Synthesis and antifungal activities of phenylhydrazono derivatives as the novel fructose-1,6-bisphosphate aldolase inhibitors
2020-06-18GUOLiGUOYanrongRENYanliangHANXinya
GUO Li,GUO Yanrong,REN Yanliang,HAN Xinya
(1.Hubei Ecological and Environmental Monitoring Center Station,Wuhan 430072,China;2.School of Chemistry and Chemical Engineering,Anhui University of Technology,Maanshan,Anhui 243002,China;3.Laboratory of Pesticide &Chemical Biology,Key Laboratary of Ministry of Education,College of Chemistry,Central China Normal University,Wuhan 430079,China)
Abstract:Fructose-1,6-bisphosphate aldolase from C.albicans (CaFBA-II) is an attractive new target for the discovery of drugs to combat invasive fungal infection.Our previous study demonstrated that β-arylhydrazono-α,γ-dicarbonylderivatives exhibit moderate inhibitory against CaFBA-II.Herein,several new phenylhydrazono derivatives were found to potently inhibit CaFBA-II.The experimental results show that the NO2 group in the R2 position is favorable,but COOH,SO3H and halogen atom are unfavorable for their CaFBA-II inhibitory activities.Especially,when the R3 was substituted by sulfonic acid group,compound 2g possessed the highest inhibitory activity (IC50=200 nmol·L-1).Furthermore,compound 2g were selected as representative molecule,the binding mode of 2g and the surrounding residues in the active site of CaFBA-II were elucidated by jointly using DOX methods and molecular dynamics (MD) simulations.Notably,antifungal experiments demonstrate that most of our designed compounds possess moderate inhibitory activities (MIC80=16-64 μg · mL-1) against C.albicans.The present results suggest that the phenylhydrazono derivatives can be used as the lead compounds of novel drugs against fungal pathogens of humans in the future.
Key words:novel inhibitors;rational drug design;phenylhydrazono derivatives;CaFBA-II;antifungal activities
Bacterial and fungal infections are an important public health problem of major concern[1].C.albicansis the primary systemic fungal affecting humans.Azoles are the most well-known antifungal drugs all over the word to combatC.albicansinfections.However,due to extensive use of these drugs in humans,the resistance of pathogenic microorganisms to current antibiotics is an important public health problem[2-3].Fructose-1,6-bisphosphate aldolase (FBA) is an essential enzyme in GNG pathway,and occurs in two distinct classes in the biosphere,and catalyzes the reversible aldol condensation of dihydroxyacetonephosphate (DHAP) and glyceraldehyde 3-phosphate (GAP) to fructose-1,6-bisphosphate (FBP)[4-6].Class I FBA (FBA-I),which is present in higher organisms (plants and animals) and some prokaryotes,forms a Schiffbase intermediate between the keto substrate (FBP or DHAP) and a lysine residue of the active site.Class II FBA (FBA-II) requires a divalentmetal ion (usually zinc or cobalt ion) to polarize the keto carbonyl group of the substrate (FBP or DHAP) and to stabilize the enediolate intermediate formed during catalysis.Because of their occurrence in many pathogenic microbes (bacteria,yeasts,parasites) and their absence in animals,FBA-II has been regarded as a particularly attractive new target and a lot of the attention was focused on the drug design of the enzyme[5,7-10].In previous work,we discovered several potent FBA-II inhibitors againstC.albicans(CaFBA-II)[11-12].Herein,a positive progress was achieved in this field,several new phenylhydrazono derivatives were found to exhibit potent CaFBA-II inhibitory activities and antifungal effects againstC.albicans.Furthermore,the probable binding-modes between the hit compounds and CaFBA-II were also analyzed by jointly using the DOX method[13](i.e.,molecular docking,ONIOM3 and XO),and molecular dynamics (MD) simulations and enzymatic assays.
1 Material and Methods
1.1 Chemistry
All synthetic work was performed in air and at room temperature.All solvents and chemicals were obtained from commercial sources and used as received.The proton (1H) and carbon (13C) NMR spectra were recorded on a Varian Mercury-Plus 400 or 600 MHz spectrometer in DMSO-d6or acetone-d6.Chemical shifts are given in parts per million (ppm) with TMS as the internal reference.Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel F-254 plates.Flash chromatography purifications were performed on Merck silica gel 60 (230-400 mesh) as the stationary phase,and MeOH and CH2Cl2were used as eluents.High-resolution mass spectrometry (HRMS) analysis was performed using a hydrid IT-TOF mass spectrometer with ESI interface (Shimadzu,Kyoto,Japan) and Agilent 6224 Accurate-Mass time-of-flight mass spectrometer with ESI interface (Agilent Technologies,Waldbronn,Germany).If necessary,the purity was determined by high performance liquid chromatography (HPLC).The purity of novel synthesized compounds was 96% or higher,as illustrated in supporting information.
General procedure for the preparation of phenylhydrazono (1,2a-g).A suspension of the substituted aniline (5 mmol) in NaOH solution (0.5 mol · L-1,10 mL) were added NaNO2(5 mmol) and 36% HCl (5 mL) at a temperature below 5 ℃.The resulting diazonium solution was used directly without purification.
A solution of NaOH (0.5 mol · L-1,5 mL) in ethanol (5 mL) was added a portion of theβ-diketone (5 mmol).The solution was cooled in an ice bath to 0 ℃,and then added with the diazonium solution mentioned above under vigorous stirring for 1 h.The mixture was filtered,and the solid product was washed with water,then recrystallized in ethanol to produce1,2a-g.Notably,this approach did engender important safety concerns due to the highly explosive diazonium salt which may decomposed intensively under a relatively high temperature while releasing heat and outgassing.In addition,the raw material sodium nitrite decomposes at 175 ℃,which may easily cause the combustion or explosion of organics in the reaction system,and the combustion or explosion may also be caused by strong oxidants.
1,1,1,5,5,5-Hexafluoro-3-(2-(2-hydroxy-5-nitrophenyl) hydrazono)pentane-2,4-dione (1).Dark red powder,yield 75%.1H NMR (400 MHz,DMSO-d6)δ:8.98 (d,J=9.7 Hz,1H),8.05 (dd,J=11.3,1.9 Hz,1H),6.59 (d,J=10.1 Hz,1H).13C NMR (101 MHz,DMSO-d6)δ:174.75,132.48,132.13,132.10,129.88,129.84,124.08,90.84.HRMS (ESI):m/zcalculated for C11H5F6N3O5+K+[M+K]+411.9765.Found:412.0574.
Ethyl 4,4,4-trifluoro-2-(2-(2-hydroxy-5-nitrophenyl) hydrazono)-3-oxobutanoate (2a).Dark red powder,yield 75%.1H NMR (400 MHz,DMSO-d6)δ:15.19 (s,1H),8.49 (s,1H),8.17 (d,J=8.5 Hz,1H),7.22 (d,J=8.9 Hz,1H),4.41 (d,J=6.7 Hz,2H),1.38 (t,J=7.0 Hz,3H).13C NMR (151 MHz,DMSO-d6)δ162.55,153.09,140.64,129.33,123.26,122.99,116.44,110.55,62.13,14.25.HRMS (ESI):m/zcalculated for C12H10F3N3O6-H+[M-H]-348.0443.Found:348.0453.
Ethyl 4,4,4-trifluoro-2-(2-(2-hydroxy-5-carbonylphenyl) hydrazono)-3-oxobutanoate (2b).Brown powder,yield 45%.1H NMR (400 MHz,DMSO-d6)δ:1H NMR (600 MHz,DMSO-d6)δ12.83 (s,1H),8.10 (s,1H),7.72 (d,J=8.4 Hz,1H),7.05 (d,J=8.5 Hz,1H),4.33 (q,J=7.1 Hz,2H),1.32 (t,J=7.1 Hz,3H).13C NMR (151 MHz,DMSO-d6)δ:176.88,167.23,165.70,163.62,152.66,138.38,128.82,123.85,122.58,116.45,116.20,89.06,61.49,14.36.HRMS (ESI):m/zcalculated for C13H11F3N2O6-H+[M-H]-347.0491.Found:347.0489.
Ethyl 4,4,4-trifluoro-2-(2-(2-hydroxy-5-sulfophenyl) hydrazono)-3-oxobutanoate (2c).Dark powder,yield 35%.1H NMR (400 MHz,DMSO-d6)δ:15.47 (s,1H),11.79 (s,1H),8.02 (d,J=2.0 Hz,1H),7.46 (dd,J=8.4,2.0 Hz,1H),6.95 (d,J=8.5 Hz,1H),4.39 (q,J=7.1 Hz,2H),1.35 (t,J=7.1 Hz,3H).13C NMR (151 MHz,DMSO-d6)δ:162.55,150.44,141.22,133.09,132.30,128.00,119.44,116.59,61.58,14.73.HRMS (ESI):m/zcalculated for C12H11F3N2O7S[M] 384.0234.Found:384.0000.
Ethyl 4,4,4-trifluoro-2-(2-(5-chloro-2-hydroxyphenyl) hydrazono)-3-oxobutanoate (2d).Dark red powder,yield 40%.1H NMR (600 MHz,DMSO-d6)δ:13.34 (s,1H),11.47 (s,1H),11.14 (s,1H),7.37 (s,1H),7.29 (d,J=8.4 Hz,1H),7.17 (d,J=8.6 Hz,1H),7.04 (t,J=9.0 Hz,1H),4.34 (dd,J=13.8,6.7 Hz,2H),1.32 (t,J=7.1 Hz,3H).13C NMR (151 MHz,DMSO-d6)δ:162.34,149.62,134.45,133.92,129.78,124.13,119.46,119.19,61.58,14.60.HRMS (ESI):m/zcalculated for C12H10ClF3N2O4-H+[M-H]-337.0203.Found:337.0208.
Ethyl 4,4,4-trifluoro-2-(2-(2-hydroxyphenyl)hydrazono)-3-oxobutanoate (2e).Red powder,yield 58%.1H NMR (600 MHz,DMSO-d6)δ:13.55 (s,1H),10.91 (s,1H),7.47 (d,J=8.0 Hz,1H),7.14 (t,J=7.7 Hz,1H),7.05 (d,J=8.0 Hz,1H),6.97 (t,J=7.6 Hz,1H),4.34 (q,J=7.0 Hz,2H),1.32 (t,J=7.1 Hz,3H).13C NMR (151 MHz,CDCl3)δ:170.61,163.82,146.33,127.76,127.49,121.46,117.43,117.18,109.99,61.97,14.06.HRMS (ESI):m/zcalculated for C12H11F3N2O4-H+[M-H]-303.0592.Found:303.0604.
Ethyl 4,4,4-trifluoro-2-(2-(3-chloro-2-hydroxy-5-nitrophenyl)hydrazono)-3-oxobutanoate (2f).Black powder,yield 43%.1H NMR (600 MHz,DMSO-d6)δ:8.73 (s,1H),6.63 (d,J=2.2 Hz,1H),6.53 (d,J=8.1 Hz,1H),6.39 (dd,J=8.1,2.2 Hz,1H),4.37 (s,2H),1.23 (d,J=4.4 Hz,3H),1.18 (s,9H).13C NMR (151 MHz,DMSO-d6)δ:166.25,162.84,136.52,129.75,125.21,124.51,124.27,122.85,120.84,109.45,61.44,14.41.HRMS (ESI):m/zcalculated for C12H9ClF3N3O6-H+[M-H]-382.0054.Found:382.0062.
Ethyl 4,4,4-trifluoro-2-(2-(2-hydroxy-5-nitro-3-sulfophenyl) hydrazono)-3-oxobutanoate (2g).Dark red powder,yield 50%.1H NMR (400 MHz,DMSO-d6)δ:15.13 (s,1H),13.23 (s,1H),12.31 - 12.09 (m,1H),8.50 (d,J=2.7 Hz,1H),8.29 (d,J=2.7 Hz,1H),4.47 - 4.34 (m,2H),1.36 (dt,J=25.0,7.1 Hz,3H).13C NMR (101 MHz,DMSO-d6)δ:167.29,155.22,145.86,137.89,137.08,132.73,124.10,121.34,66.31,19.46.HRMS (ESI):m/zcalculated for C12H10F3N3O9S-H+[M-H]-428.0012.Found:428.0281
1.2 Enzyme inhibition activity of CaFBA-II
To evaluate the inhibitory activity of hit compounds screened and synthesized in the present study,the half maximal inhibitory concentration (IC50) values of hit compounds were determined at the CaFBA-II recombinant protein level by linear regression analysis using the program package origin.The gene sequence of FBA-II fromC.albicanswas obtained from NCBI (XM_717597.1)[14].CaFBA-II was expressed inE.coliBL21(DE3) cells as described previously[15].Commercial preparations from Sigma of glycerol 3-phosphate dehydrogenase (GPDH) from rabbit muscle,triosephosphate isomerase (TIM) from rabbit muscle,and Ra-FBA-I were used.Ra-FBA-I and CaFBA-II activity and its inhibition were determined in reactions using an NADH linked enzymatic assay.The activity of the cleavage reaction was assayed in Tris-K-acetate buffer (0.1 mol·L-1,pH 7.4,with 0.2 mol·L-1potassium acetate for FBA-II),using fructose bisphosphate and inhibitor at the appropriate concentrations,NADH (0.3 mmol·L-1),GPDH (0.26 U),TIM (1 U) and aldolase (4 mU) in a cuvette to give a final volume of 0.42 mL.The decrease in absorbance of NADH at 340 nm was monitored on a spectrophotometer over 5 min.
To determine thecorresponding inhibitor constants (IC50),the initial rate data taken at a saturating substrate concentration,a fixed effector concentration,and systematically varied inhibitor concentrations were fit to a Hill equation,V=V0-(V0-V∞)/((I0.5/I)n+1)[16],whereV,V0,andV∞are the velocity,maximum velocity (atI=0),and the limiting velocity (atIsaturating),respectively;n is the Hill coefficient associated with the inhibitor;andIC50is the inhibitor concentration at 50% inhibition.All kinetic data were fit to the growth/sigmoidal model in origin 7.0 software.The inhibition assay was run under a concentration of compounds2fand2gillustrated in Fig.1.

Fig.1 The inhibition assay was run in the concentration range (0-50 μmol·L-1) of hit compounds 2f and 2g (The inhibition of DMSO was taken as blank control,for all hit compounds dissolved in DMSO)
1.3 Inhibitory Assays on Candida albicans
Invitroantifungal activity was measured as the minimum inhibitory concentration (MIC) that achieved 80% inhibition of the C.albicans using the broth microdilution method,as given in document M27-A3[17]and M38-A2[18]from the National Committee for Clinical Laboratory Standards (CLSI).TheC.albicansstrain (SC5314) were obtained from the Second Military Medical University.The test compounds were dissolved in DMSO and serially diluted in growth media.The microtiter plates were incubated at 35 ℃ for 48 h for the Candida species.DMSO was used as a growth control and fluconazole (Pfizer) and ketoconazole (Sigma) were used as controls against the tested fungi.
Inhibitory effect of hit compounds on the growth of weed roots and shoots
The inhibitory effect of hit compounds on the Growth of Weed roots and shoots was executed as described previously[19].A mixture of 4 g agar powder and 0.5 L distilled water was heated to melt and then cooled down about 45 ℃.The 20 μL solution containing 1.5 mg · mL-1,4.5 mg · mL-1,15 mg · mL-1compounds and 280 μL melting agar were mixed in 96-well plate.The seeds ofCynodondactylonL.,and Arabidopsis thaliana were put on the surface of the agar.The 96-well plates were kept in (25 ± 1) ℃,12 h in the light and 12 h in the dark alternatively,and 50%-55% relative humidity for seven days.Then the lengths of roots and shoots,and the inhibitory rate was determined.The commercial herbicide acetocblor,glyphosate,and meostrione were used as the positive control.
2 Results and Discussions
2.1 Synthesis and optimization of phenylhydrazono derivatives
Our previous work[11]suggested that the hydroxyl group of phenylhydrazonoderivatives is essential to the binding of hit compounds and its target,because it could coordinate with the Zn(II) ion of CaFBA-II.To obtain more potent hit compounds,several novel phenylhydrazono derivatives with hydroxyl group were further synthesized,as illustrated in Tab.1,together with their CaFBA-II inhibitory activities and antifungal activities.

Tab.1 The inhibitory activities of the hit compounds against CaFBA-II,together with the inhibitions against C.albicans
Compounds1,2a-gwere synthesized according to procedure described previously[20],as illustrated in Scheme 1.
Scheme 1 Synthetic Route of the hit compounds1and2a-g

Reagents and conditions:a) NaNO2/HCl,0-5 ℃;b) CF3COCH2COR1,NaOH,0-5 ℃
It is shown in our previous study that the methyl group onα,γ-dicarbonyl substructure are unfavorable to the inhibitory activities of hit compounds,thus we attempt to synthesize the new compounds with trifluoromethyl or ethyl ester groups inR1position.As listed in Tab.1,the nitro group in theR2position of the benzene ring is favorable for the inhibitory activities of hit compounds (compounds1and2a).When the nitro group was changed to a carboxyl group or a sulfonic acid group,such as2band2c,the corresponding inhibition activities against CaFBA-II decreased approximately 10-fold compared with that of2a.Notably,when the Cl and SO3H were introduced to theR3,compounds2fand2gexhibit potent CaFBA-II inhibitory activities,with theIC50of 1.5 μmol·L-1and 0.2 μmol·L-1.These results indicate that a hydrophilic group in theR3position is favorable.

The ribbon represents the helix and sheet of protein,the light blue stick represents the hit compound 2g,and the surrounding important residues were representedby the creamy white stick.The green dashed line represents the important interactions of the ligands and target enzymeFig.2 The proposed binding-mode of compound 2g into the active site of CaFBA-II optimized by MD simulations
The binding mode of2gand CaFBA-II were further predicted by using DOX strategy[13,21],and optimized by molecular dynamics (MD) simulations (Fig.2 and Fig.3) with the PMEMD module in the AMBER 12 package.Additionally,using FBA-II fromE.coli(PDB ID:1B57) as template,the plausible homology model of CaFBA-II were performed by SWISSMODEL server,which has been documented previously[11].As shown in Fig.1,the sulfonic acid and nitro group on the benzene ring of2gcan form hydrogen bonds with Asn224 and Ser268,respectively.The hydroxyl group on the benzene ring of2gcould coordinate with Zn(II) ion.The hydrogen-bonds between carbonyl group and Asn36 could also be observed in Fig.1.However,when the sulfonic acid group inR3position was substituted by H and Cl,the hydrogen bond between hit compound and Asn224 was lost,thus the inhibitory activity of compound2a(3.2 μmol·L-1and2f(1.5 μmol·L-1) will decrease approximately 10-fold compared with that of2g(0.2 μmol·L-1).This to some extend explain why2gpossess the highest inhibitory activity against CaFBA-II.
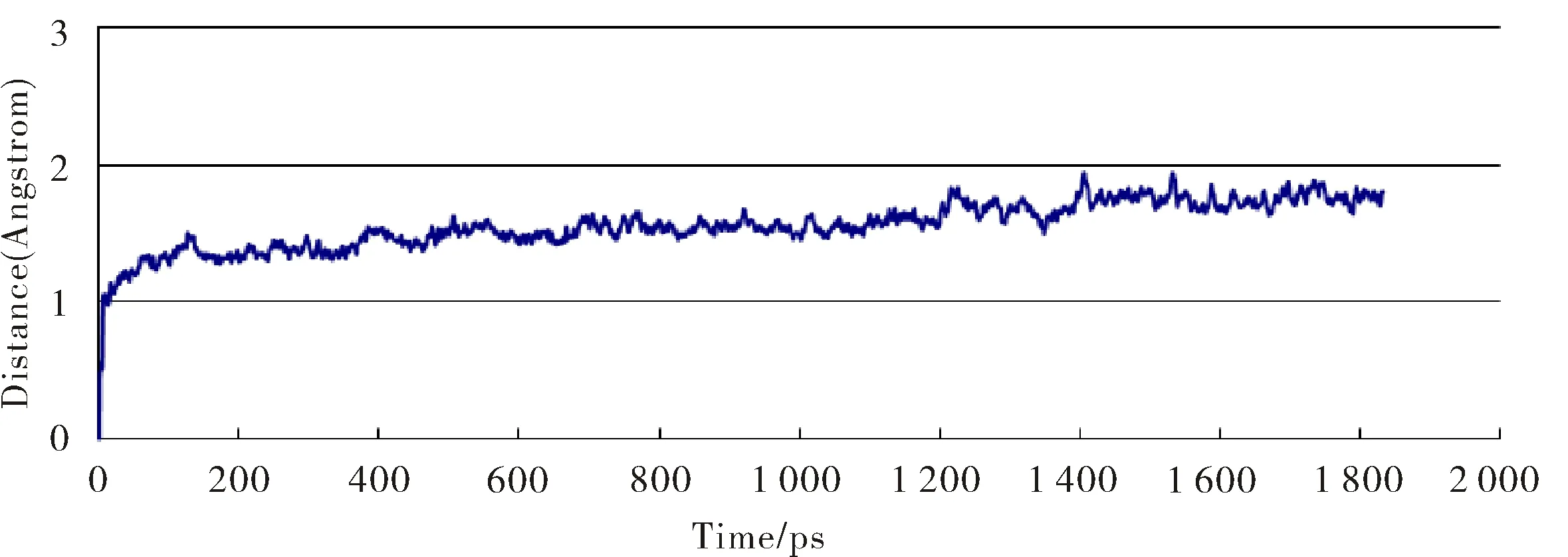
Fig.3 Plots of the molecular dynamic (MD) simulation time vs root-mean-square deviation (RMSD) of the all atoms of CaFBA-II in complex with 2g
According to the binding mode in Fig.2,the carboxyl group or the sulfonic acid group inR2position can also form hydrogen-bond with Ser268,however,our results show that theIC50values of2b(34 μmol·L-1) and2c(25 μmol·L-1) are 10-fold larger than that of2a(3.2 μmol·L-1).In order to explain this case,the solvation energies of2a,2band2cwere further calculated at wb97xd/6-31G*-smd theory level by using Gaussian 09 software package,which are of 61.45 kJ· mol-1,75.81 kJ · mol-1and 71.66 kJ· mol-1,respectively.Our current calculation results reveal that the larger salvation effect counteracts the binding of hit compounds into CaFBA-II,thus compounds2band2cexhibit weak inhibitory activities than2a,In addition,when theR2position was substituted by chlorine,the inhibitory activity of2ddecreased approximately 3-fold compared with that of2a.This is likely due to the weak interaction (halogen bond) of2dcompared to that (hydrogen bond) of2a.Taken together,the nitro-group in theR2position is favorable.In the future,the compounds with more different substituents inR3position will be designed and synthesized to further insight the structure-activity relationships (SAR) of these hit compounds.
Additionally,the compound (2g) with highest inhibitory activity against CaFBA-II were also tested against a representative of mammalian FBA-I,isozyme A from rabbit muscle.Our experimental results show that the inhibitory activity of2gagainst rabbit muscle FBA-I(Ra-FBA-I) is larger than 200 μmol·L-1,thus the inhibitory selectivity of this compound against FBA-I and FBA-II is up to 1,000.The selectivity for FBA-II validates that the compound2gexhibit pure competitive inhibition patterns.
2.2 Antifungal evaluation
All of our present compounds were assayed using a standard procedure for the inhibition of growth of cultivatedC.albicans.[17-18].The results show that compounds2eand2fexhibit moderate inhibitory activities againstC.albicanswith theMIC80values of 32 μg · mL-1.In comparison,compounds1,2a,2b,2dand2gshow more potent inhibitory activities (16 μg · mL-1) againstC.albicans.The dose responses of2dand2gin the inhibition of growth of cultivatedC.albicanswere illustrated in Fig.4.To our knownledge,several FBA-II inhibitors have been reported previously,however,few of them demonstrate inhibitory activities against microorganisms so far.This is the first report of phenylhydrazonoderivatives with antifungal effects againstC.albicans.In addition,the inhibitions of compound2gagainstC.neoforman(MIC80=16 μg · mL-1),Synechocystissp.PCC 6803 (EC50=12 μg · mL-1),Cynodondactylon L (MIC80>1000 μg · mL-1) and Arabidopsis thaliana (MIC80>1000 μg · mL-1) were tested.The results show that compound2gcould inhibit the microorganisms with FBA-II (e.g.,C.neoformanandSynechocystissp.PCC 6803),but couldn’t inhibit the organisms without FBA-II (e.g.,CynodondactylonL andArabidopsisthaliana).Thus,compound2gshows selectivity in itsinvitroactivities.

Fig.4 Dose response for compounds 2d and 2g in the inhibition of growth of cultivated C.albicans
3 Conclusion
Several new phenylhydrazono derivatives were discovered to potently inhibit CaFBA-II.The current experimental results show that compound1,2a,2fand2gexhibit potent inhibitory activities against CaFBA-II (IC50<5.0 μmol·L-1).Especially,compound2gpossessed the highest inhibitory activity (IC50=200 nmol·L-1).Furthermore,compound2gwere selected as the representative molecule,the binding mode of2gand the surrounding residues in the active site of CaFBA-II were elucidated by jointly using DOX methods and molecular dynamics (MD) simulations.The structure-activity relationships (SAR) analysis of the designed compounds can be consistently explained according to our proposed binding-modes of2gin CaFBA-II.These results further confirm and aid our understanding of the binding mode of inhibitors targeting CaFBA-II.The binding mode of2gand CaFBA-II show that the hydrophilic group in theR2position is favorable own to the hydrogen-bond between hit compound and Ser268 of CaFBA-II,but COOH,SO3H inR2position is unfavorable for their CaFBA-II inhibitory because of their larger salvation effect.It should be noticed that compound2gshows high selectivity (up to 1,000) for CaFBA-II compared with Ra-FBA-I.Notably,invitrotest results demonstrate that most of our designed compounds possess moderate inhibitory activities (MIC80=16-64 μg · mL-1) againstC.albicans.The present enzymatic and in vitro experiments suggest that the phenylhydrazono derivatives can be used as the lead compounds of novel drugs against fungal pathogens of humans in the future.
