Distillation Sequence Optimization Considering Extractive Distillation under Multiple Conditions: A Methanol to Propylene Case Study
2020-01-13YuJingQianFengWangJiming
Yu Jing; Qian Feng; Wang Jiming
(1. Key Laboratory of Adνanced Control and Optimization for Chemical Process, Ministry of Education, East China Uniνersity of Science and Technology, Shanghai 200237; 2. Coteνa AgriscienceTM, Shanghai 201203; 3. School of Chemical Engineering, East China Uniνersity of Science and Technology, Shanghai 200237)
Abstract: This study provides insights into the distillation sequence optimization of refinery system in a methanol to propylene plant with extractive distillation under multiple conditions. The simulated annealing algorithm (SA) with relative cost function was used to solve a meaningful optimization problem. It was observed that different conditions had differed on the flowsheet. Case study shows the effectiveness of the proposed method.
Key words: distillation sequence optimization; simulated annealing algorithm; MATLAB; multiple conditions
1 Introduction
Propylene has been entering the human life for 200 years. Its production and scale are second only to ethylene. It is an important raw material for manufacturing polypropylene, acrylic acid, propylene oxide, etc. In order to meet the rapid growth of China’s economy and the strong demand for chemical raw materials, the development of propylene production technology is important.
Other than the traditional route of producing propylene from naphtha[1], the methanol to propylene (MTP) process is a promising alternative way of propylene production. In the crude separation process of fixed bed MTP, in order to remove the by-product dimethyl ether (DME), it is necessary to carry out extractive distillation using methanol as an extractant. In actual production, the concentration of DME varies with the activity of the catalyst. If only a single operating condition is considered in the design, it will easily lead to higher separation energy consumption. Therefore, in order to design a coarse separation distillation sequence of the fixed bed MTP, it is necessary to consider different working conditions of the catalyst to enhance the energy utilization efficiency and operating flexibility of the process.
The synthesis of distillation sequences is an important research area for the integration of process systems[2-5]. The research efforts can be traced back to 1947, when Lockhart[6]proposed the theory of distillation sequence optimization. Heaven[7]introduced several rules for intuitive inference. Early research has tended to empirically synthesize distillation sequences. Subsequently, mathematical programming has gradually attracted the attention of researchers. Yeomans and Grossmann[8]established a superstructurebased distillation sequence optimization method. Iv and Floudas[9]and Benz and Cerda[10]respectively established a multi-period optimization model for the synthesis of distillation sequences. Gutierrez-Guerra, et al.[11]explored a step-by-step optimization strategy for the synthesis of extractive distillation sequences. Tsirlin, et al.[12]used a new method to evaluate the energy consumption of distillation sequences. Vazquez-Castillo, et al.[13]optimized the design and control of distillation sequences. However, studies, which can consider both multi-period and extractive distillation in the synthesis of distillation sequences, have not been reported. If these two situations are included in the mathematical optimization model at the same time, it will inevitably lead to the complexity of the problem, and the traditional deterministic optimization algorithm would be difficult to solve. Therefore, it is necessary to introduce a stochastic optimization method to solve the model.
The feature of the stochastic optimization method is easy to implement. It can handle the multi-extreme problem and the non-convex complex combinatorial optimization problem. Therefore, it is suitable for the synthesis problem of multi-component distillation sequence[14]. Common stochastic optimization methods include the genetic algorithms, the simulated annealing algorithms, the evolutionary algorithms, and the ant colony algorithms. In the field of distillation sequence synthesis, An and Yuan[15]optimized the heat integrated distillation sequence using the simulated annealing algorithm. Wang, et al.[16]proposed a method for synthesizing distillation sequences based on the stochastic optimization strategy.In this paper, we will focus on the distillation sequence synthesis problem in the methanol-to-propylene process, where multi-period conditions as well as the extractive distillation should also be considered. Simulated annealing algorithm will be employed to solve the problem.
The simulated annealing algorithm is derived from the principle of solid annealing. The solid is heated to a sufficiently high temperature and then is allowed to cool down slowly. In the course of heating, the internal particles of the solid become disordered, and the internal energy increases, while the particles gradually become ordered during cooling. According to the Metropolis criterion, the probability that a particle tends to equilibrate at temperature T is e-ΔE/kT, where E is the internal energy at temperature T, and k is the Boltzmann constant. When applying annealing to combination optimization problems, E is recognized as the objective function, and T stands for the control parameter t. The cooling schedule includes an initial value t0of the control parameter, an attenuation factor Δt, the number of iterations for each t value, a Mapkob chain length, and a termination condition S. There are many examples of solving the distillation sequence problem by using the simulated annealing algorithm[14-15],including those complicated distillation systems[16-21].
2 Distillation Sequence Optimization Model
The methanol is subjected to catalytic conversion to obtain a mixture of C1—C10hydrocarbons containing propylene. The product is subjected to crude separation according to different boiling ranges, and some byproducts are further recycled to the reactor for further reaction. During the catalytic conversion process, the dimethyl ether content of the product increases as the activity of the catalyst decreases. The boiling point of dimethyl ether (DME) is close to that of propylene and propane. In order to ensure the quality of product, DME should be separated from propylene and propane. This goal is achieved by extractive distillation, as shown in Figure 1. It can be seen from Figure 1 that DME is absorbed by methanol, and they are discharged together with C4+ components from the bottom of the column. Thus, there is no DME byproduct at the top of the column. To optimize the separation sequence, different conditions of DME content should be considered when the separation process is designed.

Figure 1 The flowsheet of extractive distillation
According to the content of each component, upon considering the requirements for crude separation, we divide the components into 8 groups. They are C1, C2, C3, C4, C5, C6, and C7+.
The feed composition and related parameters were simplified, as shown in Table 1, where the operating pressure was set at 2 MPa. It should be noted that the above mentioned 8 groups are assigned to different characters as shown in Table 1. The product and byproducts of the separation system are shown in Figure 2, which includes the recycle hydrocarbon, the fuel gas, and the liquid phase products. The composition of the recycle hydrocarbon and liquid phase products is shown in Table 2.
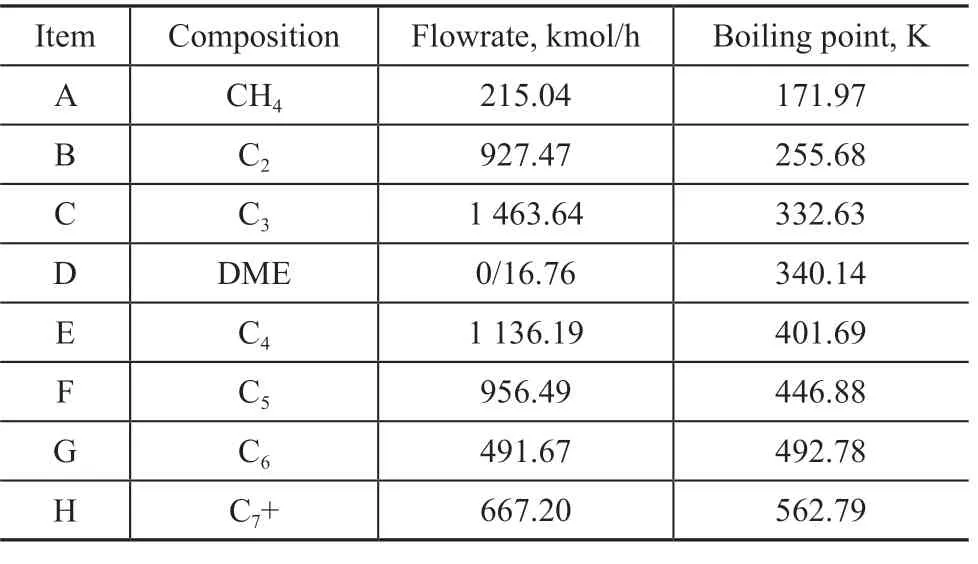
Table 1 Composition of feed
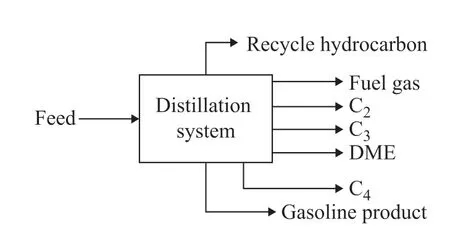
Figure 2 Products of crude separation unit
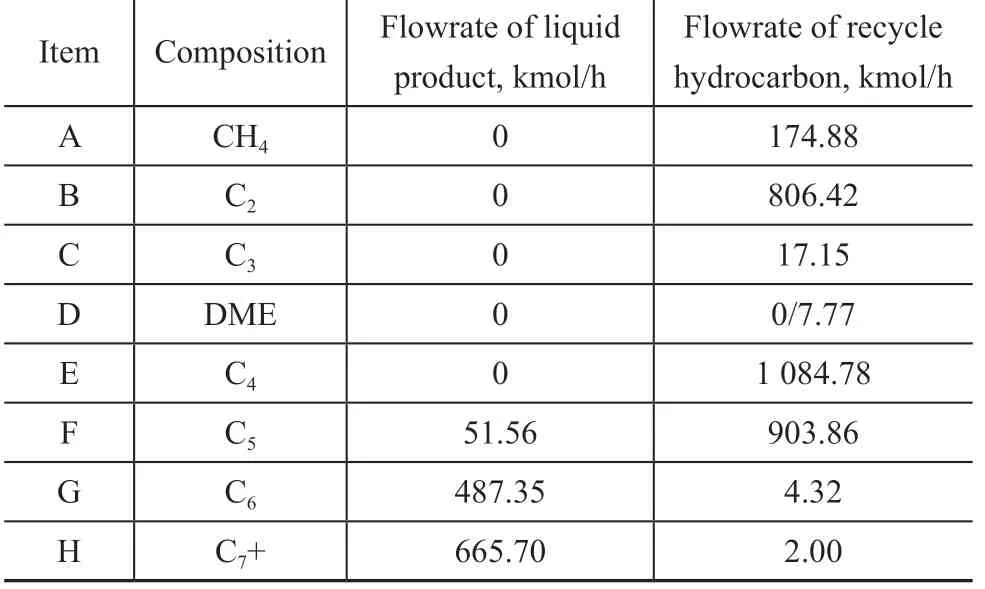
Table 2 Composition of products discharged from crude separation unit
3 Distillation Sequence
For resolving the above-mentioned eight-component separation problem, there should be a number of 7 cut points. We can arrange the cut points as the following order: A1B2C3D4E5F6G7H. The numbers 1—7 indicate the cutting position.
Different segmentation points correspond to different separation sequences. If we have N-components to separate, then possible separation sequences can be described by the ordering of N-1 segmentation points. The total number of possibility: P(N-1) = (N-1)! It should be noted that it contains all reasonably feasible separation sequences; however, it also includes the number of repeated separation sequences. When the number of components N is increased to a large level, the number of repeated separation sequences is sharply increased, resulting in an unnecessary increase in calculation time. It is noted that the repeated process structure is generated by the non-adjacent segmentation point exchange locations, and the adjacent segmentation point exchange locations will generate different process structures. Therefore, the neighborhood switch mechanism is set, requiring that only adjacent ones are selected. The points are exchanged to eliminate the redundant sequence of repetitions and reduce the calculation time.
4 Relative Cost Function
The distillation sequence optimization problem studied in this paper is described as follows: Given the mixture of N components, the flowrate of each component, and the boiling point under the operating pressure, it is assumed that all the distillation columns except the extractive distillation column are simple and clear. The mixture is separated into a series of pure components and mixtures under a certain operating pressure, and the operating cost of the separation sequence is set as an objective function. The commonly used separation sequence cost indicators include a relative cost function, a separation ease factor, and a separation difficulty coefficient. Shi and Wang[22]have found that the relative cost function is better than the other factors. Therefore, we use the relative cost function as the objective function. The relative cost function is defined as:
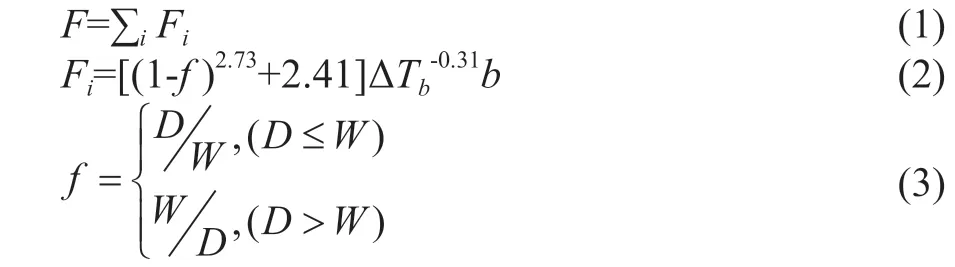
where D is the molar flow of the overhead product, W is the molar flow of the bottom product, ΔTbis the difference in boiling point between adjacent light and heavy components at the cutting point.
As regards the extractive distillation column, the extractant is treated as a separate feed and the same method is used for the estimation. The relative cost function of the distillation sequence takes the average of its two operating conditions. It is worth noting that since the recycled hydrocarbon material in the present study is a mixture, it needs to be further mixed with the separated product. Therefore, the setting of the separated product is different from the general distillation sequence optimization.
5 Algorithm Design
In this paper, the simulated annealing algorithm is used to solve the distillation sequence optimization problem. The algorithm flow is shown in Figure 3. The initial separation sequence x is randomly generated. According to different feed composition and different recycle hydrocarbon composition, the inlet flow and outlet flow of each column in accordance with the recycle hydrocarbon composition under working conditions 1 and 2 are calculated, while the corresponding relative cost functions of the respective columns are also calculated. Accordingly, the corresponding relative cost functions F1(x), F2(x), as well as the average value of F1(x) and F2(x) are obtained. A new solution x' is generated using the neighborhood transform mechanism, and the corresponding relative cost function F(x’) is calculated. If F(x’) < F(x), then the new solution x’ should be adopted, otherwise the new solution x’ with a probability of exp(-(F(x’)-F(x))/tk) should be adopted. There will be two termination conditions available. If more than five adjacent new solutions are not adopted, the control parameter t would reach the termination value. Either one of the two conditions could trigger the termination decision. If any of the termination conditions is satisfied, the loop will be ended. During the calculation, the memory variable is used to save the minimum value of the relative cost function obtained during running of the program. At the end of the loop, the solution with a minimum value of relative cost function will be the output of the algorithm. At the temperature tk, if the number of new solutions accepted or the number of new solutions generated is greater than the specified value, the temperature is lowered to tk+1for calculation. Among them, the exponential cooling method is adopted, which is: tk+1= a × tk. Separate sequences are randomly generated, the objective function value F is calculated, and then the maximum difference ΔFmaxof the objective function is taken as the initial value t0of the control parameter.
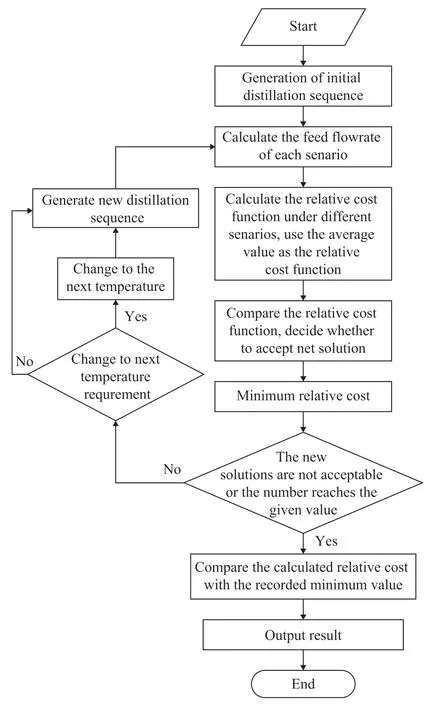
Figure 3 Procedure of the proposed algorithm
The cooling factor a is set as 0.8. The initial temperature t0set in the cooling accuracy Table is taken as 0.356, and the annealing end temperature is t = 0.00001. The total number of possible distillation sequences is calculated as: P(8-1)/2=7!/2=2 520. Therefore, we set the number of iterations per t value—the Mapkob chain length—as 100.

Table 3 Parameters of algorithm
6 Results and Discussion
The final temperature t = 0.04722, and the optimal separation sequence is coded as {4 5 6 7 2 1 3}. The corresponding isolated sequence is shown in Figure 4, where the sequence is first divided between DME and C4components. And the searching procedure for running the algorithm is shown in Figure 4.
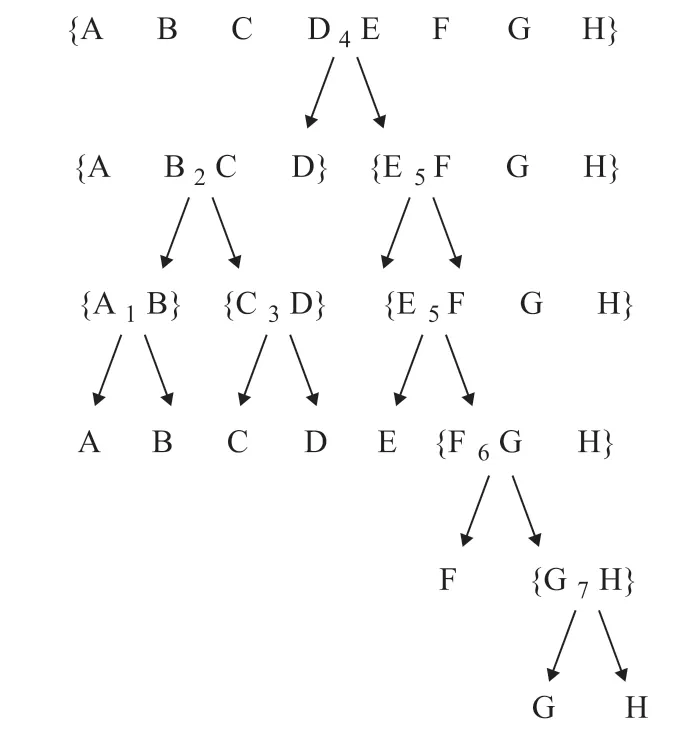
Figure 4 Representation of final result
In the algorithm, it is necessary to extract 30.03 kmol/h as the circulating hydrocarbon in the distillation column feed with the cut point of 2, and to extract 916.23 kmol/h as the circulating hydrocarbon in the distillation column feed with the cut point of 1. In the distillation column feed with a cut point of 5 204.7 kmol/h are required as the recycled hydrocarbon, and the distillation column feed with the cut point of 7 needs to extract 28.42 kmol/h as the recycled hydrocarbon. The final distillation process is shown in Figure 5.

Figure 5 The flowsheet structure under design condition
It is found in the algorithm that 745.1 kmol/h are required to be withdrawn as a recycle hydrocarbon in the distillation column feed having a cut point of 1, and 46.9 kmol/h are required to be extracted as a recycle hydrocarbon in the distillation column feed having a cut point of 7. The final distillation flowsheet is shown in Figure 6.
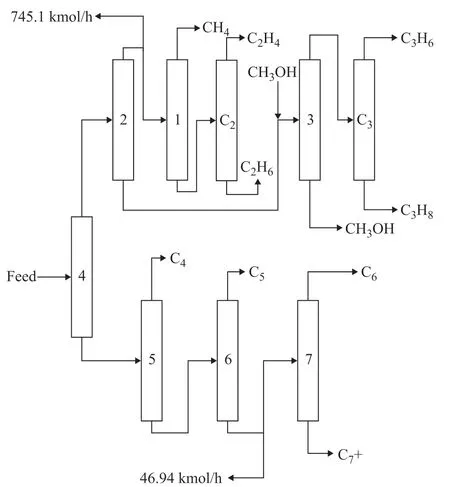
Figure 6 The flowsheet structure under initial condition
The distillation system employed in the industry can be referred to as a front end debutanization process, which first divides the C4and C5components. Since the effluent methanol contains C4component, it cannot be directly used as the recycled methanol. Thus the energy intensive separation of the two components is required.
The calculated distillation system first separates DME and C4components. Moreover, methanol and C4components are not mixed. Consequently, the extracted methanol can be directly used as the recycled methanol after being extracted from the distillation column. Therefore, the calculated process can save energy consumption for separating methanol and C4components as compared to the previous front end debutanization process.
7 Conclusions
The distillation sequence optimization problem considering the multi-period and extractive distillation is solved using the simulated annealing algorithm. The method considers the conditions with different concentrations of by-product DME, including extractive distillation, which can improve the accuracy and practicality of the system model. The results show that DME and C4are the first to divide the optimal distillation sequence, and the extraction flowrate and the feed flowrate of each column are changed under different working conditions. The calculated distillation sequence can reduce the separation energy consumption as compared to the previously used debutanization process employed in the industry.
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
