAC families are involved at the late germination stage (Wei et al, 2015). In this study, five MYB and three WRKY members e.g. OsWRKY23, OsWRKY45 and OsWRKY113, as well as five HD-Zip TFs, were identified involving in the early seed germination of rice. The MYB transcription factors regulated seed germination are involved in ABA signalling in(Reyes and Chua, 2007; Kim et al, 2015). The WRKY TFs act as regulators of seed germination via the ABA-dependent or independent pathways or via GA-dependent pathway (Zou et al, 2008; Rushton et al, 2010; Ding et al, 2014). In rice, OsWRKY45 plays important roles in the fungal and bacterial disease resistance (Shimono et al, 2012) and abiotic stress tolerance (Tao et al, 2011), and OsWRKY113 might be involved in iron stress tolerance (Viana et al, 2017). Meanwhile, rice HD-Zip TFs have been reported in the regulation of stress tolerance (Zhang et al, 2012), bulliform cell development (Chew et al, 2013) and organ or tissue differentiation in the developing embryo (Yang et al, 2002). However, the roles of here identified MYB, WRKY and HD-Zip TFs on seed germination have never been reported in rice, and more research is required in the future.
Interestingly, the largest number of rice AP2- domain-containing TFs were identified compared to the other types of TFs involving in the initial imbibition of rice seed germination in this study. AP2-domain contains 60 to 70 conserved amino acids, which are important for DNA binding activity (Magnani et al, 2004). Recently, AP2-domain-containing proteins are classified into five subfamilies including AP2-, RAV-, DREB-, ERF-subfamily and others (Gong et al, 2004; Gutterson and Reuber, 2004). In this study, at least six, five, two and one AP2-domain-containing TFs belong to AP-, DREB-, RAV- and ERF-subfamily, respectively. The functions of DREBs on abiotic stress tolerance are well investigated in plants (Shinozaki and Yamaguchi-Shinozaki, 2000; Kizis et al, 2001; Thomashow, 2001). In this study, the OsDREB1A, OsDREB1B, OsDREB1G/OsDREB1I, OsDREB1H and OsDREB1J were identified in the initial imbibition of seed germination in rice. Previously, OsDREB1A and OsDREB1B have been reported involving in the regulation of cold tolerance in rice (Challam et al, 2015; He et al, 2016), while OsDREB1G involving in drought tolerance (Chen et al, 2008). We speculated that OsDREBs might participate in the stress response pathway in the initial imbibition of seed germination when seeds from dry to imbibed states in rice.
Previous studies proposed that the AP2-domain- containing TFs negatively and positively regulate GA and ABA biogenesis respectively in fine-tuning seed dormancy and germination in(Shu et al, 2016). In rice, the AP2-domain-containing TF OsAP2-39 directly promotes the expressions of ABA-biosynthesis geneand GA-inactivating gene, thus enhancing ABA and decreasing GA levels to increase seed dormancy (Yaish et al, 2010). Meanwhile, it is well known that DREBs play important roles on abiotic stress tolerance in plants (Shinozaki and Yamaguchi-Shinozaki, 2000; Thomashow, 2001). AP2-domain-containing TF OsDREBs might regulate the expressions of large enzyme family- (e.g. UDP glucosyl and glucoronyl transferases and cytochrome P450) and stress response-related genes during seed germination (Fig. 5-C and Supplemental Table 5). In this study, DRE-elements existed in five large enzyme family genes (LOC_Os10g09110, LOC_ Os07g44140, LOC_Os07g11739, LOC_Os07g23570 and LOC_Os01g43720) encoding cytochrome P450 enzymes. Plant cytochrome P450 enzymes play important roles in hormone synthesis (Schuler and Werck-Reichhart, 2003), suggesting OsDREBs might regulate the hormone synthesis in the initial imbibition of seed germination in rice. Furthermore, the DRE-elements were also observed in the drought/salt stress-related genes in this study. It further supported our speculation that OsDREBs might participate in the stress response pathway in the initial imbibition of rice seed germination. Altogether, we assumed that OsDREBs regulate the early seed germination might be through the regulations of hormone synthesis and stress responses in rice. Further investigation is deserved to clarify this speculation.
In conclusion, we employed an RNA-Seq strategy to reveal the signalling responses of seed germination at the early seed germination stage (Phase I) in rice. A total of 563 DEGs with at least 4-fold change were identified in 8 h imbibed seeds compared to dry seeds. In which, the majority of signalling associated DEGs were hormone- and TF-related genes involving in the initial imbibition of seed germination in rice. Our study has provided new information that AP2-domain- containing signalling regulators involved in the signalling responses in the early seed germination of rice. The identified genes provide a valuable resource for the study of molecular mechanism of seed germination in the future.
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Plan (Grant No. 2018YFD0100901), the Guangdong Province Key Research and Development Program (Grant No. 2018B020202012), the Guangdong Province Key Laboratory of Plant Molecular Breeding (Grant No. GPKLPMB201903), and the Major Scientific Research Projects of General Colleges and Universities of Guangdong Province (Grant No. 2017KTSCX024).
Supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Primers used for quantitative real-time PCR analyses in this study.
Supplemental Table 2. List of differentially expressed genes with at least 4-fold changes in the initial imbibition stage in rice.
Supplemental Table 3. Information of 193 differentially expressed genes involved in the process of seed germination by MapMan analysis in rice.
Supplemental Table 4. Information of dehydration- responsive element (DRE)-elements existed in the promoter sequences of 251 differentially expressed genes in rice.
Supplemental Table 5. Functional classification information of the differentially expressed genes putatively regulated by OsDREBs during seed germination in rice.
Supplemental Fig. 1. Expression of ten randomly selected AP2/EREBP transcription factors after 8 h imbibition in rice.
The RNA sequencing data have been submitted to the Sequence Read Archive (SRA) database (https://www. ncbi.nlm.nih.gov/sra) under the accession number PRJNA544406.
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003.AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling., 15: 63–78.
Beaudoin N, Serizet C, Gosti F, Giraudat J. 2000. Interactions between abscisic acid and ethylene signaling cascades., 12(7): 1103–1116.
Bewley J D. 1997. Seed germination and dormancy., 9(7): 1055–1066.
Challam C, Ghosh T, Rai M, Tyagi W. 2015. Allele mining across DREB1A and DREB1B in diverse rice genotypes suggest a highly conserved pathway inducible by low temperature., 94: 231–238.
Chen J Q, Meng X P, Zhang Y, Xia M, Wang X P. 2008. Over-expression ofgenes lead to enhanced drought tolerance in rice., 30(12): 2191–2198.
Chen Y F, Randlett M D, Findell J L, Schaller G E. 2002. Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of., 277: 19861–19866.
Chew W, Hrmova M, Lopato S. 2013. Role of homeodomain leucine zipper (HD-Zip) IV transcription factors in plant development and plant protection from deleterious environmental factors., 14(4): 8122–8147.
Chiwocha S D, Cutler A J, Abrams S R, Ambrose S J, Yang J, Ross A R, Kermode A R. 2005. Themutation inaffects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination., 42(1): 35–48.
Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H. 2014. Ethylene, a key factor in the regulation of seed dormancy., 5: 539.
Dametto A, Sperotto R A, Adamski J M, Blasi É A R, Cargnelutti D, de Oliveira L F V, Ricachenevsky F K, Fregonezi J N, Mariath J E A, da Cruz R P, Margis R, Fett J P. 2015. Cold tolerance in rice germinating seeds revealed by deep RNAseq analysis of contrastinggenotypes., 238: 1–12.
Dave A, Hernández M L, He Z, Andriotis V M E, Vaistij F E, Larson T R, Graham I A. 2011. 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in., 23(2): 583–599.
Ding Z J, Yan J Y, Li G X, Wu Z C, Zhang S Q, Zheng S J. 2014. WRKY41 controlsseed dormancy via direct regulation oftranscript levels not downstream of ABA., 79(5): 810–823.
Feng C Z, Chen Y, Wang C, Kong Y H, Wu W H, Chen Y F. 2014.transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of,, andduring seed germination and early seedling development., 80(4): 654–668.
Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North H M, Marion-Poll A. 2012. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members., 70(3): 501–512.
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. 2000. Regulation of abscisic acid signaling by the ethylene response pathway in., 12(7): 1117–1126.
Gong W, Shen Y P, Ma L G, Pan Y, Du Y L, Wang D H, Yang J Y, Hu L D, Liu X F, Dong C X, Ma L, Chen Y H, Yang X Y, Gao Y, Zhu D M, Tan X L, Mu J Y, Zhang D B, Liu Y L, Dinesh-Kumar S P, Li Y, Wang X P, Gu H Y, Qu L J, Bai S N, Lu Y T, Li J Y, Zhao J D, Zuo J R, Huang H, Deng X W, Zhu Y X. 2004. Genome-wide ORFeome cloning and analysis oftranscription factor genes., 135(2): 773–782.
Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe W J J. 2012. Molecular mechanisms of seed dormancy., 35(10): 1769–1786.
Gutterson N, Reuber T L. 2004. Regulation of disease resistance pathways by AP2/ERF transcription factors., 7(4): 465–471.
He D L, Wang Q, Wang K, Yang P F. 2015. Genome-wide dissection of the microRNA expression profile in rice embryo during early stages of seed germination., 10(12): e0145424.
He Y A, Li Y P, Cui L X, Xie L X, Zheng C K, Zhou G H, Zhou J J, Xie X Z. 2016. Phytochrome B negatively affects cold tolerance by regulatinggene expression through phytochrome interacting factor-like protein OsPIL16 in rice., 7: 1963.
He Y Q, Cheng J P, He Y, Yang B, Cheng Y H, Yang C, Zhang H S, Wang Z F. 2019. Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice., 17(2): 322–337.
Huang Y, Feng C Z, Ye Q, Wu W H, Chen Y F. 2016.WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development., 12(2): e1005833.
Inahashi H, Shelley I J, Yamauchi T, Nishiuchi S, Takahashi- Nosaka M, Matsunami M, Ogawa A, Noda Y, Inukai Y. 2018., which encodes a member of the auxin efflux carrier proteins, is involved in root elongation growth and lateral root formation patterns via the regulation of auxin distribution in rice., 164(2): 216–225.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg S L. 2013. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions., 14(4): R36.
Kim J H, Hyun W Y, Nguyen H N, Jeong C Y, Xiong L, Hong S W, Lee H. 2015. AtMyb7, a subgroup 4 R2R3 Myb, negatively regulates ABA-induced inhibition of seed germination by blocking the expression of the bZIP transcription factor ABI5., 38(3): 559–571.
Kizis D, Lumbreras V, Pagès M. 2001. Role of AP2/EREBP transcription factors in gene regulation during abiotic stress., 498: 187–189.
Kucera B, Cohn M A, Leubner-Metzger G. 2005. Plant hormone interactions during seed dormancy release and germination., 15: 281–307.
Lee K, Lee H G, Yoon S, Kim H U, Seo P J. 2015. TheMYB96 transcription factor is a positive regulator of ABSCISIC ACID-INSENSITIVE4 in the control of seed germination., 168(2): 677–689.
Lee S, Cheng H, King K E, Wang W, He Y, Hussain A, Lo J, Harberd N P, Peng J. 2002. Gibberellin regulatesseed germination via, a-like gene whose expression is up-regulated following imbibition., 16(5): 646–658.
Li B, Dewey C N. 2011. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome., 12: 323.
Linkies A, Leubner-Metzger G. 2012. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination., 31(2): 253–270.
Liu X D, Zhang H, Zhao Y, Feng Z Y, Li Q, Yang H Q, Luan S, Li J M, He Z H. 2013. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediatedactivation in., 110: 15485–15490.
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod., 25(4): 402–408.
Magnani E, Sjolander K, Hake S. 2004. From endonucleases to transcription factors: Evolution of the AP2 DNA binding domain in plants., 16(9): 2265–2277.
Mahender A, Anandan A, Pradhan S K. 2015. Early seedling vigour, an imperative trait for direct-seeded rice: An overview on physio-morphological parameters and molecular markers., 241(5): 1027–1050.
Matilla A J, Matilla-Vazquez M A. 2008. Involvement of ethylene in seed physiology., 175: 87–97.
Narsai R, Secco D, Schultz M D, Ecker J R, Lister R, Whelan J. 2017. Dynamic and rapid changes in the transcriptome and epigenome during germination and in developing rice () coleoptiles under anoxia and re-oxygenation., 89(4): 805–824.
Nee G, Xiang Y, Soppe W J J. 2017. The release of dormancy, a wake-up call for seeds to germinate., 35: 8–14.
Nonogaki M, Sall K, Nambara E, Nonogaki H. 2014. Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds., 78(3): 527–539.
Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E. 2009. Temporal expression patterns of hormone metabolism genes during imbibition ofseeds: A comparative study on dormant and non-dormant accessions., 50(10): 1786–1800.
Ren H, Park M Y, Spartz A K, Wong J H, Gray W M. 2018. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in., 14(6): e1007455.
Reyes J L, Chua N H. 2007. ABA induction of miR159 controls transcript levels of two MYB factors duringseed germination., 49(4): 592–606.
Rushton P J, Somssich I E, Ringler P, Shen Q X J. 2010. WRKY transcription factors., 15(5): 247–258.
Schuler M A, Werck-Reichhart D. 2003. Functional genomics of P450s., 54: 629–667.
Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, Kurihara T, Matsushita A, Sugano S, Jiang C J, Kaku H, Inoue H, Takatsuji H. 2012. Rice WRKY45 plays important roles in fungal and bacterial disease resistance., 13(1): 83–94.
Shinozaki K, Yamaguchi-Shinozaki K. 2000. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways., 3(3): 217–223.
Shu K, Zhang H W, Wang S F, Chen M L, Wu Y R, Tang S Y, Liu C Y, Feng Y Q, Cao X F, Xie Q. 2013. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in., 9(6): e1003577.
Shu K, Liu X D, Xie Q, He Z H. 2016. Two faces of one seed: Hormonal regulation of dormancy and germination., 9(1): 34–45.
Spartz A K, Ren H, Park M Y, Grandt K N, Lee S H, Murphy A S, Sussman M R, Overvoorde P J, Gray W M. 2014. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in., 26(5): 2129–2142.
Stortenbeker N, Bemer M. 2019. The SAUR gene family: The plant’s toolbox for adaptation of growth and development., 70(1): 17–27.
Subbiah V, Reddy K J. 2010. Interactions between ethylene, abscisic acid and cytokinin during germination and seedling establishment in., 35(3): 451–458.
Tao Z, Kou Y J, Liu H B, Li X H, Xiao J H, Wang S P. 2011.alleles play different roles in abscisic acid signalling and salt stress tolerance but similar roles in drought and cold tolerance in rice., 62(14): 4863–4874.
Thomashow M F. 2001. So what’s new in the field of plant cold acclimation? Lots!, 125(1): 89–93.
Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M. 2009. A guide to using MapMan to visualize and compare omics data in plants: A case study in the crop species, maize., 32(9): 1211–1229.
Viana V E, Marini N, Finatto T, Ezquer I, Busanello C, dos Santos R S, Pegoraro C, Colombo L, Costa de Oliveira A. 2017. Iron excess in rice: From phenotypic changes to functional genomics of WRKY transcription factors., 16(3): gmr16039694.
Wang L L, Guo M X, Li Y, Ruan W Y, Mo X R, Wu Z C, Sturrock C J, Yu H, Lu C G, Peng J R, Mao C Z. 2018., encoding OsPIN2, is involved in root system architecture in rice., 69(3): 385–397.
Wang Z F, Wang J F, Bao Y M, Wu Y Y, Zhang H S. 2011. Quantitative trait loci controlling rice seed germination under salt stress., 178(3): 297–307.
Ward J M, Smith A M, Shah P K, Galanti S E, Yi H, Demianski A J, van der Graaff E, Keller B, Neff M M. 2006. A new role for theAP2 transcription factor, LEAFY PETIOLE, in gibberellin-induced germination is revealed by the misexpression of a homologous gene,., 18(1): 29–39.
Wei T, He Z L, Tan X Y, Liu X, Yuan X, Luo Y F, Hu S N. 2015. An integrated RNA-Seq and network study reveals a complex regulation process of rice embryo during seed germination., 464(1): 176–181.
Wilson R L, Kim H, Bakshi A, Binder B M. 2014. The ethylene receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 have contrasting roles in seed germination ofduring salt stress., 165(3): 1353–1366.
Wuriyanghan H, Zhang B, Cao W H, Ma B, Lei G, Liu Y F, Wei W, Wu H J, Chen L J, Chen H W, Cao Y R, He S J, Zhang W K, Wang X J, Chen S Y, Zhang J S. 2009. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice., 21(5): 1473–1494.
Yau C P, Wang L, Yu M, Zee S Y, Yip W K. 2004. Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions., 55: 547–556.
Yaish M W, El-kereamy A, Zhu T, Beatty P H, Good A G, Bi Y M, Rothstein S J. 2010. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice., 6(9): e1001098.
Yamauchi Y, Takeda-Kamiya N, Hanada A, Ogawa M, Kuwahara A, Seo M, Kamiya Y, Yamaguchi S. 2007. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibedseeds., 48(3): 555–561.
Yang J Y, Chung M C, Tu C Y, Leu W M. 2002. OSTF1: A HD-GL2 family homeobox gene is developmentally regulated during early embryogenesis in rice., 43(6): 628–638.
Yu M, Yau C P, Yip W K. 2017. Differentially localized rice ethylene receptors OsERS1 and OsETR2 and their potential role during submergence., 12(8): e1356532.
Zhang A P, Qian Q, Gao Z Y. 2018.Research advances on rice seed vigor., 32(3): 296–303. (in Chinese with English abstract)
Zhang S X, Haider I, Kohlen W, Jiang L, Bouwmeester H, Meijer A H, Schluepmann H, Liu C M, Ouwerkerk P B F. 2012. Function of the HD-Zip I genein ABA-mediated drought and salt tolerances in rice., 80: 571–585.
Zheng Y, Schumaker K S, Guo Y. 2012. Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in., 109: 12822–12827.
Zou X L, Neuman D, Shen Q J. 2008. Interactions of two transcriptional repressors and two transcriptional activators in modulating gibberellin signaling in aleurone cells., 148: 176–186.
24 August 2019;
20 November 2019
Wang Zhoufei(wangzf@scau.edu.cn); Ying Jifeng (yingjifeng@caas.cn)
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.05.006
(Managing Editor: Li Guan)
杂志排行
Rice Science的其它文章
- Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding
- Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases
- Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars
- RNAi-Mediated Silencing of ITPK Gene Reduces Phytic Acid Content, Alters Transcripts of Phytic Acid Biosynthetic Genes, and Modulates Mineral Distribution in Rice Seeds
- Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis
- Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth
