CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
2020-01-13JiangRuiyuWangMinZhuTaoZhouYudongSongXinyuZhangFengDingLiangGaoJian
Jiang Ruiyu; Wang Min,2; Zhu Tao; Zhou Yudong; Song Xinyu; Zhang Feng; Ding Liang; Gao Jian
(1. Jiangsu Collaboratiνe Innoνation Center for Ecological Building Material and Enνironmental Protection Equipment, Yancheng Institute of Technology, Yancheng 224051; 2. School of Enνironmental and Chemical Engineering, Jiangsu Uniνersity of Science and Technology, Zhenjiang 212000; 3. School of Management and Economics, Gdańsk Uniνersity of Technology, Gdańsk 80-233, Poland)
Abstract: The X-CoMnMgAl hydrotalcite-like precursors (X-LDHs) were first synthesized by the coprecipitation method using Cl-, and as the corresponding guest anions, which were further used to prepare X-CoMnMgAl complex oxides (X-LDOs) through calcination. The structure and the surface morphology of the as-prepared samples were characterized by FT-IR, XRD, N2 adsorption-desorption and SEM. These as-prepared X-LDOs could act as sulfur-transfer catalysts for desulfurization. The activity of SOx adsorption and regeneration was evaluated via a self-assembled fixed-bed reactor simulating the conditions found in the fluid catalytic cracking units. These four types of sulfur-transfer catalysts with the same phase but different structure displayed the following order of desulfurization efficiency: CO3-LDO >Cl-LDO >NO3-LDO > SO4-LDO.
Key words: coprecipitation method; hydrotalcite-like compounds; sulfur-transfer catalyst; desulfurization efficiency
1 Introduction
SOxare one of the main pollutant gases produced by the combustion of sulfur-containing fuel. Excessive emission of SOxinto the atmosphere has caused great harm to the eco-environment, animal, plant, and human being[1-4]. Acid rain formed via the combination of SOxwith water in the atmosphere affects the normal growth of plant and leads to the direct damage to the humans’ hearts and lungs, brains and nerves, while sulfur oxides can lead to chronic corrosion of the refinery-owned equipment[5-8].
Since these gases are among the major atmospheric pollutants and are acid rain precursors, their emissions are increasingly restricted by environmental legislation. Currently, the main methods used for desulfurization in refineries include the feed hydrodesulfurization, flue gas scrubbing, and sulfur-transfer catalysts[9-12].
LDHs are layer-structured materials and the metal oxide after calcination has several advantages, such as large specific surface area, uniform dispersion of metal ions and high thermal stability. The redox promoters introduced are mostly oxides of Ce, V, Cu, Fe, and Mn[13-15]. Among these metals, Mn is a cheap metal, and its oxides show great catalytic oxidation performance although its reductive regeneration performance is not satisfactory[16]. Therefore, the sulfur-transfer catalyst prepared by a combination of multiple metallic oxides with Mg-Al hydrotalcite-like compounds could display its unexpected desulfurization efficiency.
Herein, CoMnMgAl quaternary hydrotalcite-like compounds were first synthesized by the coprecipitation method using cheap elements, Mn and Co, which were introduced as the oxidation and reduction promoters on the basis of Mg-Al LDHs system, respectively. Four kinds of different inorganic anions (,,, and Cl-) were intercalated into the quaternary hydrotalcitelike compounds, respectively, to finally obtain the corresponding composite oxides (LDOs) after calcination, which could be used as four types of sulfur transfer catalysts for desulfurization. The effects of intercalated anions on the phase structure and sulfur transfer property were investigated.
2 Experimental
2.1 Preparation of complex oxides
2.1.1 Synthesis of the hydrotalcite-like precursors (X-LDHs)
Four kinds of X-CoMnMgAl hydrotalcites (X=,,, a n d C l-) were prepared by the coprecipitation method. Solution A was prepared by dissolving a certain amount of Co(NO3)2, Mn(NO3)2, MgSO4and Al(NO3)3with their molar ratios equating to: n(Mg2++Mn2+)/n(Al3+) =3, n(Mg2+)/n(Mn2+) =2, and m (CoO dry basis) = 10%, respectively. Solution B was prepared by dissolving a certain amount of NaOH with n(OH-)=2[n(M2+) + n(M3+)]. Solution C was prepared by dissolving a certain amount of Na2CO3, Na2SO4, NaNO3and NaCl, respectively. Solutions A and B were added simultaneously dropwise to solution C under vigorous stirring at 35 °C in a nitrogen atmosphere, while maintaining a constant pH value of around 10. The mixture was subjected to crystallization for 12 h, and then was filtered, washed several times with deionized water until pH = 7 prior to being dried at 60 °C for 10 h. The four synthesized samples were labeled as CO3-LDHs, SO4-LDHs, NO3-LDHs, and Cl-LDHs, respectively.
2.1.2 Synthesis of complex oxides (X-LDOs) for sulfur transfer catalysts
Samples of CO3-LDHs, NO3-LDHs, and Cl-LDHs were calcined at 800 °C for 2 h. Sample of SO4-LDHs were calcined at 900 °C for 2 h and then were sieved to 80 mesh for further use. The composite oxides obtained were labeled as CO3-LDO, SO4-LDO, NO3-LDO, and Cl-LDO, respectively.
2.2 Characterization of complex oxides
All of the samples were characterized by X-ray diffraction (XRD) on a Netherlands X’Pert PRO diffractometer and were also subjected to inductively coupled plasma mass spectrometry (ICP-MS) detection. The functional groups contained in the sample were qualitatively analyzed by an RX-1 Fourier transform infrared (FT-IR) spectroscope with a scanning range of between 4 000 cm-1and 400 cm-1. The adsorption-desorption curves were measured at a liquid nitrogen temperature (-196 °C) using a Beckman Coulter SA3100 specific surface area and pore size analyzer.
2.3 Activity evaluation of complex oxides as sulfur transfer catalysts
The SO2adsorption-reduction abilities of the four samples were tested via a self-assembled fixed-bed reactor simulating the conditions found in FCC units. 0.2 g of four kinds of sulfur transfer catalyst samples were added to the tube reactor with a diameter of 6 mm, with the reactor temperature maintained at 700 °C. Then a mixed stream consisting of 1 600 μL/L of SO2, 80.46% of N2and 19.4% of O2was passed over the catalysts at a flow rate of 200 mL/min. The time needed for sulfur absorption was 30 min. After the temperature was lowered to 530 °C, the regeneration process was performed with a stream of nitrogen containing 30% of H2at a flow rate of 100 mL/min. During the test, the effluent SO2gas concentration was measured using a Testo flue gas analyzer. The SO2desulfurization rate (E) can be calculated using the following formula:

where C0is the SO2concentration in the feed gas, and C is the SO2concentration in the effluent gas.
3 Results and Discussion
3.1 Characterization of hydrotalcite-like precursors
Based on the above synthetic steps, four kinds of X-CoMnMgAl hydrotalcite-like precursors (X-LDHs) were first obtained. The structures of the products were confirmed by FT-IR and XRD. Figure 1 shows the FTIR spectra of these precursors, and the characteristic absorption peaks of the hydrotalcite phase in all four samples at 3 450 cm-1, 1 640 cm-1, and 630 cm-1were attributed to the stretching vibration of OH-group, interlayer water and the lattice vibration of the Me-O bonds between the layers of hydrotalcites, respectively[17]. We can also clearly observe the absorption bands at 1 370 cm-1, 1 110 cm-1, and 1 380 cm-1, which corresponded to the stretching vibration peaks of,and, respectively[18-20]. Furthermore, the samples SO4-LDHs and Cl-LDHs also exhibited weak stretching vibration peaks at 1 380 cm-1, indicating that a small amount ofstill existed between the layers of the SO4-LDHs and Cl-LDHs samples during the synthesis process despite the reduced exchange capacity of anions between the hydrotalcite layers, which decreased in the following order:
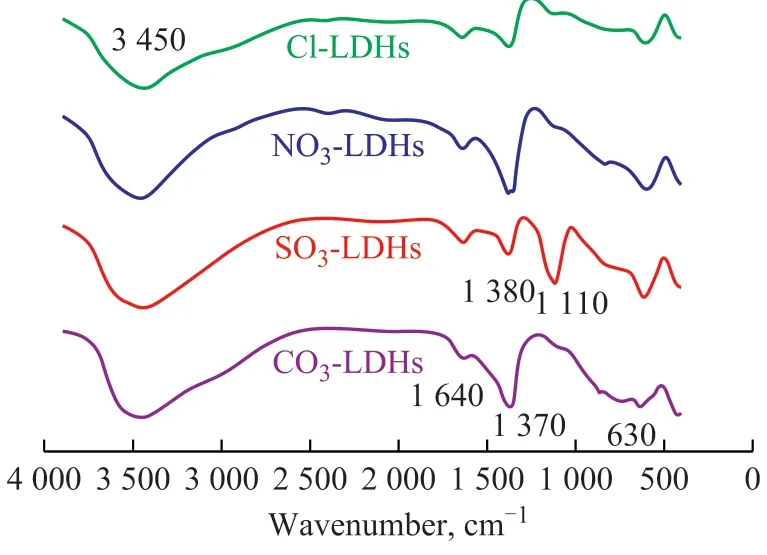
Figure 1 FT-IR spectra of four kinds of hydrotalcite-like precursors
The XRD patterns of X-LDHs were used to further characterize their structure. Figure 2(a) exhibits the characteristic diffraction peaks of the hydrotalcite-like compounds at the 2θ value of 11°, 22°, 35°, and 60°, which were associated to the planes (003), (006), (009), and (110) of the hydrotalcite phase, respectively. Upon comparing the diffraction peak of plane (003) with others, we can clearly observe that this diffraction peak of plane was sharp, indicating the good crystallinity and regular interlayer structure for hydrotalcite-like compounds[14]. Moreover, the diffraction peak of plane (003) gradually shifted to a smaller angle, meanwhile the interlayer distances d (003) of all four samples decreased from 0.85 nm to 0.77 nm with a reducing radius of the intercalated anionsin LDHs. All of these points can also verify that X-LDHs have been successfully prepared. According to the ICP test results in Table 1, the molar percentages of Mg, Al, Mn, and Co elements in the four types of hydrotalcite precursors are almost the same.
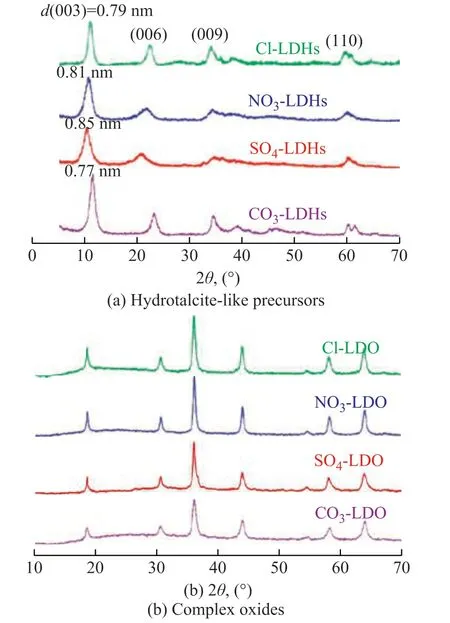
Figure 2 XRD patterns of four types of hydrotalcite-like precursors, and the corresponding complex oxides

Table 1 Molar percentage of MgAlMnCo elements in four kinds of hydrotalcite precursors
3.2 Structure and morphology characterization of sulfur transfer catalysts
The X-CoMnMgAl complex oxides (X-LDOs) were prepared by calcination of four kinds of X-LDHs for 2 h. After aerobic calcination, inorganic anions and H2O between the layers were removed, leading to the formation of complex metal oxides. The XRD patterns of these four X-LDOs are shown in Figure 2(b). The peaks of four samples are almost in the same position, the 2θ values at about 19°, 31°, 36°, 58°, and 64° were attributed to the MnMgAl spinals[21]. The difference of anions between layers did not lead to the significant different phases of complex oxides. Moreover, the characteristic peak of Co-oxide was not observed in all four samples, indicating that the cobalt component was dispersed highly in the samples.The N2adsorption-desorption and pore size distribution data were also used to characterize the structure of X-LDOs after calcination. As illustrated by the N2adsorption-desorption isotherms of all four samples in Figure 3(a), the sample CO3-LDO exhibited the H3-type hysteresis loop, which was usually characterized by the slit-shaped pores formed by aggregates of platy particles. However, the SO4-LDO, NO3-LDO, and Cl-LDO samples presented H1-type hysteresis loops, which were characterized through particle accumulation and similar pore size. According to the IUPAC classification, all four X-LDOs samples exhibited the type IV isotherms, which were characteristic of mesoporous materials[22]. As it can be seen from Figure 3b, the double peaks of the pore distribution located at about 42 nm and 105 nm for CO3-LDO indicated that the mesopores and macro-pores were mainly distributed in this sample. As for other three samples SO4-LDO, NO3-LDO and Cl-LDO, the pore sizes were relatively uniform (20—30 nm), which belonged to a series of mesopores.

Figure 3 (a) N2 adsorption-desorption isotherms, and (b) pore size distribution for four types of complex oxides
It is well-known that the anions between the layers of the hydrotalcite-like compounds have a great influence on the structure of their derivatives after calcination. Table 2 shows the structural parameters of the four complex oxides. The results revealed that CO3-LDO had the largest specific surface area, pore volume and average pore size. The pore structure of industrial catalysts is characteristic of wide pore size distribution and unfixed pore volume, so that the tortuosity factor cannot be determined. Although SOxis the small gas molecule, the molecular size is much smaller than the pore size of the catalytic material we made, and the pore structure of the catalyst has an important influence on the effective diffusion of reactant molecules in the catalyst pores[11]. However, all these parameters for SO4-LDO are the smallest when compared with others, which may be caused by the higher thermal stability of its precursor, SO4-LDHs (the complex metal oxide, SO4-LDO was prepared by calcining SO4-LDHs at 900 °C for 2 h). Therefore, SO4-LDHs used as the precursors actually are not suitable for preparing the sulfur transfer catalysts.
Further evidence for the formation of mesoporous complex oxide materials was obtained by SEM analysis. The typical SEM images of four X-LDHs before and after calcination are shown in Figure 4. It can be seen that CO3-LDHs take the approximately sheet-like particles by accumulation, and form the aggregates of sheet-like particles after calcination, leading to the collapse of originally layered structure. This is also consistent with the characteristics in H3-type hysteresis loop. As for the remaining three X-LDHs precursors, all the surface morphologies were irregular, especially for SO4-LDHs, which exhibited a wrinkled sheet-like structure. The particles were pushed to finally form ball-like morphology. After high-temperature calcination, all these three samples SO4-LDO, NO3-LDO, and Cl-LDO displayed the characteristics of particle accumulation on their surfaces, which are consistent with the characteristics of H1-type hysteresis loop.

Table 2 Structural parameters of four types of the complex oxides and the SO2 uptakes over two cycles
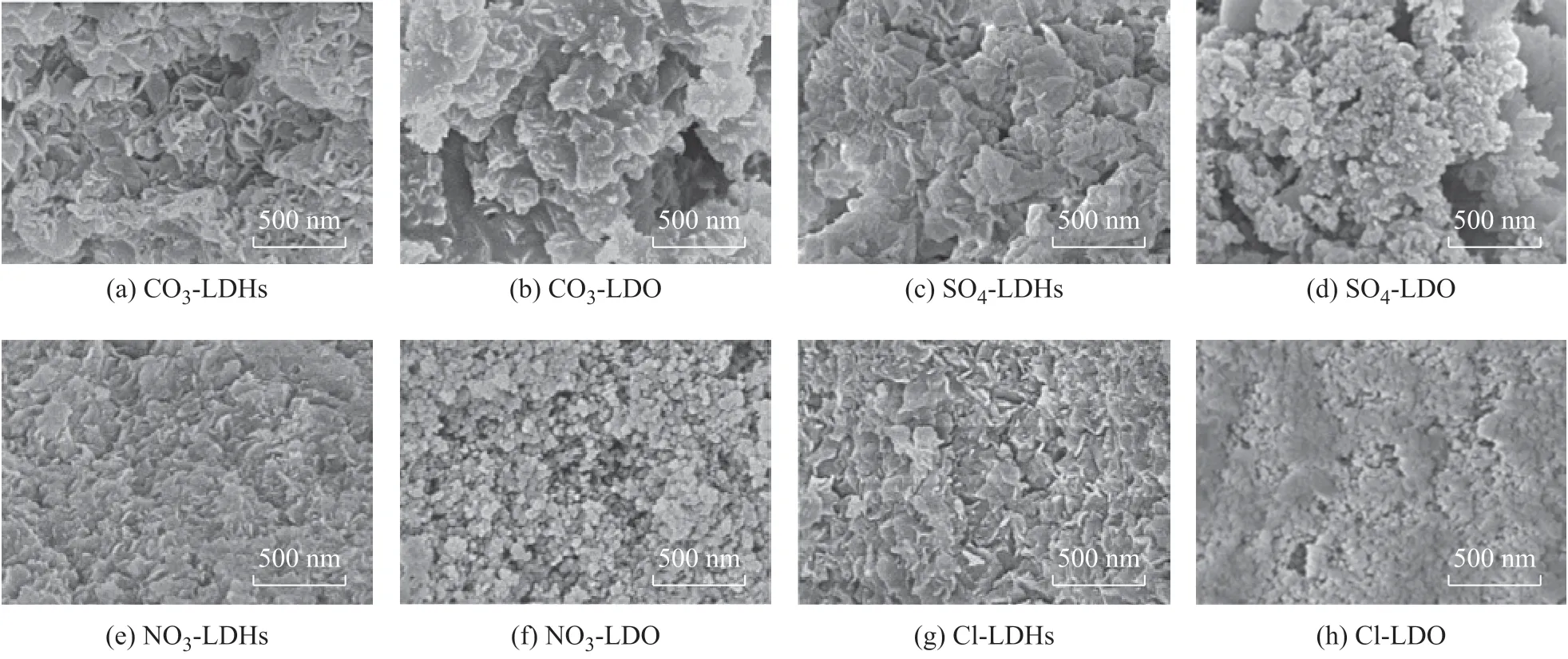
Figure 4 SEM images of hydrotalcite-like precursors and the corresponding complex oxides
3.3 Performance of complex oxides as sulfur transfer catalysts
Once four types of X-LDOs were obtained, and meanwhile, the structure and morphology were also determined, we finally could examine the performance of these as-prepared X-LDOs functioning as sulfur transfer catalysts for SO2uptake and regeneration. Generally, the desulfurization efficiency of sulfur transfer catalysts is attributed to their surface area and pore structure of materials. The larger the specific surface area of the sulfur transfer catalyst is, the more favorable the SO2adsorption would be.
Figure 5(a) shows the primary oxidation desulfurization performance of four X-LDOs samples. As it can be seen, the sample CO3-LDO demonstrated an optimal desulfurization efficiency that reached more than 90% within 30 minutes. As for other three samples, the continuous and significant decline of the desulfurization rates, which reduced from more than 90% to less than 50%, was observed. Besides, all the desulfurization curves gradually become flat after 30 min, suggesting that the sulfur absorption capacity tends to reach saturation. It should be noted that the desulfurization efficiency of sample SO4-LDO is lowest (lower than 45% after 10 min), which might be ascribed to its relatively small surface area, pore volume, and average pore size. However, after combining the structural parameters with specific SO2uptake of four samples in Table 2, we discovered that the specific surface area of sample NO3-LDO is slightly larger than that of Cl-LDO, while the desulfurization performance of the former is lower than that of the latter, suggesting that the pore structure would play a major role when the materials have similar surface area. This is because the pore volume would expand after absorbing SO2and further form the sulphate that densifies the surface of material and even plugs the original pore structure to reduce seriously the internal utilization of the sulfur transfer catalyst[23-24]. In addition, the diffusion resistance against admitting SO2through the pores would also increase when the pore size becomes small, resulting in a decrease in the content of SO2. It can be inferred that the large pore volume and size are conducive to SO2adsorption and enhance the desulfurization efficiency.
The secondary desulfurization performance of four X-LDOs samples after H2reductive regeneration is shown in Figure 5(b). Compared with the primary desulfurization performance curves and two-cycle SO2uptakes shown in Figure 5(a) and Table 2, it can be found that there is no obvious difference in the trend of change although the desulfurization efficiency and SO2uptakes were less than those obtained in the first cycle, suggesting that X-LDOs used as sulfur transfer catalysts exhibit efficient SO2adsorption and regeneration abilities.
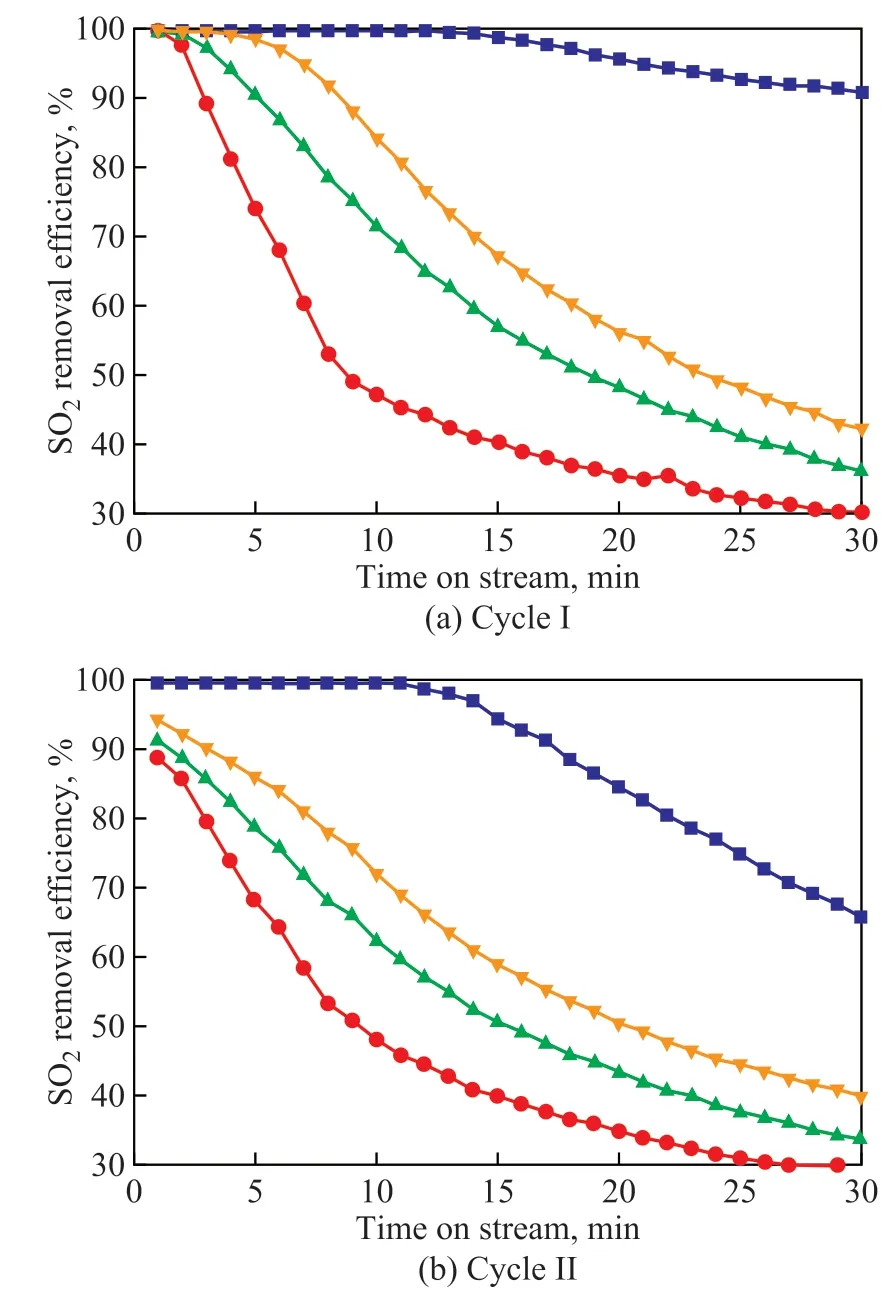
Figure 5 Comparison of the desulfurization performance of four sulfur transfer catalysts measured in (a) the primary cycle and (b) the secondary cycle
4 Conclusions
Four types of X-CoMnMgAl complex oxides (X-LDOs) were prepared by calcining the X-CoMnMgAl hydrotalcite-like precursors (X-LDHs) intercalated with four kinds of inorganic anions Cl-,,, andvia the coprecipitation method, which showed the same phase but different structure of these samples. All these four X-LDOs could be used as sulfur transfer catalysts for SO2uptake and regeneration. The results show that the initial structural parameters of X-LDOs could affect the desulfurization efficiency of the regenerated samples. The higher the pore volume and pore size of the sulfur transfer catalysts are, the better the performance of SO2uptake is. The sulfur transfer catalyst prepared by CoMnMgAl LDH intercalated with(CO3-LDO) exhibited an optimal SO2absorption capacity after regeneration and presented a satisfactory sulfur transfer performance.
Acknowledgements:The authors thank the Natural Science Foundation of Jiangsu Province (No. BK20171273), the National Natural Science Foundation of China (No. 21774107), the High-level Talent Project “Talents in Six Peak Disciplines” (JNHB-068), the Major Fundamental Research Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJA540001), the “Qing Lan Project” of Jiangsu Province, the Jiangsu Provincial Government Scholarship Program, and the Initial Scientific Research Foundation of Yancheng Institute of Technology (No. KJC2014002) for financial support of this research.
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effects of Blending Long-Chain Alcohols with Jet Fuel on the Macroscopic Spray Characteristics
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
