氧化石墨烯负载磁性纳米复合材料(Fe3 O4 /rGO):氧化降解三氯乙烯的有效来源
2020-02-18乌斯曼庄景果王霏陈玖妍王新海
乌斯曼庄景果王 霏陈玖妍王新海
(河南大学 化学化工学院,河南 开封,475004)
Chlorinated aliphatic hydrocarbons (CAH) for instance trichloroethylene (TCE) have been frequently found in groundwater because of their extensive use in dry cleaning and process industries[1]. United States Environmental Protection Agency (USEPA) listed TCE as a priority groundwater pollutant as inhalation of TCE vaporized from groundwater may cause chronic diseases[2-3]. Numerous remediation techniques such as bio⁃remediation, reduction, adsorption, catalytic oxidation have been utilized to remove these recalcitrant pollutants from groundwater[4-6]. Sulfateradical based advanced oxidation technique can be promising for various CAH’s[7-8].
Cobalt (Co) based nanoparticles for activation of PMS found efficient for degradation of various contaminants but the leaching and toxicity of Co are threatening for the quality and safety of water[13]. Iron is an environmentally friendly and low⁃cost material and possesses extraordinary properties to deal with environmental contaminants[14]. Nano magnetite(Fe3O4) has a spinal structure and exhibits excellent magnetic and electric properties that favor the transformation of iron species around its octahedral structure[15]. Fe3O4has been applied for the activation of H2O2and PS to generate ROS and efficiently degrade contaminants but the acidic conditions,instability of H2O2,and high oxygen demand should be considered before application[16]. However, facile preparation, high stability due to excellent magnetic characteristics make Fe3O4makes it more suitable to activate PMS for TCE degradation[17]. But under strong magnetization and high surface energy the Fe3O4tends to aggregate and may lose its potency to activate PMS.
A promising material, graphene oxide (GO) has attracted hefty attention in current era due to its valuable thermal, surface, electric, and other material properties[18]. The GO nanosheet can immobilize nanoparticles which can help to enhance the activity of nanoparticles towards environmental application by reducing agglomeration[19]. At present, we synthesized Fe3O4nanoparticles immobilized on the surface of the GO sheet (Fe3O4/rGO) used for activation of PMS for degradation of TCE. Fe3O4/rGO was characterized using various analytic techniques. The dosage Fe3O4/rGO and PMS were optimized for TCE removal and finally, the extent and stability of the process was elucidated through mineralization, dechlorination and recyclability. Fe3O4/rGO system can prove to be a promising technique in advance oxidation process for remediation of environmental contaminants.
1 Chemicals and methods
1.1 Chemicals
Hydrochloric acid ( HCl, 30%), sodium persulfate (Na2S2O8), hydrogen peroxide (H2O2),Ferrous sulfate ( FeSO4· 7H2O), sulfuric acid( H2SO4), andn⁃hexane were purchased from Shanghai Lingfeng Reagent Co. Ltd., China. Ethanol,sodium nitrate ( NaNO3), and sodium hydroxide(NaOH) were purchased from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China.
1.2 Synthesis of Fe3O4/rGO nanocomposite
The GO was synthesized from graphite powder using modified hummers method described in our previous study[20]. The Fe3O4/rGO nanocomposite was synthesized using the co⁃precipitation method. Initially,0.5 g of pre⁃synthesized graphene oxide was sonicated for about 30 min with 2.5 g of FeSO4·7H2O in 150 mL water. The mixture was transferred to magnetic stirrer system and NaOH was added dropwise until solution pH reached 8.5. Then Teflon lined autoclave was used to heat this mixture at 180 ℃for about 17 h.The black solution was filtered and washed several times with deionized water and ethanol to remove the impurities. The resultant precipitates were dried overnight at 50 ℃in vacuum drier.
1.3 Experimental procedures
The TCE stock solution was prepared using non⁃aqueous phase liquid pure TCE and mixed it with ultra⁃pure water in a dark. A specified quantity from this solution was used to prepare the desired TCE (0.15 mmol/L) solution. A 150 ml borosilicate Erlenmeyer flask with a stopper was used for the process. The temperature was controlled using water bath. The aqueous samples (1.0 mL) were collected at desired time (10, 30, 45, 60 min) interval andn⁃hexane added as a solvent to stop the reaction. This solution was placed at the vortex mixer about 3-4 min for further mixing. The upper layer was separated and transferred to a vial using dropper and sent for analysis to gas chromatography (GC).
1.4 Analytical methods
The surface morphology of the synthesized nanomaterial was evaluated using a scanning electron microscope (SEM, JSM⁃630LV, Tokyo, Japan) and transmission electron microscope (TEM, JEM⁃1400 electron microscope operated at accelerating voltage at 80 kV). The crystalline characterization was done by X⁃ray diffraction (XRD) analysis using a Rigaku D/max diffractometer (2550VB/PC) with Cu Kα(λ=0.154 nm) radiation. The Brunauer⁃Emmett⁃Teller( BET ) method ( Quantasord Jr. Instrument Quantachrome Co; UK) was used for textural properties of prepared nanocomposite. The TCE degradation analysis was performed using GC having electron captured detector ( ECD) and DB⁃VRX column (60 m length, 250 μm internal diameter, 1.4 μm thickness). The temperature of the oven, detector,and injector was maintained at 90, 240 and 260 ℃,respectively. All the experiments were performed duplicate and mean value along with standard deviations were reported.
2 Results and discussion
2.1 Characterization of Fe3O4/rGO nanocomposite
The surface properties of the synthesized nanomaterial were investigated using SEM and TEM analysis. The SEM image confirmed a spherical shaped cluster particle (Fig.1) distributed on the surface of graphene oxide that is validated by TEM (Fig.2a).Furthermore, well⁃distributed spherical shaped Fe3O4particles were found on surface of rGO (Fig.2b), and size of the particles ranged from 100-120 nm. The crystalline structure of the synthesized composite confirmed using XRD analysis as shown in Fig.3. A broad peak at 10. 9° confirmed the formation of graphene oxide from oxidation of graphite powder. The XRD analysis of Fe3O4/rGO showed characteristics peaks at 30. 10°, 35. 46°, 43. 16°, 57. 03°, and 62.65°, confirming the formation of magnetite Fe3O4.This diffraction pattern is consistent with the Joint Committee on Powder Diffraction Standards (JCPDS)75-0033[21]. A broad peak at 24.5° showed the presence of reduced GO in Fe3O4/rGO nanocomposite synthesis. BET analysis conducted and it confirmed the Fe3O4/rGO has a particle size of 79.27 nm, specific surface area of 176.32 m2/g, a pore volume of 0.955 cm3/g, and pore size of 12. 35 nm. There is a difference between BET and TEM particle size, which may be due to change took place in pores during drying and degassing of sample during BET analysis[22].
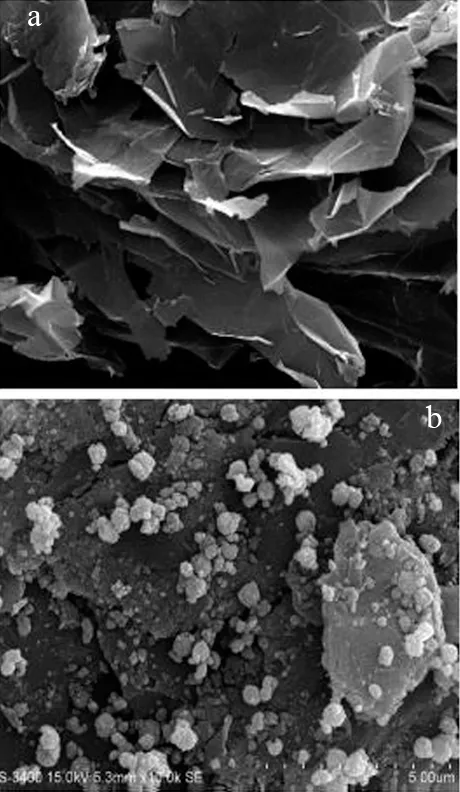
Fig.1 SEM image of a) GO and b) Fe3O4/rGO
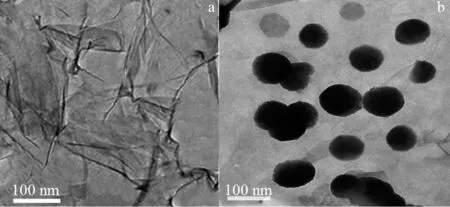
Fig. 2 TEM image of a) graphene oxide and b) Fe3O4/rGO
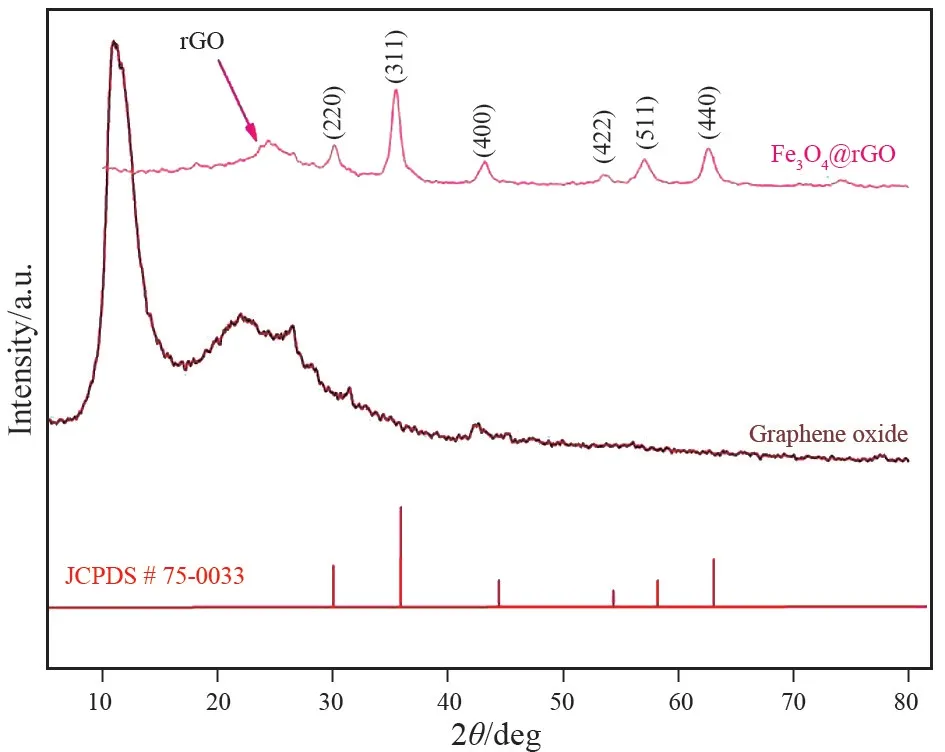
Fig.3 X⁃ray diffraction of synthesized Fe3O4/rGO
2.2 TCE removal and catalyst dosage optimization
The degradation performance of TCE was investigated under the Fe3O4/rGO catalytic environment. The TCE dosage was fixed at 0.15 mM in all sets of experiments. The control tests were performed for TCE only and observed about 4%decrease in concentration due to the volatility of the compound. Initially, sorption of the prepared catalyst was checked individually, and found that Fe3O4, GO and Fe3O4/rGO sorbed about 19%, 17%, and 22%respectively (not shown here). The performance of TCE degradation at various dosages (0.05, 0.1, 0.15 and 0.2 g/L) investigated at fixed PMS concentration(2.0 mmol/L). It can be seen from the results that TCE removal efficiency increases with Fe3O4/rGO dosage. The TCE degradation found to be 85.3%, 92.5%, 99.6% and 98.2% at 0.05,0.1,0.15 and 0.2 g/L respectively as shown in Fig.4. The slight decrease in TCE removal at 0.2 g/L is due to quenching effect of Fe2+at higher concentrations (Eq.1)[23].
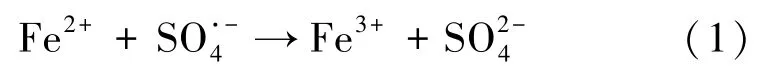
The initial and final pH was also observed during the degradation process and it was found within the acidic range (3-5). This confirmed that TCE degradation in Fe3O4/rGO activated PMS favors acidic conditions[24].
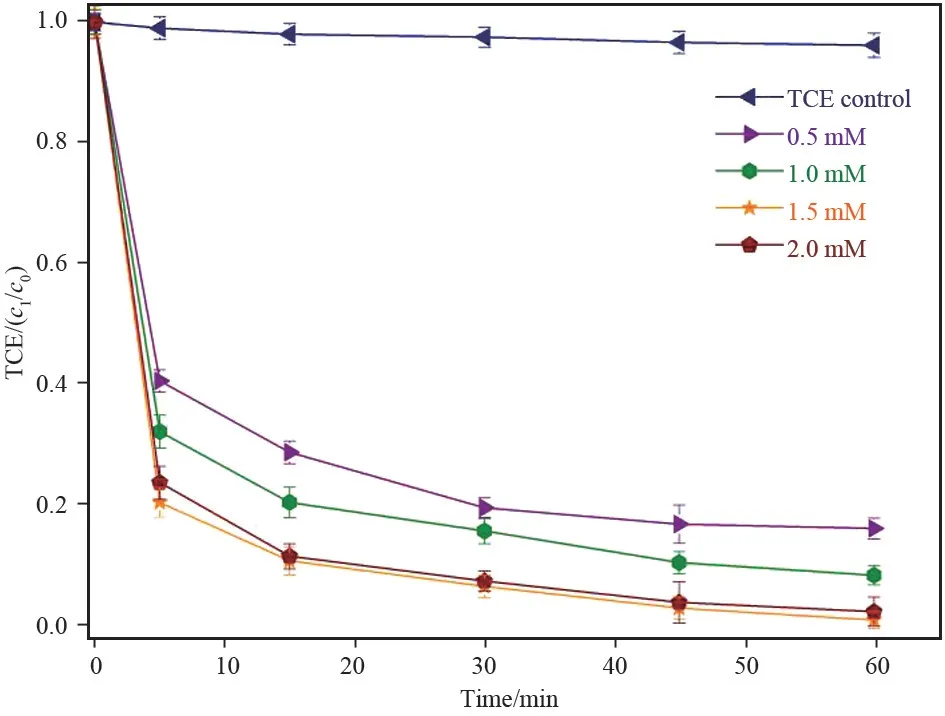
Fig.4 Effect of catalyst dose on TCE degradation at[TCE]o =0.15 mmol/L and [PMS] =2.0 mmol/L
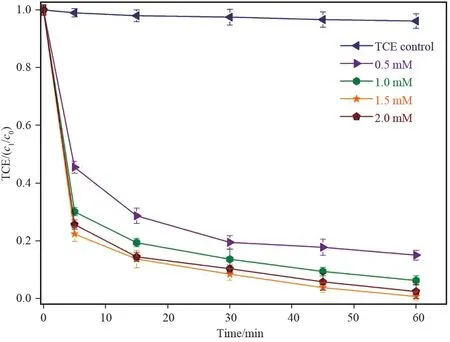
Fig.5 TCE degradation at different PMS dose using[TCE]o =0.15 mmol/L and [Fe3O4/rGO] =0.15 g/L
2.3 Effect of PMS concentration on TCE removal
The concentration of oxidant (PMS) is crucial for the degradation efficiency of environmental contaminants. Herein we evaluated the effect of various concentrations on the removal of TCE at an optimized Fe3O4/ rGO dosage( Fig.5) . A control test of TCA degradation in absence of PMS performed and found only 4% decrease in TCA concentration that may be due to evaporation. Various PMS dosages (0.5, 1.0,1.5 and 2.0 mmol/L) considered to investigate its relationship with TCE degradation. The results affirmed that TCE removal efficiency improves with increase in
PMS dosage and a maximum. The maximum efficiency obtained was 99.4% at 1.5 mmol/L and beyond this dosage (2.0 mmol/L) TCE removal slightly decreased(97.6%). This tenuous decline in removal efficacy at higher doses may be due to scavenging effect produced at higher PMS dosage[25].The pH during this test found more towards higher acidic value as PMS concentration increased and the highest final pH 2.76 was observed at 2.0 mmol/L PMS dosage.
2.4 Mineralization, dechlorization and stability tests
The evaluation of non⁃toxic chemical formation in the degradation of TCE can be verified by examining the mineralization and chlorine removal process. In this regard dechlorination and TOC analysis were performed. It is theoretically known that 1 mol of TCE produces 3 mol of Cl-. The release of chlorine was observed to be 89.3% at optimum parameters (1.5 mmol/L PMS,0.15 g/L catalyst) obtained in previous experiments. Furthermore, TOC analysis was conducted to investigate the extent of mineralization and it was found about 85% (Fig.6a). Unfortunately,due to the low concentration of TCE, no intermediate products were detected in GC⁃MS analysis. The studies validated that acids (oxalic, formic, dichloroacetic,and glyoxylic) could be intermediates at the initial stage of degradation that could be eventually converted to chloride and CO2due to oxidation finally[26]. The stability of the catalyst was also checked and found about 18% degradation of TCE in 4thcycle, (Fig.6b)this may be owing to the deterioration of iron active species on surface of GO. There is need of a coating material for this catalyst to strengthened the stability and recyclability of synthesized Fe3O4/rGO.
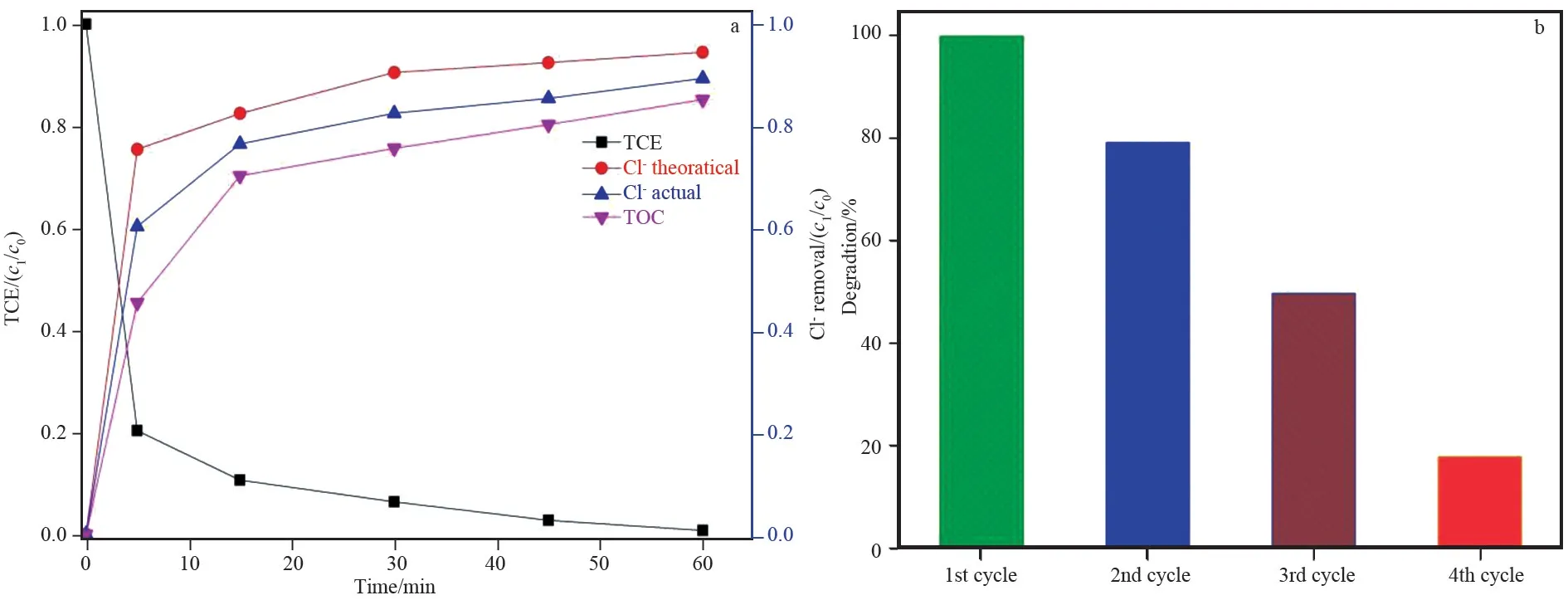
Fig.6 (a) Dechlorination and TOC analysis; (b) stability of Fe3O4/rGO
3 Conclusions
This study emphasizes on the synthesis of magnetic Fe3O4nanocomposite supported on rGO for the degradation of groundwater pollutant. The results revealed that Fe3O4/rGO efficiently activate PMS to remove TCE. The analytical characterization analysis evaluated the formation of higher surface area and crystalline spherical nanoparticles around a size range of 80-120 nm. It can be seen that 0.15 g/L Fe3O4/rGO and 1.5 mmol/L PMS dose can degrade about 99% TCE. A higher dose of either PMS or Fe3O4/rGO produce a scavenging effect to retard removal performance. The mineralization ( TOC ) and
dechlorination experiments observed reasonable values that support the nontoxic intermediates and by⁃products. The stability of catalyst needs to be improved in future study as around 18% degradation performance observed after 4thcycle that may be due to the corrosion of active species.
