Effect of Guanxinkang medicated serum on efferocytosis of ox-LDL-loaded macrophages by activating ERK5 in vitro
2020-06-17WANGJianruZHANGYifanMAOMeijiaoDUWentingXUHuayingLIUPing
WANG Jianru, ZHANG Yifan, MAO Meijiao, DU Wenting, XU Huaying, LIU Ping
(Department of cardiology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China)
[Abstract] Objective: To explore the potential molecular mechanism of Guanxinkang(GXK)against atherosclerosis through ERK5 activation.Methods: RAW264.7 cells were incubated with ERK5 inhibitors(ERK5-IN-1 and XMD8-92)to explore the effect of ERK5 inactivation on efferocytosis.RAW264.7 cells were subjected to ox-LDL to build a cell model with impaired efferocytosis.The cell model was treated with GXK medicated serum in the presence or absence of XMD8-92.The efferocytosis rate of macrophages was detected by flow cytometry.RT-qPCR and Western blot were used to determine the mRNA and protein expressions of ERK5 and C1qA, respectively.Results: The results showed that ERK5 inhibitors suppressed efferocytosis and ERK5 phosphorylation accompanied by decreased mRNA and protein expression of C1qA in RAW264.7 cells.Moreover, GXK medicated serum enhanced declining macrophage efferocytosis induced by ox-LDL, which was associated with activating ERK5, thereby upregulating the mRNA and protein expression of C1qA.Conclusion: Our findings suggested that ERK5 inactivation impaired efferocytosis by downregulating C1qA expression in macrophages and GXK improved efferocytosis of ox-LDL-loaded macrophages by activating ERK5 to upregulate the expression of C1qA.
[Key words] atherosclerosis; efferocytosis; macrophages; extracellular signal-regulated kinase 5(ERK5)
Atherosclerosis is a dynamic and progressive inflammatory process[1].The development of necrotic cores has long been a hallmark of the vulnerable atherosclerotic plaque that can lead to acute ischemic atherosclerotic cardiovascular events[2-3].Efferocytosis is a process through which phagocytic cells(mainly macrophages)timely and efficiently clear apoptotic cells(ACs)[4].The process is a complex multistep one and closely regulated by many signal molecules.The molecules, also called efferocytosis-related signal molecules, include Find-Me signals, Eat-Me signals, and Don’t Eat-Me signals.Each type of efferocytosis-related signal molecules, including many members, have different irreplaceable functions in efferocytosis.Defective efferocytosis can lead to the accumulation of ACs and debris in atherosclerotic lesions, which further cause secondary necrosis, expansion of plaque lesions, and eventual plaque rupture[2,5].Thus, efficient efferocytosis is extremely critical in the progression and vulnerability of atherosclerotic plaque.Efferocytotic capacity is compromised in human atherosclerotic lesions and genetically engineered atherosclerotic mice[3,6].
Extracellular signal-regulated kinase 5(ERK5), also termed mitogen-activated protein kinase 7(MAPK7)or big mitogen-activated protein kinase-1(BMK1), is the latest identified member of the MAPK family.ERK5, which is widely expressed in different tissues of mammalian, regulates many cellular functions such as cellular proliferation, migration, differentiation, apoptosis, and cytoskeletal remodeling and participates in the pathological process of some diseases[7].ERK5 deficiency in macrophage impairs efferocytosis and accelerates atherosclerotic plaque progressinvivo[8].Simvastatin is effective in treating atherosclerotic cardiovascular diseases and has been widely applied in clinical practice.It has been also used as a positive control in many animal studies to demonstrate the effectiveness of the drug to be researched.In our previous studies, simvastatin has been used as a positive control drug to interfere with ApoE-/-mouse for many times, and its effectiveness has been proved[9-10].Therefore, we chose simvastatin in this study to be positive drug.
Complement component C1q is a hexamer of trimers made up of three similar but distinct polypeptide chains, A, B, and C, which are coded by C1qA, C1qB, and C1qC gene, respectively[11-12].The function of C1q as an Eat-Me signal in efferocytosis has been appreciated for years.C1q enhances efferocytosis via three distinct pathways:(1)activating classical complement pathway[13-14],(2)serving as a bridging molecule(opsonin)between phosphatidylserine(PS)on ACs and a common receptor complex(calreticulin/CD91 complex)on efferocytes[15-16],(3)upregulating the expression of Mer tyrosine kinase(MerTK), an Eat-Me signal that is involved in efficient efferocytosis, and growth arrest-specific 6(Gas6), which participates in linking PS to the Tyro3-Axl-MerTK family of receptors on efferocytes[17-18].A study has shown that C1q enhances the efferocytotic ability of macrophage foam cellinvitro[19].C1qA depletion leads to larger and more complex atherosclerotic lesions accompanied by an accumulation of more ACs in lesions[20].The involvement of C1qA in atherosclerosis has been well established.Notably, loss of ERK5 in the macrophage reduces the expression of a broad range of efferocytosis-related signal molecules, including C1qA[8].ERK5 activation enhances the efferocytotic capacity of macrophages by upregulating the expression of C1qA[8].Thus, ERK5 activation and its roles in C1qA-related signaling in macrophages may be potential targets for suppressing atherosclerotic diseases.
Guanxinkang(GXK), a Chinese herbal formula, is composed of six different herbs, includingRadixAstragali,Trichosanthes,RadixSalviaeMiltiorrhizae,Alliummacrostemon,Pinelliatuberifera, andMotherwort.Our previous studies have shown that GXK has an action of anti-myocardial ischemia, which is connected with the opening of KATP channel[21-22]and also an anti-atherogenic effect, which occurs partly through anti-inflammation and regulating lipid metabolism[9-10].However, the potential cardiac vascular protection role of GXK has not been fully described.Serum pharmacology, advanced by Tashino in 1988, is more scientific and more suitable for the study of Chinese Traditional Medicine Compound(CTMC)than traditional pharmacology given that crude drugs are directly added into a culture system of cells or organsinvitro[23-24].In method of serum pharmacology analysis, CTMC is administered to animals orally and undergoes a series of biotransformation in animals, and then the blood of animals is then collected to separate the serum which can be added into a culture system of cells or organsinvitro[23].The above method is called serum pharmacology analysis.A lot of studies using this method have been widely published in pharmacological research on CTMC[23-24].In addition, based on a large number of previous studies on GXK medicated serum in China, the research method of serum pharmacology was selected in this study.
Macrophages, which play crucial roles in all stages of atherosclerosis, are the main efferocytes in the arterial intima and are vital for efficient efferocytosis in atherosclerotic lesions[6].Therefore, we hypothesized that GXK exerted an anti-atherogenic effect by activating ERK5 to upregulate the expression of efferocytosis-related signal molecules to enhance macrophages efferocytosis.Thisinvitrostudy was designed to investigate the effect of GXK medicated serum on impaired efferocytosis of macrophages induced by oxidative low-density lipoprotein(ox-LDL).The level of ERK5 activation and expression of C1qA in ox-LDL-loaded macrophages in the presence or absence of GXK medicated serum and ERK5 inhibitor was measured to explore potential molecular mechanisms of GXK against atherosclerosis.
1 Materials and methods
1.1 Materials
1.1.1 Animals and cells
Male Sprague-Dawley rats and female C57BL/6 mice were supplied by Shanghai Sippr-BK laboratory animal Co., Ltd.(Shanghai, China).RAW264.7 cell line was purchased from cell resource center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences(Shanghai, China).
1.1.2 Reagents and drugs instruments
ox-LDL was purchased from Shanghai Yisheng Biological Technology Co., Ltd.(Shanghai, China).Dexamethasone was bought from Sigma-Aldrich Corp.(St.Louis, MO USA).Cell TrackerTMGreen CMFDA was obtained from Molecular Probes(Life Technologies, Eugene, USA).Anti-F4/80 antibody was obtained from BD Biosciences(NJ, USA), anti-TIM-4 antibody from Santa Cruz Biotechnology Inc.(CA, USA), anti-C1qA antibody from Abcam Company(Cambridge, UK), and anti-ERK5, anti-p-ERK5, and anti-GAPDH antibodies from Cell Signaling Technology(MA, USA).Fetal bovine serum(FBS), Dulbecco’s modified Eagle’s medium(DMEM)and penicillin-streptomycin solution were purchased from Hyclone(Logan, USA).SDS-PAGE Sample Loading Buffer, BCA Protein Assay kit and BeyoECL Star were provided by Beyotime(Shanghai, China).Prime Script®RT reagent kit and SYBR premix EX Taq were obtained from Takara Biotechnology(Dalian, China).ERK5 inhibitors(ERK5-IN-1 and XMD8-92)were purchased from Selleck Chemical(Houston, TX, USA).Simvastatin was bought from Merck Sharp & Dohme Limited(Northumberland, United Kingdom).PVDF membrane was purchased from Millipore(Darmstadt, Germany).TRIzol was purchased from Invitrogen Corporation(CA, USA).Ultraviolet spectrophotometer was purchased from Eppendorf(Hamburg, Germany).ABI 7500 real-time PCR system was purchased from Applied Biosystems(Foster City, USA).The primers used were synthesized by Shanghai Sangon Biological Engineering Technology(Shanghai, China)(Table 1).We bought all herbs of GXK formula from the Chinese medicine pharmacy of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine.
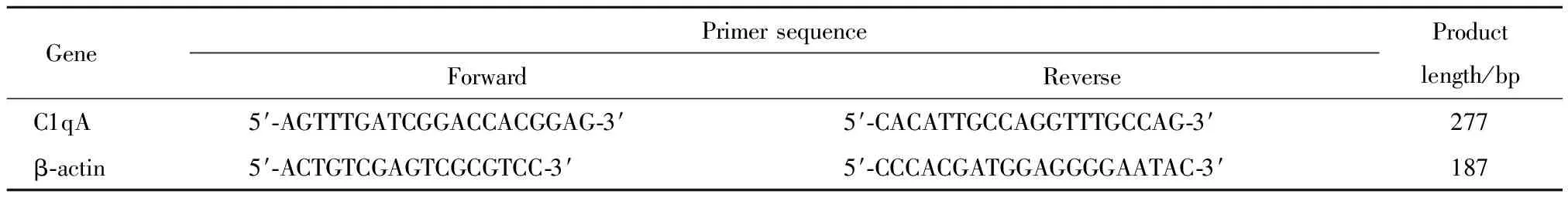
Table 1 Primer sequence of RT-qPCR
1.2 Experimental design
1.2.1 Preparation of GXK decoctions and rat medicine serum
All herbs were immersed in water for 1 h and boiled for 1 h.Subsequently, the water extraction solution was filtered.Another round of the extraction process was performed as mentioned above.The filtrate of both extraction was combined and concentrated to a final concentration of 1 g/mL(equivalent to the amount of raw herbal material), then packaged and stored at 4 ℃ before use.
After 5 days of acclimatization, SD rats were randomly divided into three groups(12 rats per group).One group was normal control group, which received intragastric administration of normal saline twice a day for 5 days, while the other two groups were treated intragastrically with GXK[9.99 g/(kg·d)], which dosage of this medicine in rats was equivalent to 6.3 times that in humans, and simvastatin[1.8 mg/(kg·d)]twice a day for 5 days respectively.At 1 h after the last administration, rat abdominal aortic blood was collected, left at room temperature for 1 h, and centrifuged at 3 000 r/min for 10 min at 4 ℃.Serum from the same group was mixed, filtered using a 0.22-μm microfiltration membrane, bathed in 56 ℃ water for 30 min, dispensed, and then stored at-80 ℃.
1.2.2 Treatment of RAW264.7 cells
RAW264.7 cells were incubated in DMEM medium with 10% FBS and 1% penicillin-streptomycin Solution in the incubator(5% CO2, 37 ℃).When cells were spread over the bottom of a Petri dish, they were digested with 0.25% trypsin and passaged.RAW264.7 cells with no more than five passages were inoculated into 6-well plate at a density of 3×105cells/well for the subsequent experiments.
1.2.3 Effect of ERK5 inhibitors on RAW264.7 cells
RAW264.7 cells were divided into blank control group, XMD8-92 group and ERK5-IN-1 group, and the latter two groups were treated with XMD8-92(5 μmol/L)and ERK5-IN-1(2.5 μmol/L), respectively.Cells were harvested for quantitative reverse transcription PCR(RT-qPCR)assay after 10 h, and western blot and efferocytosis assay after 24 h.
1.2.4 Complement component C1q effect of ox-LDL on RAW264.7 macrophage efferocytosis
RAW264.7 cells were incubated with ox-LDL at concentration of 0, 10 and 20 μg/mL, and were collected for efferocytosis assay after 24 h.
1.2.5 Effect of medicated serum on ox-LDL-loaded macrophages
RAW264.7 cells in logarithmic growth phase were inoculated into a 6-well plate at a density of 3×105cells/well.The cells were divided into control group in which cells were treated with rat serum of normal control group; ox-LDL group with rat serum of normal control group and 20 μg/mL ox-LDL; GXK group with rat serum of GXK group and 20 μg/mL ox-LDL; simvastatin group with rat serum of simvastatin group and 20 μg/mL ox-LDL; ox-LDL + XMD8-92 group with rat serum of normal control group, 20 μg/mL ox-LDL and XMD8-92; GXK + XMD8-92 group with rat serum of GXK group, 20 μg/mL ox-LDL and XMD8-92; simvastatin + XMD8-92 group with rat serum of simvastatin group, 20 μg/mL ox-LDL and XMD8-92.In groups with XMD8-92, the RAW264.7 cells were pretreated with XMD8-92(2.5 μmol/L)for 1 h.Serum concentration in each group was 10%.After 12 h, cellular RNA was extracted for RT-qPCR assay.After 24 h, the total protein was extracted for WB assay, and macrophage efferocytosis was tested.
1.3 Experimental methods
1.3.1 Efferocytosis assay
Thymocytes isolated from 4~6 week-old C57BL/6 mice were treated with 1 μmol/L dexamethasone for 6 h to undergo apoptosis and then incubated with 2 μmol/L CMFDA for 30 min at 37 ℃[8,25].The CMFDA-labeled apoptotic thymocytes were added to the RAW264.7 cells, which were stained with F4/80-PE antibody(Vapoptotic thymocytes∶Vmacrophages=10∶1).Afterwards, they were co-cultured in the incubator for 90 min to allow efferocytosis to occur.After incubation, RAW264.7 cells were washed 2~3 times with PBS to remove apoptotic thymocytes not ingested and then collected.Efferocytosis assay was performed by flow cytometry(BD Biosciences, NJ, USA).The percent of efferocytosis was calculated as the number of macrophages positive for both F4/80 and CMFDA, which was considered as efferocytosis of the apoptotic thymocytes by RAW264.7 cells, divided by the total number of F4/80-positive macrophages and then multiplied by 100.The number of F4/80+/CMFDA+cells was quantified by flow cytometry using FAC Suite software.
1.3.2 RT-qPCR
Total RNA of RAW264.7 cells was extracted from each sample using TRIzol.The concentration and purity of RNA were measured using an ultraviolet spectrophotometer.cDNA was synthesized using Prime Script®RT reagent kit according to the manufacturer’s instructions.RT-qPCR was performed in a 20 μL final volume which containing 10 μL of SYBR premix EX Taq, 0.5 μL of forward and reverse primer, 2 μL of cDNA and 7 μL of H2O.RT-qPCR was performed using an ABI 7500 real-time PCR system under the following conditions: initial denaturation at 95 ℃ for 1 min, then 40 cycles of denaturation at 95 ℃ for 3 s, annealing at 58 ℃ for 15 s and extension at 72 ℃ for 30 s.The mRNA expression level of C1qA was normalized to that of β-actin in the same sample.The relative mRNA expression level of C1qA was calculated using the 2-ΔΔCTmethod.
1.3.3 Western Blot analysis
After 24 h treatment, the supernatant of each group was removed and subsequently washed with PBS.The cells were lysed with RIPA lysis buffer, phenylmethylsulphonylfluoride(PMSF), protease inhibitor, and phosphatase inhibitors.Cell lysates were collected and centrifuged at 15 000gfor 30 min at 4 ℃.The supernatant fluids were collected and the overall protein concentrations were determined using the BCA protein assay kit according to the protocol provided by the manufacturer.The proteins(20 μg)were mixed with SDS-PAGE sample loading buffer and bathed in 95 ℃ water for 10min.Equal amount of protein was separated on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane(Millipore, Darmstadt, Germany).The membrane was subsequently blocked with 5% BSA for 1 h and probed with specific antibodies ERK5(VERK5∶Vdiluent=1∶1 000), p-ERK5(Vp-ERK5∶Vdiluent=1∶1 000), C1qA(VC1qA∶Vdiluent=1∶1 000)and GAPDH(VGAPDH∶Vdiluent=1∶500)overnight at 4 ℃.The membrane was washed six times for 5 min with TBST.The membrane was incubated with the secondary antibody on a shaker for 60 min at room temperature and washed again according to the above conditions.The blots were visualized using BeyoECL Star and exposed in a ChemiDoc MP Imaging System.Protein density was quantified using Image Lab software.The variation in protein density was expressed as fold change compared to the control in the blot.
1.4 Statistical analysis
All date were presented as(mean±SD)and analyzed using IBM SPSS statistics 21.0 software.One-way analysis of variance(ANOVA)with least significant difference(LSD)post hoc test was used when differences among three or more groups were analyzed.APvalue <0.05 was accepted as statistically significant.
2 Results
2.1 ERK5 inhibitors suppressed macrophage efferocytosis in vitro
To explore the effect of ERK5 inhibitors on efferocytosis, RAW264.7 cells were treated with XMD8-92(5 μmol/L)and ERK5-IN-1(2.5 μmol/L)for 24 h.Efferocytosis assay of RAW264.7 cells was performed.The results showed that, compared with control group, the efferocytotic ratios considerable decreased in XMD8-92 group and ERK5-IN-1 group(Fig.1), and no significant difference was observed between the two groups(Fig.1).This suggested that ERK5 inhibitors, XMD8-92 and ERK5-IN-1, could markedly suppress macrophage efferocytosis.
A: Representative flow cytometry scatter plots.B: Quantitative analysis of efferocytotic capacity of macrophages determined by flow cytometry.1)Compared with control group,P<0.05.
Fig.1 Inhibitory effect of ERK5 inhibitors on efferocytosis
2.2 ERK5 inhibitors suppressed ERK5 activation and expression of C1qA in macrophages
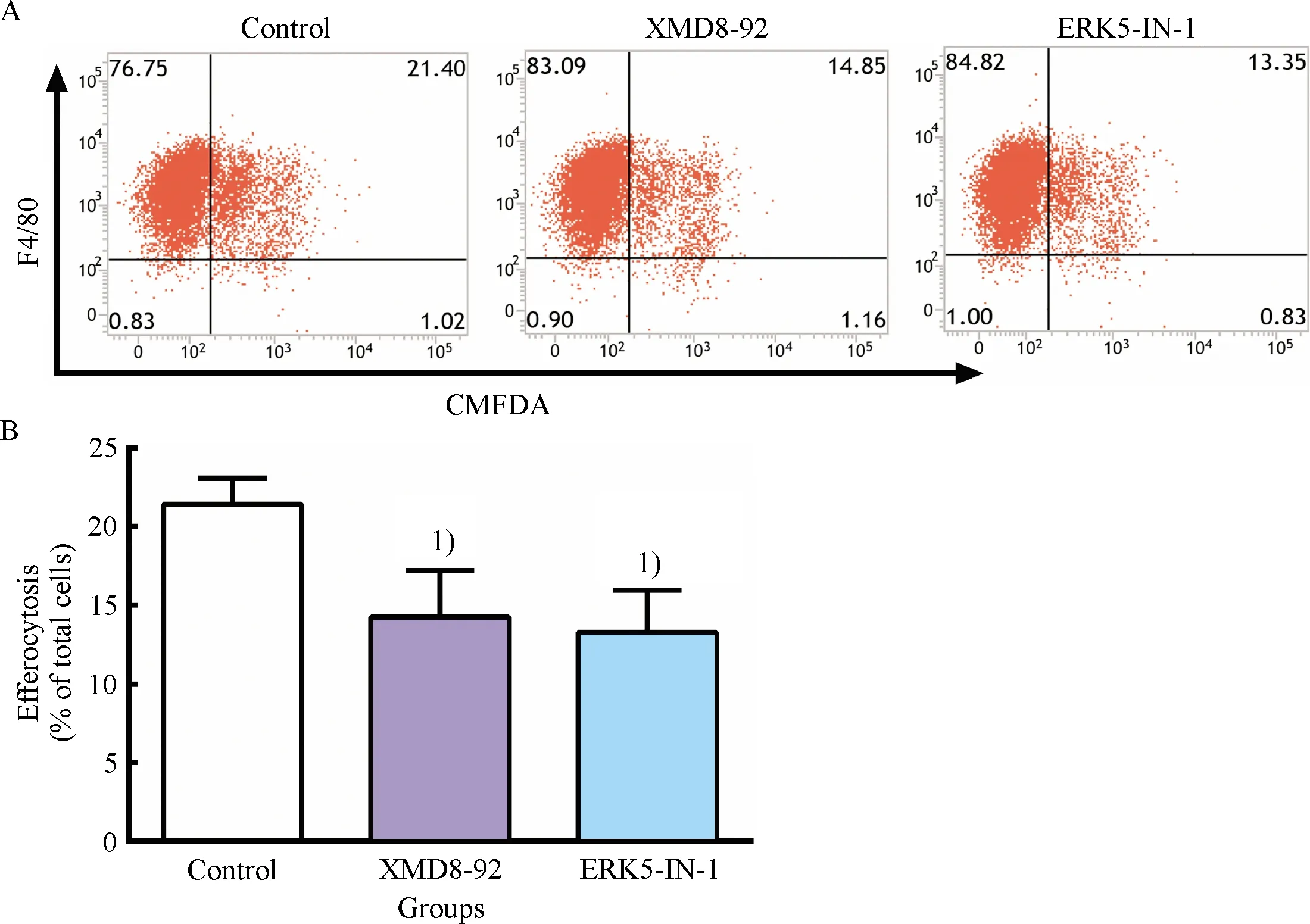
To further explore the effect of ERK5 inhibitors on ERK5 activation and the expression of efferocytosis-related signal molecule C1qA, RT-qPCR assay and western blot assay were performed.In this study, we observed significant downregulation of ERK5 phosphorylation and a concomitant reduction of C1qA mRNA and protein after treatment with XMD8-92 and ERK5-IN-1 in RAW264.7 cells(Fig.2).These data demonstrated that XMD8-92 and ERK5-IN-1 could obviously suppress ERK5 activation and expression of C1qA in macrophages.Combined with the results of inhibitory effect of ERK5 inhibitors on efferocytosis, a causal relationship between defective efferocytosis and the downregulation of C1qA could be indicated.Thus, the downregulation of C1qA may be a potential molecular mechanism for impaired efferocytosis induced by ERK5 inhibitors in macrophages.
A: Representative western blot results of p-ERK5, ERK5, GAPDH; B: Relative protein expression level of p-ERK5 were analyzed by Image Lab; C: Representative western blot results of C1qA; D: Relative protein expression level of C1qA was analyzed by Image Lab; E: The mRNA level of C1qA was determined by RT-qPCR.1)Compared with control group,P<0.01, 2)Compared with control group,P<0.05.
Fig.2 Inhibitory effect of ERK5 inhibitors on ERK5 activation and the expression of C1qA in RAW264.7 cells
2.3 Ox-LDL weakened macrophage efferocytosis
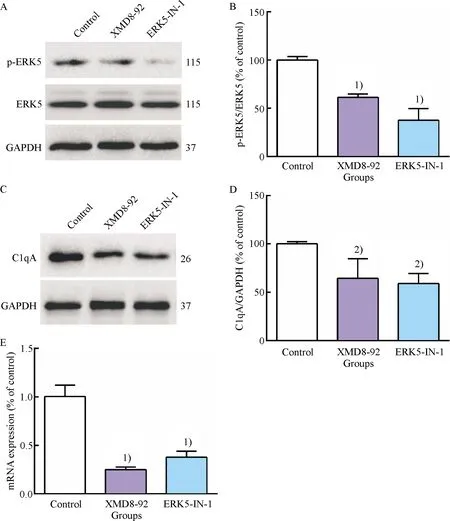
To determine the concentration of ox-LDL used to impair macrophage efferocytosis, RAW264.7 cells were incubated with different concentrations of ox-LDL(0, 10, and 20 μg/mL)for 24 h.The results showed that impaired efferocytosis of RAW264.7 cells induced by ox-LDL distinctly aggravated and cells incubated with 20 μg/mL ox-LDL had a lower efferocytotic capacity compared to that with 10 μg/mL ox-LDL(Fig.3).Thus, this was considered as the optimal condition, and RAW264.7 cells were treated with 20 μg/mL ox-LDL for 24 h in the following experiments.
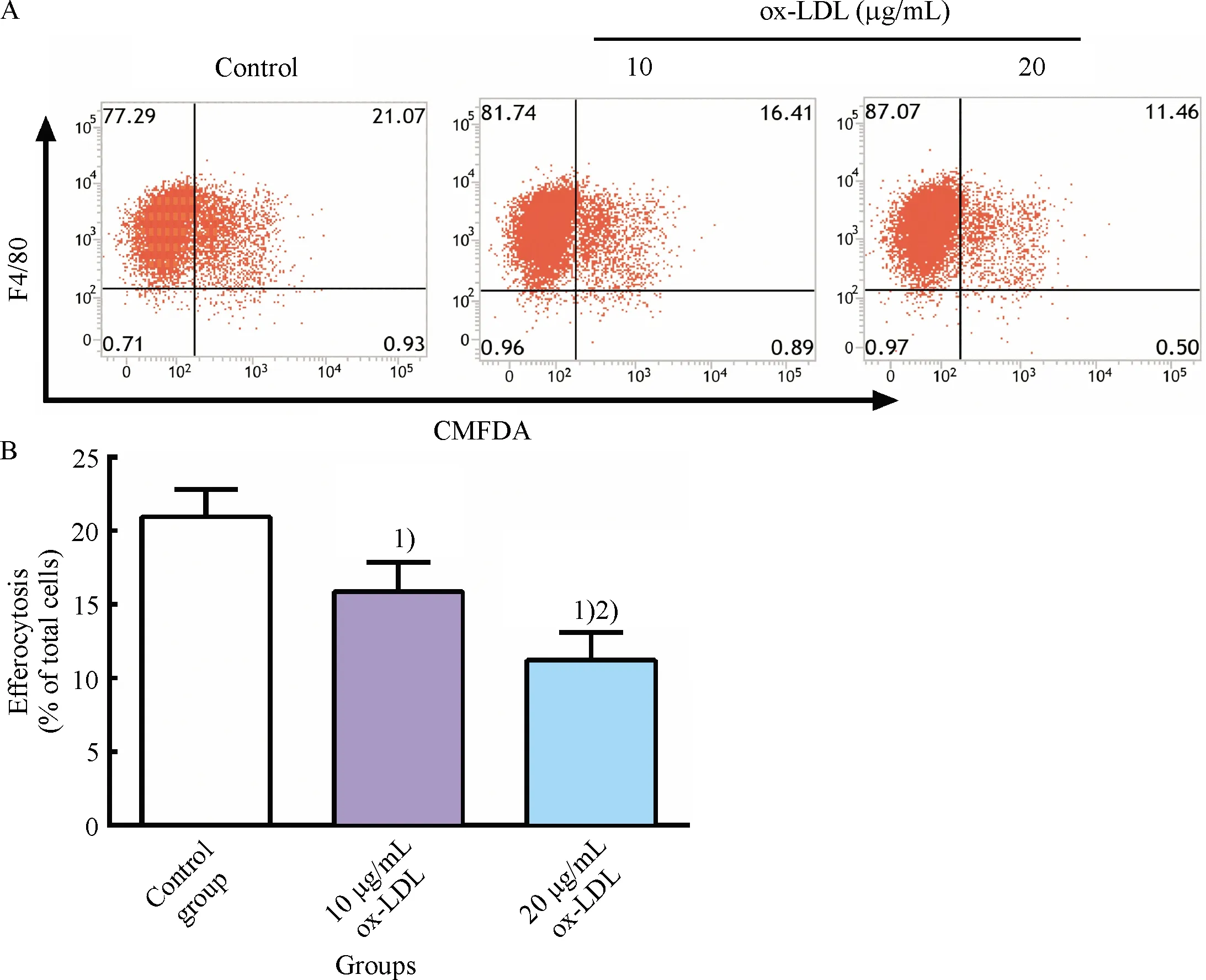
A: Representative flow cytometry scatter plots; B: Quantitative analysis of efferocytotic capacity of macrophages determined by flow cytometry.1)Compared with control group,P<0.05; 2)Compared with 10 μg/mL ox-LDL group,P<0.05.
Fig.3 Inhibitory effect of ox-LDL on RAW264.7 cells efferocytosis
2.4 GXK improved efferocytosis of ox-LDL-loaded macrophages by activating ERK5
Fig.4(a-b)showed that the degree of efferocytosis in GXK and simvastatin groups markedly increased compared to that in ox-LDL group(P<0.01), and pretreatment with XMD8-92 significantly decreased the efferocytotic degree compared to GXK and simvastatin group(P<0.05).There was difference in ERK5 phosphorylation between the control group and ox-LDL group[Fig.4(c-d)].It was found that treatment with GXK and simvastatin medicated serum markedly increased ERK5 phosphorylation compared to the ox-LDL group[Fig.4(c-d)]and ERK5 phosphorylation was downregulated compared to ox-LDL, GXK, and simvastatin groups after pretreatment with XMD8-92.The above data showed that GXK and simvastatin improved efferocytosis of ox-LDL-loaded macrophages, at least partly, by activating ERK5.
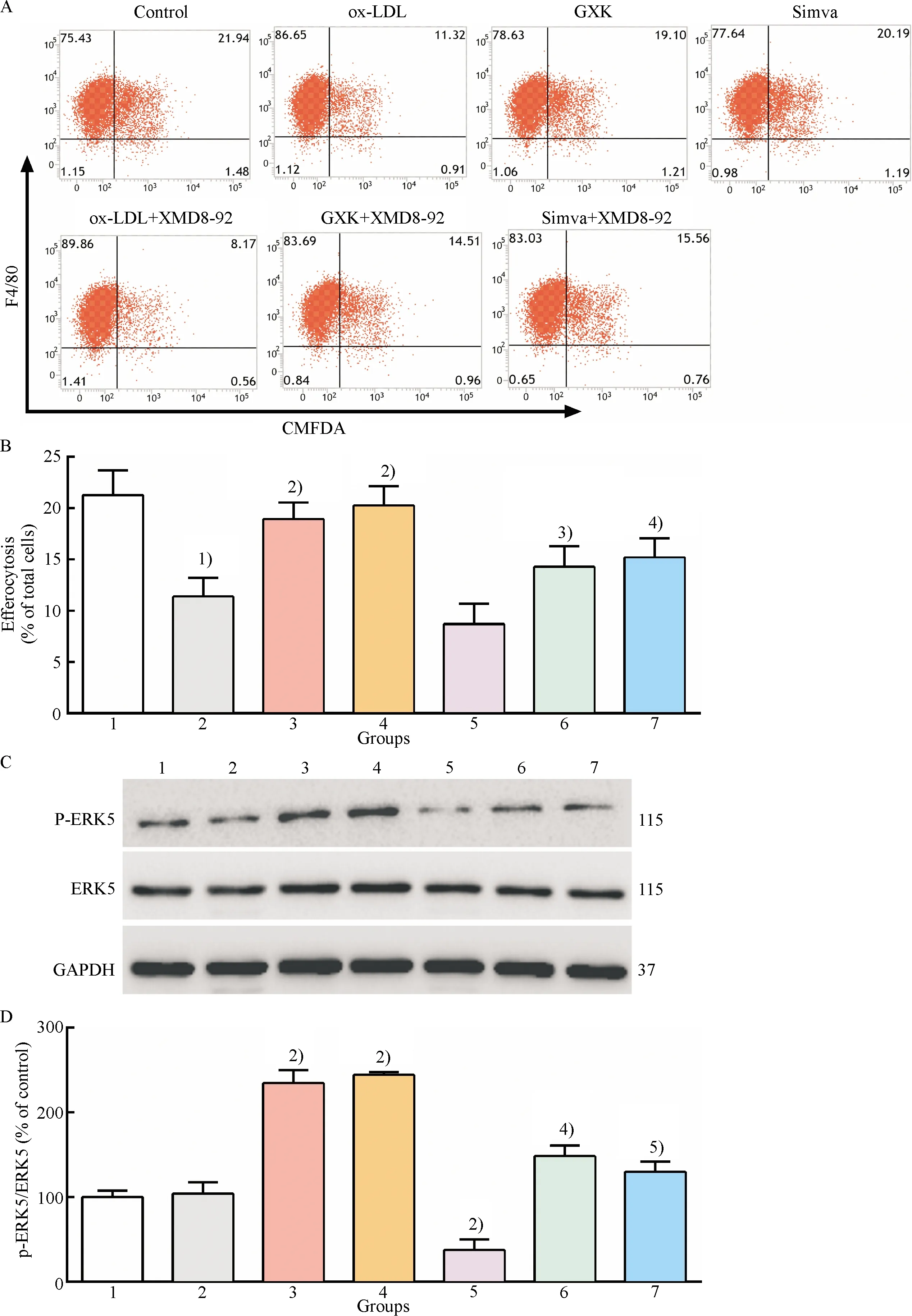
1: control group; 2: ox-LDL group; 3: ox-LDL + GXK group; 4: ox-LDL + Simva group; 5: ox-LDL + XMD8-92 group; 6: ox-LDL + GXK + XMD8-92 group; 7: ox-LDL + Simva + XMD8-92 group.A: Representative flow cytometry scatter plots; B: Quantitative analysis of efferocytotic capacity of macrophages; C: Representative western blot results of p-ERK5, ERK5, GAPDH; D: Relative protein expression levels of p-ERK5 were analyzed by Image Lab.1)Compared with control group,P<0.01; 2)Compared with ox-LDL group,P<0.01; 3)Compared with ox-LDL + GXK group,P<0.05; 4)Compared with ox-LDL + GXK group,P<0.01; 5)Compared with ox-LDL + Simva group,P<0.01.
Fig.4 Effect of GXK on the efferocytosis and ERK5 activation in ox-LDL-loaded macrophages
2.5 GXK upregulated C1qA expression via ERK5 activation in ox-LDL-loaded macrophages
The above observation demonstrated that ERK5 inactivation induced by ERK5 inhibitors could inhibit the expression of C1qA in macrophages.GXK could improve efferocytosis of ox-LDL-loaded macrophages by activating ERK5.Therefore, we propose that the mechanism of GXK promoting efferocytosis might be due to the upregulation of C1qA expression by activating ERK5.Thus, XMD8-92, an ERK5 inhibitor, was used to treat ox-LDL-loaded macrophages.As shown in Fig.5, ox-LDL downregulated the expression of C1qA, and treatement of GXK and simvastatin medicated serum significantly restored the downregulated mRNA and protein expression level.However, the effect was found to be significantly suppressed by pretreatment with XMD8-92(Fig.5).The results suggested that GXK and simvastatin were, at least in part, dependent on activation of ERK5 to upregulate the expression of C1qA.
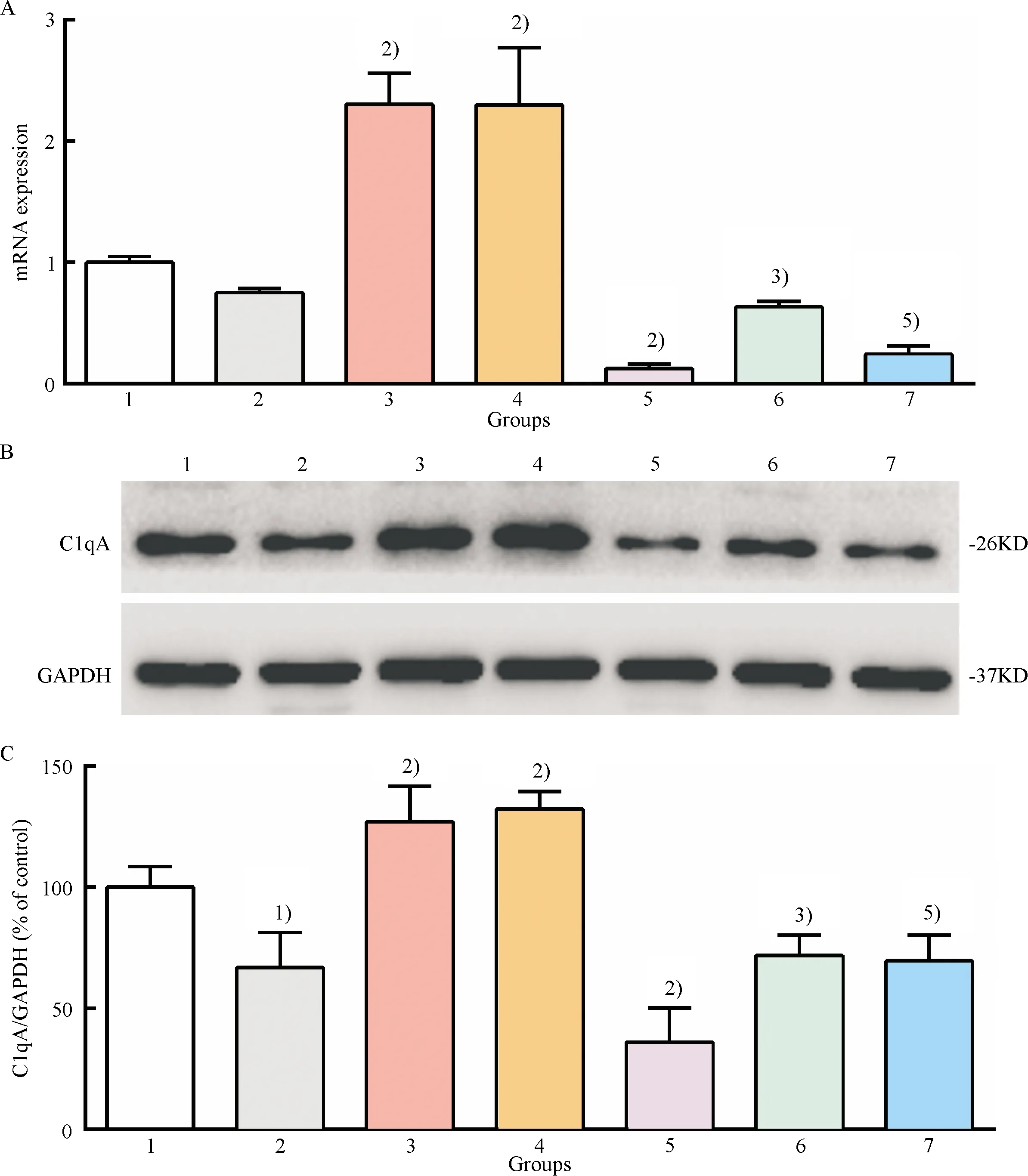
1: control group; 2: ox-LDL group; 3: ox-LDL + GXK group; 4: ox-LDL + Simva group; 5: ox-LDL + XMD8-92 group; 6: ox-LDL + GXK + XMD8-92 group; 7: ox-LDL + Simva + XMD8-92 group.A: The mRNA level of C1qA was determined by quantitative RT-qPCR; B: Representative western blot results of C1qA; C: Relative protein expression level of C1qA was analyzed by Image Lab.1)Compared with control group,P<0.01; 2)Compared with ox-LDL group,P<0.01; 3)Compared with ox-LDL + GXK group,P<0.01; 4)Compared with ox-LDL + Simva group,P<0.01.
Fig.5 Effect of GXK on the mRNA and protein expression of C1qA in ox-LDL-loaded macrophages
3 Discussion
ERK5 inhibitors(XMD8-92 and ERK5-IN-1)are effective tools for exploring the multifunctional biological characteristics of ERK5[26-27].In many studies[27-31], ERK5 kinase inactivation caused by XMD8-92 has been used in parallel to knockdown ERK5 to verify the role of ERK5.In this study, using the treatment with XMD8-92 and ERK5-IN-1, we found that efferocytotic capacity and ERK5 activation in macrophages decreased with downregulated expression of C1qA.The results were highly consistent with a study which reported knockout of ERK5 by Cre-lox recombination technology led to deficient efferocytosis and decreased expression of C1qA in macrophages[8].Thus, we could conclude that C1qA was a downstream target of ERK5 in macrophage efferocytosis.
Lipid oxidation theory proposed the pathogenicity of ox-LDL in the pathological process of AS[32].Clinical studies showed that the level of serum ox-LDL was not only correlated with atherosclerotic cardiovascular disease(ASCVD), but also predicted the future progress of subclinical AS[33-34].Many experimental data from cell and animal models showed the pathogenic role of ox-LDL in AS.Ox-LDL,as a causal risk factor of AS, can damage macrophages efferocytosis through multiple pathways.On the one hand, ox-LDL having similar epitopes to ACs[35]can inhibit efferocytosis via competition with ACs for binding to Eat-Me signal receptor scavenger receptor class B type I(SR-BI)[36]and opsonin milk fat globule epidermal growth factor 8(MFG-E8)[37].On the other hand, it also directly inhibits the expression of efferocytosis-related molecule MerTK[38].The apoptosis of RAW264.7 cells incubated with 25 μg/mL ox-LDL for 24 h has been confirmed using the MTT assay and flow cytometry[39].Therefore, for building cell model with impaired efferocytosis, ox-LDL at concentration below 20 μg/mL was selected in the present study.The results showed that ox-LDL suppressed macrophage efferocytosis in a dose-dependent manner and the effect of 20 μg/mL ox-LDL was the most effective.Moreover, we also found that ox-LDL downregulated the expression of C1qA in macrophages, which could be a mechanism of impaired efferocytosis induced by ox-LDL.
The factors and the pathways involved in efferocytosis can be used as novel therapeutic targets to lower the risk of AS and its clinical complications[1].ERK5 activation of macrophages inhibits AS by increasing the expression of efferocytosis-related molecules to promote efferocytosis[8].Therefore, activating ERK5 in macrophages may be a good drug target.The proefferocytic action of simvastatin has been found in alveolar macrophages[40-41].Furthermore, simvastatin can trigger ERK5 phosphorylation in endothelial cells[42].Thus, simvastatin was selected as a positive control drug.In the present study, we found that simvastatin improved efferocytosis of ox-LDL-loaded macrophages by activating ERK5 to increase the expression of its downstream target C1qA.
Traditional Chinese medicines(TCM), mainly Chinese herbal medicines, have been widely used to treat atherosclerotic diseases for more than 2,000 years, getting more and more attention[43].GXK, which is an empirical formula, has an obvious role in replenishing qi, activating blood, and eliminating phlegm based on TCM.In our experiments, GXK medicated serum enhanced efferocytotic ability of ox-LDL-loaded macrophages, and ERK5 inhibitor(XMD8-92)suppressed the promoted action of GXK.When, simultaneously treated with ERK5 inhibitor, the auxo-action of GXK medicated serum on ERK5 phosphorylation and the mRNA and protein expression of C1qA were also inhibited, which proved that GXK medicated serum increased the expression of C1qA by activating ERK5.The results showed that, similarly to simvastatin, GXK had an effect on efferocytosis of ox-LDL-loaded macrophages.In addition, pro-inflammatory conditions could impair efferocytosis by mediating the expression of efferocytosis-related molecules[1-2].Therefore, we speculated that the anti-inflammatory action of GXK might be also a possible mechanism of the pro-efferocytic effect.
4 Conclusion
In conclusion, we found that ERK5 inhibitors suppressed efferocytosis and ERK5 activation accompanied by decreased expression of C1qA in macrophages.Our results further illustrated that GXK and simvastatin medicated serum improved efferocytosis through ERK5 activation to upregulate the expression of C1qA in ox-LDL-loaded macrophages.Moreover, ERK5 activation in macrophage efferocytosis may be a potential therapeutic strategy for restraining the progression of atherosclerosis.Our findings described a novel anti-atherosclerosis mechanism of GXK.Nevertheless, the effect of GXK is still needed to be further verified in a rodent model of atherosclerosis.
