Molecular investigation on the compatibility of epoxy resin with liquid oxygen
2020-03-27MingfRenLeiWngTongLiBingqingWei
Mingf Ren, Lei Wng, Tong Li, Bingqing Wei*
a Department of Engineering Mechanics, Dalian University of Technology, Dalian 116024, China
b Department of Mechanical Engineering, University of Delaware, Newark 19711, NJ, USA
Keywords:Molecular dynamics ReaxFF Epoxy resin Oxygen compatibility
ABSTRACT Conventional fiber reinforced plastics (FRPs) have compatibility issues with solid oxygen while used as a fuel tank, which might cause combustion and explosion. To study the compatibility of different epoxy resins with liquid oxygen, molecular dynamics was used to simulate the phase changes of cross-linked epoxy resins under the impact of solid oxygen. Three curing resin systems,which are bisphenol A epoxy resin (DGEBA), bisphenol F epoxy resin (DGEBF), and tetrahydrophthalate diglycidyl ester (epoxy resin 711), are modeled to investigate the rational material system for the application of fuel tanks in launching vehicles. The simulation results show that the order of solid oxygen compatibility of these epoxy resins is DGEBA > DGEBF > epoxy resin 711 at the same density of crosslinking. The selection of curing agent also has an impact on the compatibility, with the same epoxy, diaminodiphenyl methane (DDM) has more advanced performance comparing to diaminodiphenyl sulfone (DDS).
Improvement of the carrying capacity of spacecraft and deep reduction of the launch cost are the main challenges in space exploration. Most of the propeller fuel tanks are made of metals that take a large proportion of the total structural weight, which is not ideal for the development of reusable spacecraft [1]. Compared to traditional aluminum alloy tanks, fiber reinforced plastics (FRPs) tanks can reduce the weight by 20%-30% [2],which is promising for the future development of launching vehicles [3]. However, for a liquid oxygen tank that is made of FRPs, the direct contact between the matrix material and liquid oxygen will not only affect its mechanical properties [4, 5], but also easily explode under impact, friction and static load conditions.
The explosive failure of FRPs tanks is believed to be caused by the strong redox between resin materials and the liquid oxygen, which strongly depends on the molecular content of matrix material of the FRPs. In the development of mature matrix materials for the FRPs tanks, laboratory trial-and-error is the most commonly adopted strategy to validate the performance of new materials. To assist the material evaluation, NASA researchers have developed a series of testing methods [6], such as liquid oxygen impact sensitivity test, friction test, vibration test, and electrostatic test. Wang et al. [7] considered that the compatibility of polymer with liquid oxygen is consistent with the difficulty of thermal oxygen reaction that could be characterized by measuring some parameters of oxidation resistance and combustion performance, such as oxidation weight gain, weight loss and flash point, etc. In addition to using thermogravimetric analysis to measure the oxidation resistance of modified polymer materials, Wu et al. [8] also measured its chemical composition by Xray photoelectron spectroscopy to investigate the oxidation reactions or oxidative degree. However, the cost of the trial-and-error strategy is high due to the cost of material fabrication. Theoretically, a large scale screening of the molecular contents of the FRPs can benefit the development of advanced fuel tanks by providing more options for high-performance polymers.
Molecular dynamics (MD) [9, 10] is a computer simulation method developed in recent years and has been widely used in many fields such as chemistry [11] and material science [9, 12,13]. With ReaxFF force fields, Zeng et al. [14] simulated the degradation of Polyvinylidene fluoride (PVDF), neat polyhedral oligomeric silsesquioxanes (FP-POSS), and their composites under atomic oxygen impact. They evaluated the stability of these materials by means of temperature evolution, mass loss, and erosion yields, which provided valuable information for the design of protective materials. Rahnamoun and van Duin [15]simulated the degradation of Kapton, POSS polyimide, amorphous silicon, and Teflon under atomic oxygen impact, and compared the effects of different material contents on their performances. However, the resins of composite liquid oxygen tank are thermosetting resins with a complex three dimensional (3D)network structure, and the oxygen-compatibility simulation of a thermosetting resin is rarely studied.
Epoxy resins are widely used in various fields due to their good material properties and price advantages. Among them, bisphenol epoxy resin is a widely used high-performance resin[16], while epoxy resin 711 has good low-temperature resistance.Therefore, in this study, bisphenol A epoxy resin (DGEBA), bisphenol F epoxy resin (DGEBF), and epoxy resin 711 were selected, at the same time, two heat-resistant curing agents diaminodiphenyl sulfone (DDS) and diaminodiphenyl methane(DDM) were selected. These materials constitute the main part of the curing system. In addition, the problems of solid oxygen with polymer materials are more prominent when compared with liquid oxygen, so solid oxygen is selected for impact.
In this paper, the reaction process of solid oxygen with epoxy resin under impact-loading was simulated by MD. First, the compatibility of epoxy resin and curing agent with oxygen, two kinds of epoxy resin systems, i.e., the same curing agent with different epoxy resin systems, the same epoxy resin with different curing agent systems, were constructed. Second, the temperature evolution and mass loss of materials were analyzed, and the compatibility of materials with oxygen was evaluated by these two indexes and compared with existing experiments.
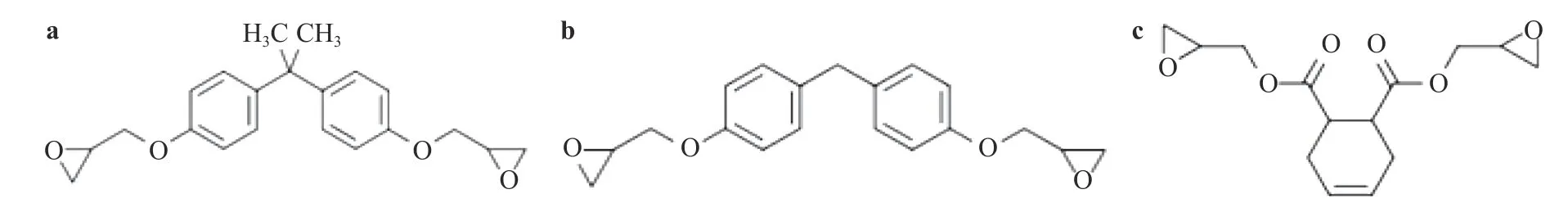
Fig. 1. Epoxy resin monomer. a DGEBA, b DGEBF, and c epoxy resin 711.
The ReaxFF force fields can effectively combine theoretical calculations of quantum chemistry with classical force fields simulations, and the MD based on this does not require a predefined chemical reaction path for the simulation of chemical reactions used in molecular systems [17]. The interaction between atoms is defined as a function, which is divided into key energy and bond angle energy by complex function calculation.Except for non-bond interactions, each part of the intramolecular energy is expressed by a bond level. The system energy is partitioned into the several partial energy contributions as
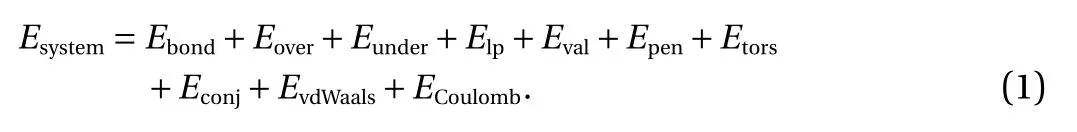
The development of the ReaxFF force fields lays a foundation for simulating chemical reactions in large systems. It can be used in large-scale system research and widely used in the combustion of alkanes [18, 19] and pyrolysis of polymers [20, 21].
The crosslinking reaction between the epoxy resin and curing agent can produce a complex 3D network structure. The Schematic diagram of epoxy resin monomer and curing agent molecules are shown in Figs. 1 and 2, respectively.
To make the constructed models closer to the real state, the crosslinking reaction between the epoxy resin and curing agent was simulated using the algorithm that comes with Materials Studio software packages from Accelrys Inc.
Each DDS or DDM curing agent molecule has four reaction nodes, while DGEBA, DGEBF, and epoxy resin 711 monomer molecules have two reaction nodes so that one curing agent molecule can react at the most with two epoxy resins. In order to allow the crosslinking reaction to proceed sufficiently, the ratio of epoxy resin molecules to curing agent molecules added to the model is 2:1. The chemical reaction between the two molecules is shown in Fig. 3.
There are currently six known forms of solid oxygen; here, we adopt α-phase, which is closer to the actual working condition.The α-phase structure is stable at atmospheric pressure and the temperature below 24 K, its crystal structure is shown in Fig. 4.
In order to analyze the compatibility of different epoxy resins with oxygen, the impact models of three curing systems, EBADDS(Fig. 5), EBFDDS (Fig. 6), and E711DDS (Fig. 7), were constructed.Among them, the epoxy resins in EBADDS, EBFDDS, and E711DDS are DGEBA, DGEBF and epoxy resin 711, respectively, and the curing agents are DDS.
The three curing systems mentioned above are all crosslinked by 60 epoxy monomers and 30 DDS curing agents, and the models with a crosslink density of 88.3% were chosen. After equilibrium, the densities of EBADDS, EBFDDS, and E711DDS are 1.229 g/cm3, 1.274 g/cm3, and 1.286 g/cm3, respectively. The reference density of epoxy resin (25 °C) is 1.1-1.25 g/cm3, and the density of the curing system is substantially the same.
To analyze the compatibility of different curing agents with oxygen, the impact models of two curing systems, EBFDDM(Fig. 8) and EBFDDS (Fig. 9) were constructed. The curing agents used in the above curing systems are DDM and DDS, respectively, and DGEBF is used for the resins.
The two curing systems mentioned above are all crosslinked by 60 DGEBF monomers and 30 curing agents, and at last, the models with a crosslink density of 89.2% were chosen. After equilibrium, the densities of EBFDDM and EBFDDS are 1.132 g/cm3and 1.264 g/cm3, respectively, and this also corresponds to the reference density of epoxy resin.
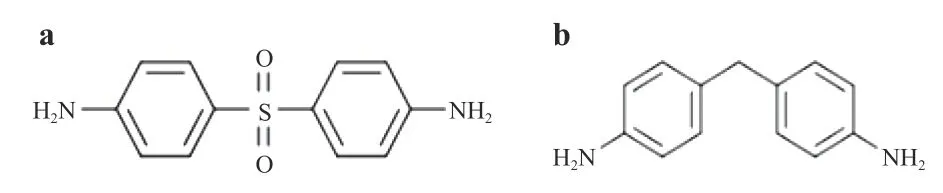
Fig. 2. Curing agent molecule. a DDS and b DDM.
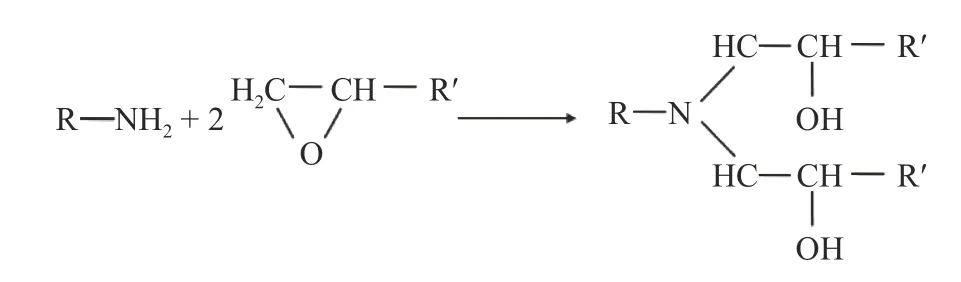
Fig. 3. Reaction process between the epoxy resin and curing agent.
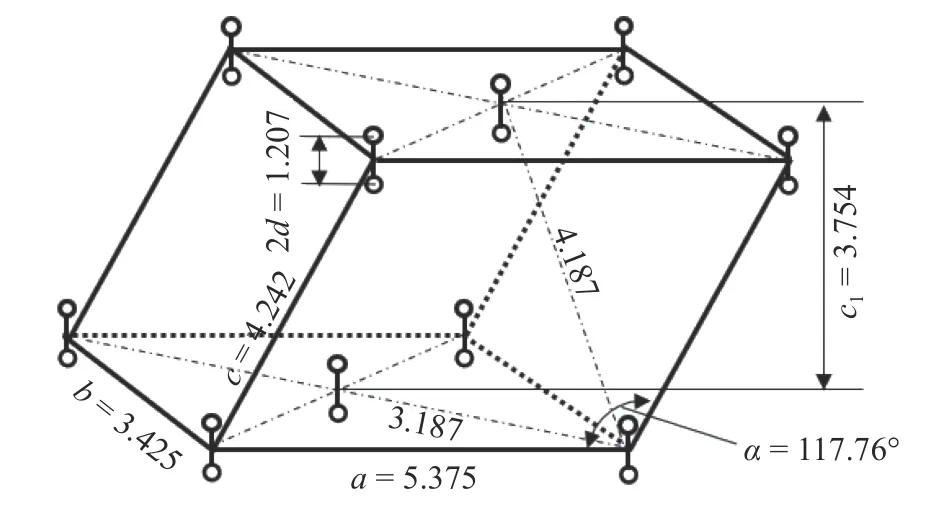
Fig. 4. Crystal structure of α-phase oxygen.
After the initial crosslinked model is constructed, a series of steps are implemented to obtain a more reasonable model, as shown in Fig. 10.
In the first step, a thermal annealing process is performed to further release the strain energy of the system. The specific settings are as follows: heat the system from 300 K to 500 K, then cool down the system from 500 K to 300 K, do the cycle three times where each cycle lasts 100 ps (1 ps = 1×10-12s), a total of 300 ps. In the second step, a canonical ensemble (NVT) dynamic equilibration runs 500000 steps (a total of 500 ps) at 300 K, followed by a isothermal-isobaric ensemble (NPT) dynamic equilibration runs 500000 steps (a total of 500 ps) at 1 atmosphere(1.01×105Pa) and 300 K. Then, an NPT dynamic equilibration runs 500000 steps (a total of 500 ps) at 1 atmosphere and 10 K,and this temperature is similar to the temperature at which liquid oxygen converts to solid oxygen. The time step for all the steps is 1 fs (1 fs = 1×10-15s).
After that, an NVT dynamic equilibration was running for 40000 steps (a total of 40 ps) at 10 K, then the materials are ready for the simulations. In the last step, the solid oxygen was placed on the upper surface of the material at the height of about 3 Å(1 Å = 1×10-10m). The x and y directions of the model are set as periodic boundary conditions, and the z-direction is set to a free boundary condition. To avoid the entire model flying away, the lower surface of the material is fixed in the range of about 10 Å during the simulation. The simulation of the model was performed in microcanonical ensemble (NVE). A downward velocity of 0.1was applied to solid oxygen, and the subsequent reaction within 20 ps was observed, the time step is 0.1 fs.
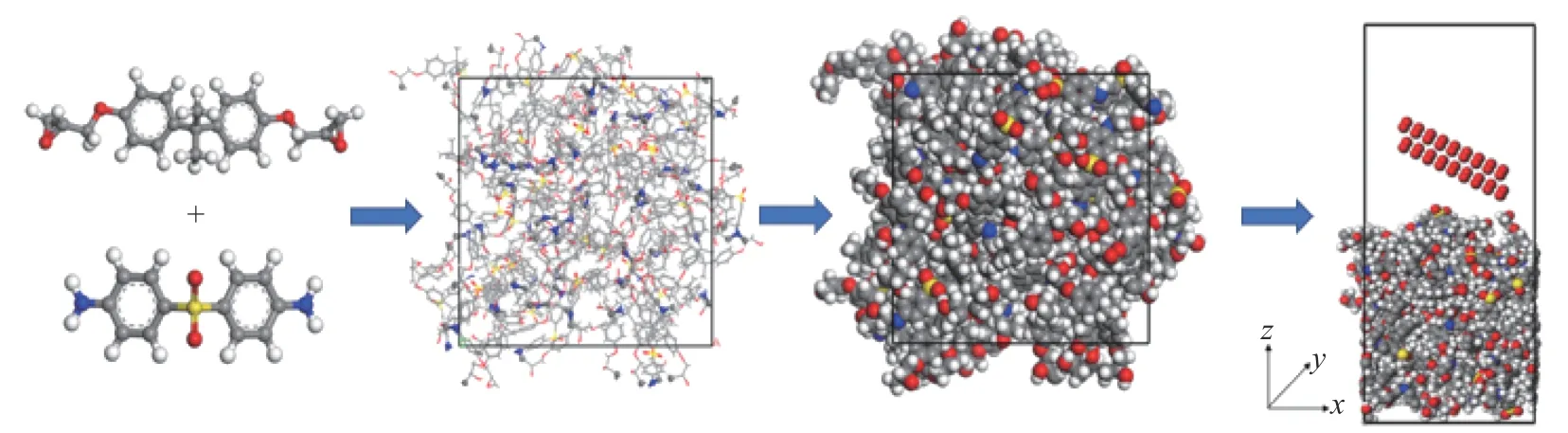
Fig. 5. Solid oxygen impact model of the curing system composed of DGEBA and DDS. There are 4046 atoms in the model, and 3810 atoms in the preparation, the white atoms represent H, the gray atoms represent C, the blue atoms represent N, the red atoms represent O, and the yellow atoms represent S.
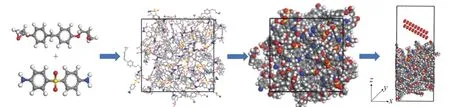
Fig. 6. Solid oxygen impact model of the curing system composed of DGEBF and DDS. There are 3686 atoms in the model, and 3450 atoms in the preparation, the white atoms represent H, the gray atoms represent C, the blue atoms represent N, the red atoms represent O, and the yellow atoms represent S.
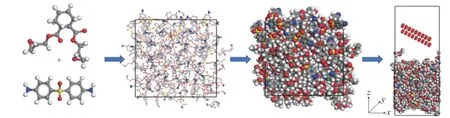
Fig. 7. Solid oxygen impact model of the curing system composed of epoxy resin 711 and DDS. There are 3386 atoms in the model, and 3150 atoms in the preparation, the white atoms represent H, the gray atoms represent C, the blue atoms represent N, the red atoms represent O, and the yellow atoms represent S.
Fig. 8. Solid oxygen impact model of the curing system composed of DGEBA and DDM. There are 3686 atoms in the model, and 3450 atoms in the preparation, the white atoms represent H, the gray atoms represent C, the blue atoms represent N, and the red atoms represent O.
Fig. 9. Solid oxygen impact model of the curing system composed of DGEBA and DDS. There are 3686 atoms in the model, and 3450 atoms in the preparation, the white atoms represent H, the gray atoms represent C, the blue atoms represent N, the red atoms represent O, and the yellow atoms represent S.
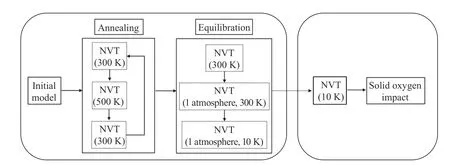
Fig. 10. Flow chart of the simulation process
All models mentioned above are constructed and equilibrated in Materials Studio (2017 version, Accelrys Inc.), and all simulations are performed in the LAMMPS molecular dynamics package (stable version).
Two models containing different epoxy resins and curing agents were constructed to verify the feasibility of using MD for qualitative analysis compatibility, and the simulation of solid oxygen impact was carried out. The compatibility was judged by the established index and compared with the existing test results of liquid oxygen impact sensitivity.
Temperature evolution:Figure 11 shows the temperature evolution of the curing systems with three different epoxy resins during solid oxygen impact. As can be seen from the figure,when oxygen impinges on the surface of the curing system, the material will have a larger temperature rise. With the development of time, the reaction will reach dynamic equilibrium and the system temperature will gradually stabilize.
Generally speaking, the temperature rise of E711DDS is the highest, followed by EBFDDS and finally EBADDS, which is determined by the molecular structure of the three epoxy resins.Compared with the molecular structure of the epoxy resin 711,both DGEBA and DGEBF contain a large amount of a benzene ring, and since the benzene ring is a stable heat-resistant group,the thermal stability of DGEBA and DGEBF is good. The epoxy resin 711 not only does not have any heat-resistant groups, but contains many carbonyl groups with poor heat resistance, therefore, although the 711 resin has good low temperature resistance, it has poor thermal stability. In addition, compared with DGEBA, the hydrogen atoms (H) linked by carbon atoms between the two benzene rings in the molecular structure replace methyl (CH3), so the thermal stability of DGEBF is lower than that of DGEBA.
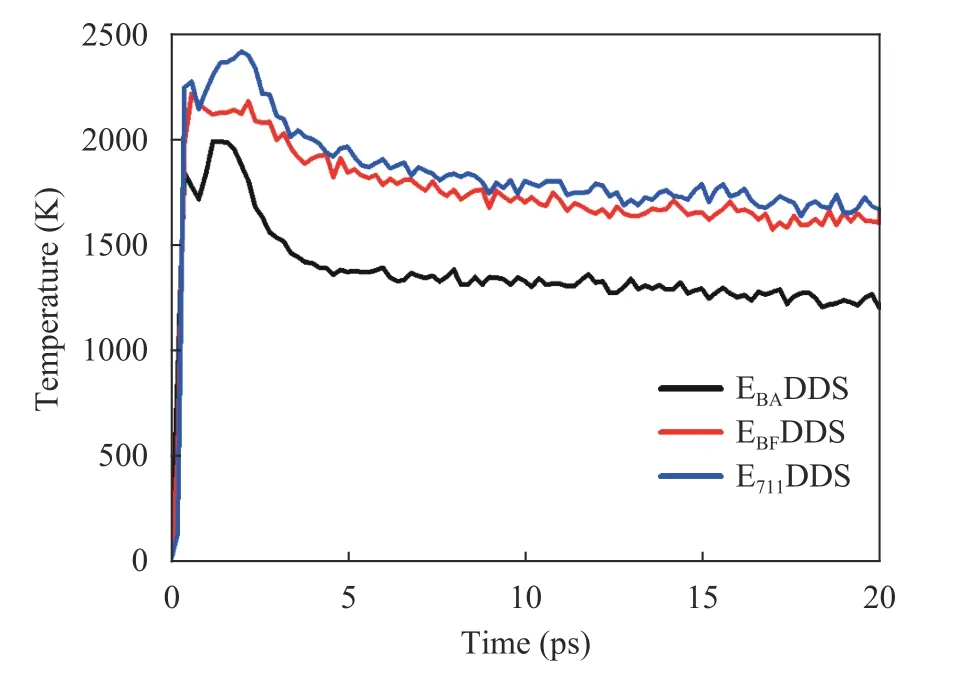
Fig. 11. Temperature evolution of the curing systems with three epoxy resins during solid oxygen impact.
Mass loss:The total remaining mass of curing systems with three different epoxy resins during solid oxygen impact is shown in Fig. 12a. To more intuitively observe the mass loss of various resin systems, we normalized the remaining relative molecular mass, as shown in Fig. 12b. Due to the molecular structure of epoxy resin 711, the small molecular structure is more easily separated from the surface of the polymer under the same impact energy, so its mass loss is more serious. In EBADDS and EBF-DDS, there is a limited difference in the mass loss in the early stage of the reaction. Although the mass loss of EBADDS is greater than that of EBFDDS in the later stage of the reaction, the mass loss of EBFDDS begins to increase and gradually exceeds that of EBADDS.
Snapshot:The stability of the curing systems can also be reflected by snapshots. Figure 13 shows a snapshot of the models(without unreacted oxygen molecules) in the middle (10 ps) and late (20 ps) stages of the reaction. It can be seen from the snapshots that over time, the macromolecular structure reacts with oxygen to produce a series of small molecule structure under the impact of solid oxygen. Chemical reactions become more and more intense and eventually reach dynamic equilibrium, and the mass loss of epoxy resin 711 is the most serious.
From the temperature evolution and mass loss of the three curing systems, it can be seen that the order of thermal stability is EBADDS, followed by EBFDDS, and finally E711DDS. Because the incompatibility between resin and oxygen is essentially an oxidative combustion reaction [7], its thermal stability is positively correlated with the compatibility of liquid oxygen, so the order of compatibility between resin and oxygen is EBADDS, followed by EBFDDS, and finally E711DDS.
To verify the simulation results, it is compared with the existing experiments (Table 1). In this experiment, the impact response sensitivity (IRS) was used to characterize the compatibility of the three curing systems. The test results showed that the order of liquid oxygen compatibility was EBADDS > EBFDDS >E711DDS, which is the same as the simulation results of MD.Therefore, the feasibility of using MD simulation to qualitatively analyze the compatibility of materials with oxygen has been verified.
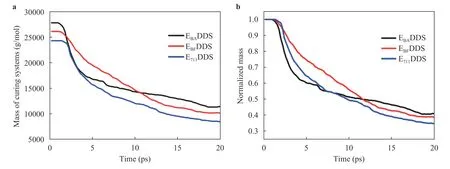
Fig. 12. Total remaining mass of curing systems with three epoxy resins during solid oxygen impact. a Absolute remaining mass, b normalized remaining mass.
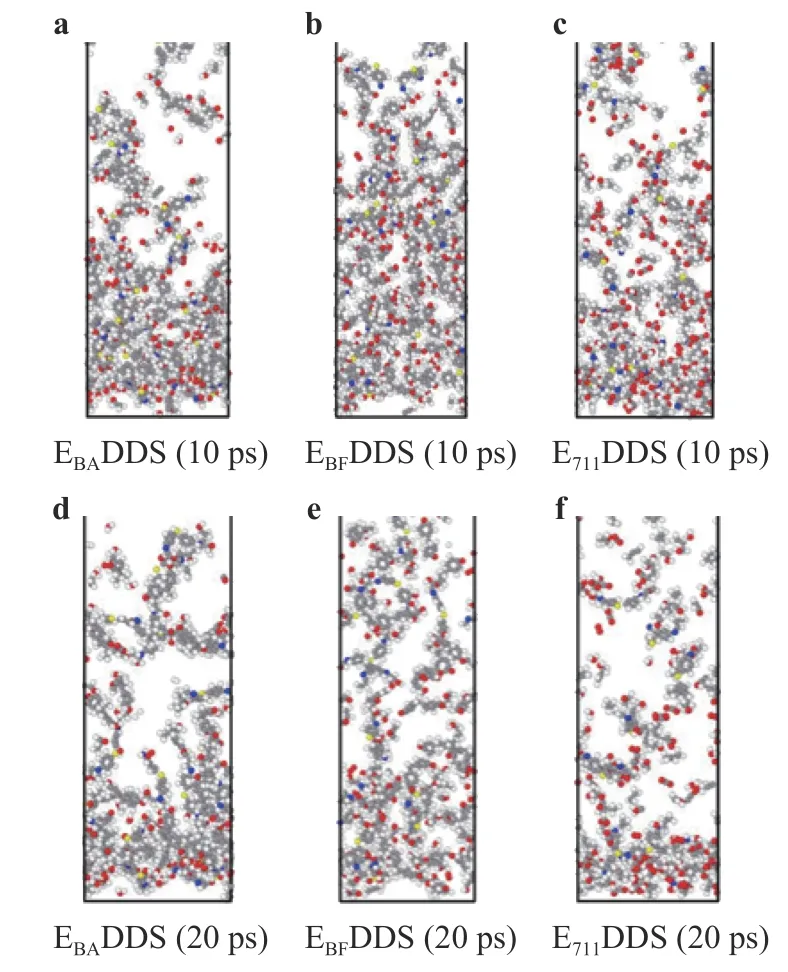
Fig. 13. Reaction snapshot of the curing systems with three different epoxy resins during solid oxygen impact (without unreacted oxygen molecules).
To further verify the rationality of the method proposed in this paper, the reaction of curing systems with different curing agents under the impact of solid oxygen was analyzed. The temperature evolution and mass loss were also discussed.
Temperature evolution:Figure 14 shows the temperature evolution of the curing systems with two different curing agents during solid oxygen impact. Compared with EBFDDM, the temperature rise of EBFDDS is more significant, which also shows that EBFDDM has better thermal stability.
Mass loss:The total remaining mass of the curing systems with two different curing agents during solid oxygen impact is shown in Fig. 15a. To more intuitively observe the mass loss of various curing systems, the remaining relative molecular mass was also normalized, as shown in Fig. 15b. When the reaction reaches dynamic equilibrium, the remaining masses are not much different, but the residual quality of EBFDDM is slightly higher than that of EBFDDS.
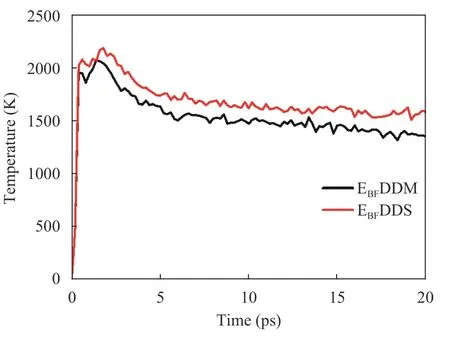
Fig. 14. Temperature evolution of a curing epoxy system with two different curing agents during solid oxygen impact.
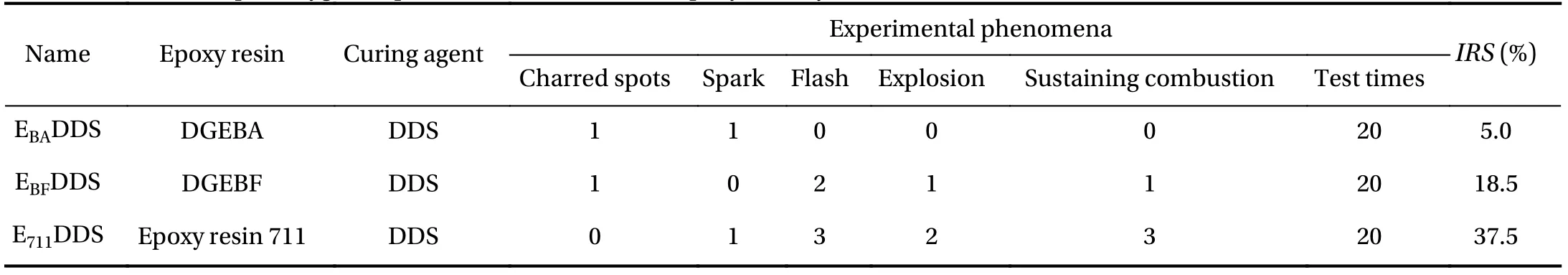
Table 1 Results of liquid oxygen impact test of three different epoxy resin systems [22].
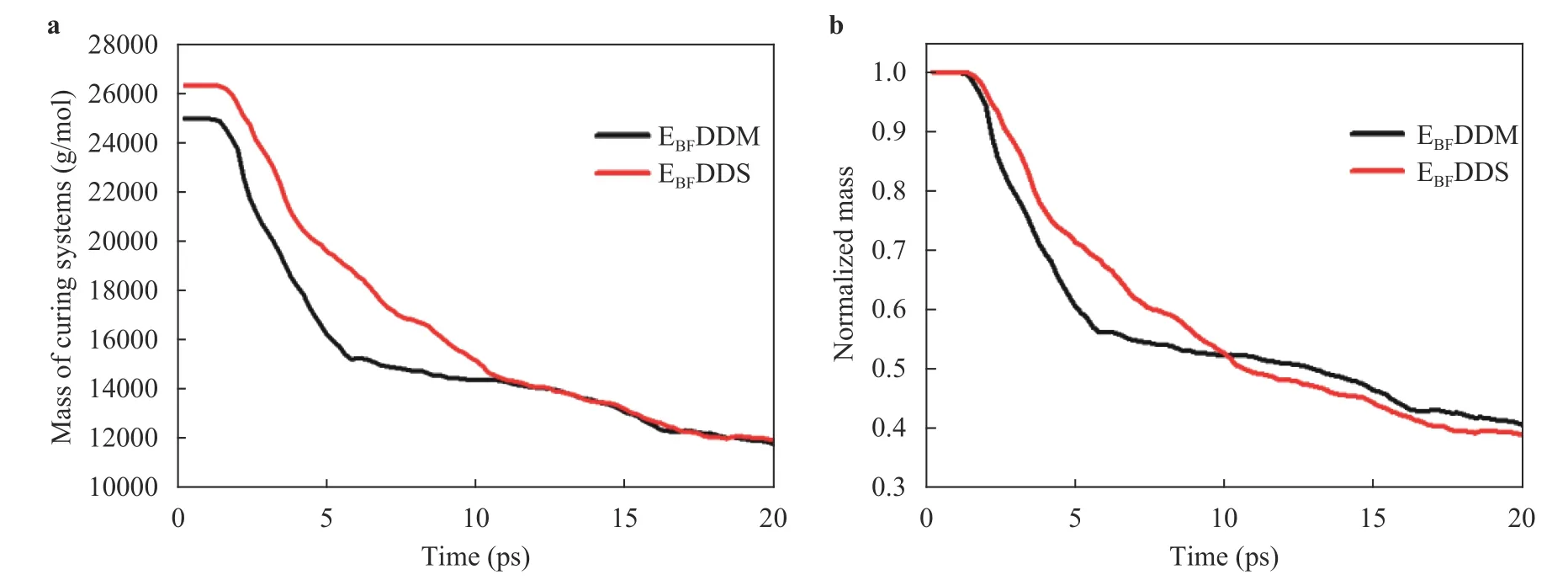
Fig. 15. Total remaining mass of curing epoxy resin systems with two different curing agents during solid oxygen impact. a Absolute remaining mass, b normalized remaining mass.
Snapshot:Figure 16 shows a snapshot (without unreacted oxygen molecules) of EBFDDM and EBFDDS in the middle (10 ps)and late (20 ps) stages of the reaction, which can also be used to illustrate the mass loss of the curing systems.
It can be seen from the temperature change and mass loss of the two curing systems above that the thermal stability of EBFDDM is better than that of EBFDDS. Based on the conclusions,we infer that the compatibility of EBFDDM with oxygen is better than that of EBFDDS.
In order to verify the simulation results, it is compared with the existing experiments (Table 2). In this experiment, only the frequency of impact sensitivity of the material is given. To scientifically characterize the compatibility of the material with oxygen, the IRS is also calculated. The formula is as follows

where nirepresents various reaction intensities, αirepresents the weighting factor at this reaction intensity, and m represents the total number of tests.
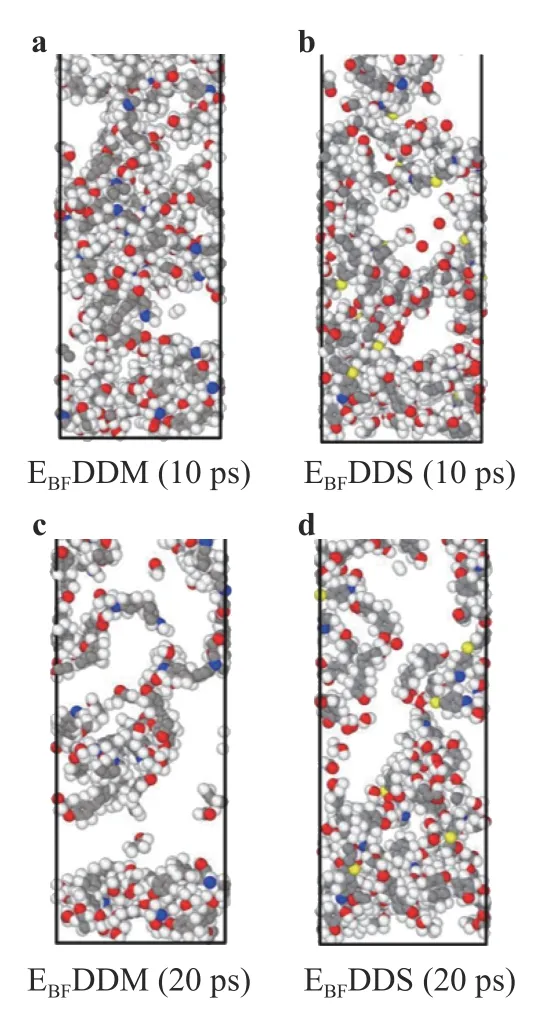
Fig. 16. Reaction snapshot of two curing agents curing epoxy resin system during solid oxygen impact (without unreacted oxygen molecules).
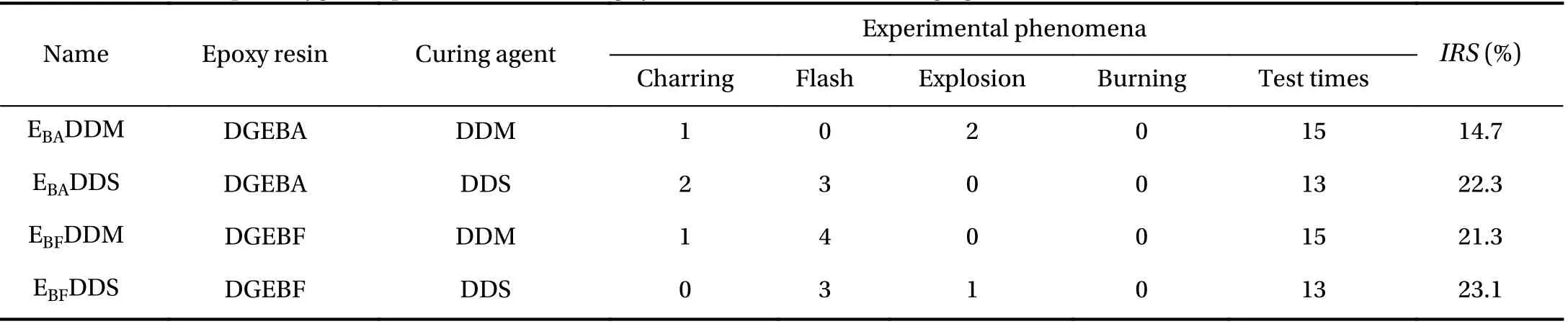
Table 2 Results of liquid oxygen impact test of the curing system with differents curing agents [23, 24].
According to the above experimental data, it can be seen that for the same type of epoxy resin, whether DGEBA or DGEBF, the DDM curing system has better liquid oxygen compatibility than the DDS curing system, which verifies the feasibility of qualitative analysis of material and oxygen compatibility by the MD simulation.
The chemical essence of the compatibility between polymer and liquid oxygen is oxidation, and the strength of its antioxidant oxidation resistance is positively correlated with its liquid oxygen compatibility. The MD simulation is used to simulate the chemical reaction of epoxy resin system under the impact of solid oxygen in this paper, and two indicators, temperature evolution and mass loss, are obtained to qualitatively judge the compatibility between polymer materials and oxygen. The simulation results show that DGEBA has better thermal stability than the other two epoxy resins, and the liquid oxygen compatibility of the cured product of DDM is relatively good, which is also verified by test results from the literature. The molecular modeling techniques open a new way for the subsequent modification of epoxy resin and the improvement of its oxygen compatibility.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (Grants U1837204 and 11802053) for the financial support of this research.
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- Micromechanical analysis on tensile properties prediction of discontinuous randomized zalacca fibre/high-density polyethylene composites under critical fibre length
- Neurodynamics analysis of cochlear hair cell activity
- Prolonged simulation of near-free surface underwater explosion based on Eulerian finite element method
- Minimizing electrostatic interactions from piezoresponse force microscopy via capacitive excitation
- Spatial artificial neural network model for subgrid-scale stress and heat flux of compressible turbulence
- An analytical model to predict diffusion induced intermetallic compounds growth in Cu-Sn-Cu sandwich structures
