An analytical model to predict diffusion induced intermetallic compounds growth in Cu-Sn-Cu sandwich structures
2020-03-27YuexingWngYoYoLeonKeer
Yuexing Wng, Yo Yo*, Leon Keer
a Institute of Electronic Engineering, China Academy of Engineering Physics, Mianyang 621999, China
b School of Mechanics, Civil Engineering and Architecture, Northwestern Polytechnical University, Xi'an 710072, China
c Department of Civil and Environmental Engineering, Northwestern University, Evanston, IL 60208, USA
Keywords:Intermetallic compounds Polarity effect Electromigration Diffusion Size effect
ABSTRACT A mass diffusion model is developed to describe the growth kinetics of Cu6Sn5 intermetallic compounds (IMC) in the Cu-Sn-Cu sandwich structure. The proposed model is based on the local interfacial mass conversation law where interfacial Cu/Sn reactions and atomic diffusion are considered. Theoretical analysis shows that the IMC thickness growth is proportional to the square root of the product of the diffusion coefficient and time. The proposed model can explain the polarity effect of electromigration on kinetics of IMC growth where all the parameters have clear physical meaning. The theoretical predictions are compared with experimental results and show reasonable accuracy.
The formation and growth of the intermetallic compounds(IMC) between bulk solder and the substrate is one of the most critical issues for the reliability of the solder interconnects [1, 2].The Cu6Sn5phase, which provides electrical and mechanical interconnection, will nucleate at the Cu-Sn interface during the soldering process. However, with the miniaturization trend of the microelectronic device and the integrated circuits approaching the limit of the Moore's Law, novel structures such as system in package (SiP) and 3D packaging have been developed. The three dimensional integrated circuit (3D IC) is one of the most promising solutions towards extending Moore's Law [3, 4]. In the 3D IC, the thickness or height of the solder joints will be reduced to only a few microns. The proportion of IMCs in the total solder joint will be much larger thus the failure mechanism will be controlled by the interface properties. It is of significance to clarify the IMC growth mechanism under different conditions especially under reflowing and high current density conditions.
It is recognized Cu-Sn-Cu structure is typically adopted in microelectronic packaging and many experiments [5-8] were conducted to investigate the IMC formation and growth in the structure. By fitting the experimental data, a corresponding semi-experimental formula is proposed to predict the IMC thickness growth for engineering application [8]. However, this phenomenological model is not physical based. Considering the effects of electromigration and thermomigration [9, 10] which is the key physical mechanism of the micro solders in the nextgeneration 3D electronic packaging, the current semi-experiment models can hardly describe the physical behavior.
In addition to the phenomenological approaches, numerical approaches such as phase field methods and finite element methods were proposed to analyze the IMC thickness growth [11, 12]. Analytical models [5, 13] were established based on the diffusion flux equilibrium relation. However, some of the numerical or analytical models are too complex for engineers to apply. An analytical solution with clear physical meaning is therefore required to better describe the IMC thickness growth under different load conditions.
It is well accepted that the IMC thickness growth is motivated by the advancement of the Cu/Sn interface and the growth of the Cu6Sn5phase, which is mainly controlled by the diffusion of Sn atoms. The present IMC growth model is established by analogy with the grain boundary precipitant nucleation and growth issue [14]. When the IMC layer is formed, an atom concentration gradient will exist at the IMC/Sn interface. The Sn atom concentration gradient can enhance the formation of the Cu6Sn5phase since Sn atoms will be driven to the interface by the chemical potential. Based on the Fick's first and second law,a mass diffusion controlled Cu6Sn5IMC growth model is developed in the current study.
A typical Cu-Sn-Cu sandwich structure is investigated and for simplification, only the Cu6Sn5IMC phase is considered as illustrated in Fig. 1. The formation of Cu6Sn5is driven by the reaction between Cu and Sn atoms at the Cu/Sn interface. The consumed Sn atoms are made up by the continuous diffusion of Sn atoms from the matrix phase. Based on the symmetrical characteristics of the whole process, a one dimensional interface diffusing reaction model can be established. The concentration of Cu6Sn5and Sn atoms at the interface are assumed to be Cβand CI, respectively. The saturation concentration of Sn atoms is Cα,which is located away from the interface. When the Cu6Sn5interface advances by dx during time dt, the growth of Cu6Sn5must be equal to the consumed Sn atoms based on the mass conservation principle, which is written as:

Fig. 1. a SnAgCu/Cu IMC interface, b simplified analytical model, and c mass diffusion conservation during the development of IMC interface

where D is diffusion coefficient of Sn atoms.
Based on the Fick's first law which relates the diffusive flux to the concentration under steady state assumption, the mass flux per unit area and per time is written as:

If denoting IMC growth velocity as v and v = dx/dt, Eq. (1)can be rewritten as:

From Eq. (3), the parameters D, Cβand CIare material constants determined from experiment. Once the differential term dC/dx is known, the IMC growth rate v can be obtained. To solve the differential term dC/dx, we assume concentration distribution of Sn atoms is linear along the diffusion path. Based on the solute conservation law, the total diffusing Sn atoms in the diffusion path is equal to the amount that remained in the IMC layer.As shown in Fig. 1(c), the solute conservation law [14] requires that the area of two colored parts should be equal:

where L is the total diffusing path length of Sn atoms and ΔC equals Cα-CI.
The differential term dC/dx can be defined by:

Substituting Eq. (5) into Eq. (3), the IMC growth velocity v is given by:

For simplification, we assume Cβ-CI≈Cβ-Cα. Integrating both sides of Eq. (6) gives:

By solving Eq. (7), the final IMC thickness growth is given by:

Rewriting Eq. (8) gives:

If the initial IMC thickness is x0, the IMC thickness growth can also be determined by:

On the other hand, mass diffusion is a thermally activated process. Taking the Arrhenius dependence of the diffusion coefficient into account, the IMC growth rate as a function of temperature is written as:

where v2describes the growth rate for the second power of the IMC thickness. D0is the diffusion constant and Q is the activation energy for Sn atoms. R is the Boltzmann constant and T is temperature.
The experimental results performed by Ousama and Ladani[5] are adopted to verify the accuracy of proposed IMC thickness growth model. In their experiments, the Cu6Sn5growth at the Sn/Cu interface was investigated at 260 °C, 310 °C and 360 °C, respectively. The experimental results are treated and shown in Fig. 2(a), the vertical and horizontal axises are lnv2and 1/T, respectively. It is noted that the three experimental points are close to a linear relationship, and the IMC growth process shows Arrhenius dependence. This predicted IMC growth by Eqs. (10)and (11) matches well with the experimental observations. It proves that IMC growth is motivated by the mass diffusion process and the diffusion coefficient D plays a key role. The observation that the second power of IMC thickness is proportional to the diffusion coefficient and time is also proved by the experiments of Zhang et al. [8].
In addition to the qualitative verification, a quantitative verification is conducted by comparing with the experimental results. In the experiments, the intermetallic compound layers growth kinetics between SnAgCu solder and Cu Substrate were investigated at temperature ranges from 100 °C to 140 °C [8].Figure 2(b) illustrates the experimental results at three temperatures, which shows that the IMC thickness (μm) is linear with respect to the square root of time (h1/2). The slope is 0.1785 μm·h-1/2, 0.06425 μm·h-1/2and 0.02397 μm·h-1/2for 140 °C, 125°C and 100 °C, respectively. Based on the proposed model, the slope should be equal toTaking D0= 7×10-4m2/s, Q =110 kJ/mol and M = 5 [5], the theoretical IMC growth slope can be obtained. Comparison of the experimental and theoretical result for the IMC growth velocity is shown in Fig. 2(c). In general, the theoretical predictions match well with the experimental results. It is noted that the relative ratios of the slope between different temperatures are consistent, proving that the diffusing Sn atoms determine the IMC thickness growth. In summary, the proposed model can predict the IMC layer growth mechanism at different aging conditions.

Fig. 2. a Arrhenius dependence of the IMC thickness growth [8] and b, c comparison between the proposed model and experimental results [8]
On the other hand, the proposed model can be extended to explain the mechanism of size and electromigration effects on the IMC layer growh [6]. Experimental analysis has shown that the IMC layer growth rate will increase with decreasing of the solder length because of the size effect, which could be a serious concern for micro solder joint reliability in 3D electronic packaging. The micro solder joint may be totally transformed to the brittle IMC layer, which will deteriorate the fatigue life of the solder interconnect [15]. Based on the proposed model, with smaller size of the solder joint, the diffusion length of the Sn atoms will be decreased corrospondingly. Thus, the ΔC = Cα-CIwill increase based on the mass conservation law. From Eq. (8),the IMC layer growth rate will increase as well as illustrated in Fig. 3. Thus, the size effect on the IMC layer growth can be well explained by the developed model.
It is noted that the IMC layer growth will be strongly affected by electromigration under high current density. Experimental analysis shows that growth of IMC layer will be enhanced at the anode side and inhibited at the cathode side, which is known as the electromigration polarity effect on IMC layer growth [16].Electromigration is the migration of metallic atoms in the conductor due to the momentum transfer from moving electrons along the electric current direction [17]. During electromigration process, Sn atoms will be migrated by the electronic wind force, inducing concerntration increases at the anode side and decrease at the cathode side, which will affect the IMC growth.Research has been performed to clarify the mechanism of the eletromigration polarity effect on IMC growth. For example, Gan and Tu [16] explained that the polarity growth of IMC growth is caused by the unsymmetric back stess induced by mass diffusion on both cathode and anode sides. However, electromigration induced back stress will be relaxed in the solder interconect.In the current study, the proposed model is to clarify the electromigration polairty effect on the mechanism of IMC layer thickness growth.

Fig. 3. Explanation of the IMC growth size effect during mass diffusion
Based on the classic Einstein relation [18], Sn atoms diffusion flux along the current direction during electromigration is given by:

where Z*is the effective charge numer of Sn atoms, e is the charge of an electron, ρ is electronic resistivity and j is the current density. For pure Sn material, the bulk concentration of Sn atoms can be written as 1/Ω, where Ω is the volume of an indiviual Sn atom.
From Eq. (12), the mass flux is not related to positions whose magnitue is constant in the total Cu-Sn-Cu sandiwich structures:i.e meaning the concentration change at the anode and cathode end will be the same. Based on Eq. (8), the sum of IMC growth rate on the cathode and anode end is half of the IMC growth rate, under no current condition, it can be written as:
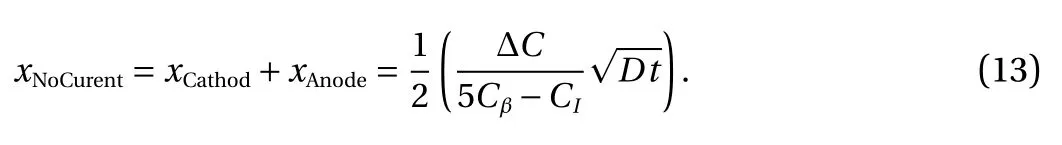
The experiment conducted by Gan and Tu [16] is adopted to verify Eq. (13). In their experiment, the IMC growth rate at 180 °C and 120 °C under three different electric current conditions were investigated. As show in Fig. 4, under lower current density, the theoretical and the experimental results match well. At the highest current density of 4×103A/cm2, the experimental and theoretical results show some gaps and the theoretical model predicts a higher value. This can be explained by that at the high current density, the atoms concentration variation will be enhanced and inducing the Cu6Sn5be reacted into Cu3Sn phase [19]. Thus the experimental result of the IMC growth rate is lower than the theoretical prediction, for which the gap value should be caused by the materials transformed into Cu3Sn Phase. On the other hand, at lower current density, there are no reactions to form Cu3Sn and the theoretical result matches the experimental result well. In the traditional back stress model, the IMC polairty effect is caused by the electromigration back stress.However, it is recognized that the back stress exists only in confined conditions. In solder interconnects, the eletromgration induced back stress will be released by the underfill showing the eletromigration induced IMC thickness polariy phoenmonen exists without back stress. Thus, the proposed approach can be regarded as an improved back stress IMC growth model.

Fig. 4. Comparison between predictions of the proposed model with experimental data [16]
A theoretical model with clear physical meaning is developed to describe the IMC thickness growth characteristics under temperature aging and high current density conditions. It is noted that the IMC growth is a mass diffusion controlled process, which depends on the Arrhenius relations. The temperature and mass diffusion coefficient show strong influence on the IMC growth. Based on the developed model, the electromigration induced polarity effect on the IMC growth thickness is analyzed and shows reasonable accuracy compared with the experimental results.
Acknowledgments
The authors would like to acknowledge the financial support by the National Natural Science Foundation of China (Grants 11572249 and 11772257) and the Fundamental Research Funds for the Central Universities (Grant G2019KY05212).
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- Micromechanical analysis on tensile properties prediction of discontinuous randomized zalacca fibre/high-density polyethylene composites under critical fibre length
- Neurodynamics analysis of cochlear hair cell activity
- Prolonged simulation of near-free surface underwater explosion based on Eulerian finite element method
- Minimizing electrostatic interactions from piezoresponse force microscopy via capacitive excitation
- Spatial artificial neural network model for subgrid-scale stress and heat flux of compressible turbulence
- Molecular investigation on the compatibility of epoxy resin with liquid oxygen
