Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
2020-01-13KongRuiqiJiangHaoWangDanTanJialunRenDanniSunHuiShenBenxianLiuJichangTangShengZhaoDeyinChangXiaohu
Kong Ruiqi, Jiang Hao, Wang Dan, Tan Jialun, Ren Danni, Sun Hui,2, Shen Benxian,2, Liu Jichang,2, Tang Sheng, Zhao Deyin, Chang Xiaohu
(1. Petroleum Processing Research Center, East China Uniνersity of Science and Technology, Shanghai 200237; 2. International Joint Research Center of Green Energy Chemical Engineering, East China Uniνersity of Science and Technology, Shanghai 200237; 3. Sinopec Northwest Oilfield Branch, Urumqi 830011)
Abstract: Binderless zeolite is considered to be a potential alternative for binder-containing zeolite in the industrial applications of adsorptive separation process. Synthesized binderless zeolite and commercial binder-containing product were used in adsorptive separation of n-paraffins from a model oil, with their performance compared. It is indicated that the binderless zeolite exhibits by 25%—35% higher in saturated adsorption capacity and by 115%—130% more adsorption amount at the breakthrough point with much shorter length of mass-transfer zone. Adsorptive separation of n-paraffins from naphtha was carried out in a fixed-bed adsorber containing the synthesized binderless zeolite 5A under the operating conditions covering a feed space velocity of 90 h-1 and an adsorption temperature of 573 K. As compared to original naphtha, the raffinate shows by 34 units more in research octane number and by around 10% more of potential aromatic content, while the desorption oil exhibits by 13.3% more ethylene yield and by 11.7% higher in total olefins yield.
Key words: binderless zeolite 5A; adsorptive separation; n-paraffins; naphtha
1 Introduction
Effective utilization of naphtha is of great concern nowadays since naphtha is an important resource involved in the production of various industrial raw materials, including mainly the light olefins[1-4]and gasoline blending fractions for octane improvement[5-6]. Distinct hydrocarbon composition of naphtha, which commonly consists of normal paraffins (n-paraffins), isomeric paraffins (i-paraffins), naphthenes, and aromatics, is used for different purposes[7]. Specifically, the n-paraffinexcluded fraction can be used as a promising component for blending gasoline due to its high octane number[8]or for producing aromatics in the catalytic reforming process[2,9]. Meanwhile, the n-paraffin-enriched fraction contributes to higher yields of ethylene and propylene in steam cracking process[2,10]. As a result, the effective separation of n-paraffins from naphtha has been receiving wide academic and industrial interest[11-13]. Several separation processes based on shape selective adsorption over zeolite materials have been developed during the past several decades[14-16].
The successful application of adsorptive separation of n-paraffins from naphtha absolutely depends on the involved adsorbent—zeolite 5A[7,13,17-18]. In order to be successfully adopted in industrial application, the zeolite powder needs to be converted into macroscopic pellets to maintain a relative low pressure drop across the adsorber. In the commercial binder-containing zeolite, 20% or more of inert binders such as clay, kaolin, and silica sol are used to provide the pellets with necessary mechanical strength[19-20]. Consequently, the binderless zeolite has higher adsorption capacity compared to the bindercontaining zeolite product. In addition, the shaping process is usually accompanied by negative impact on pore system due to the powder compaction, leading to an inhibition of the components diffusion inside the adsorbent[21]. Besides, the binderless zeolite has also been confirmed to exhibit lower coking tendency[22]. Thus, the binderless zeolite 5A is considered to be a promising candidate for the target adsorbent[23-24].
In this paper, the synthesized binderless zeolite and the commercial binder-containing product were used in adsorptive separation of n-paraffins from the model oil, with their performance compared. Both adsorption kinetics and dynamic adsorption of n-paraffins on the binderless zeolite 5A and the binder-containing zeolite 5A were tested to compare the adsorption performance between two samples. Moreover, the optimized utilization performance of naphtha via the adsorption process based on the binderless zeolite 5A was evaluated.
2 Experimental
2.1 Materials
The binderless zeolite 5A pellets with a Si/Al ratio of 1.03 were synthesized in-situ on the silica gel pellets, which were prepared via the previously reported method[25]. Typically, the pellets were synthesized with a diameter of 3—4 mm and the size of the zeolite crystals was about 0.5—1 μm. As a reference, the commercial binder-containing zeolite 5A pellets were supplied by the Honeywell International Inc. (Shanghai, China). The binder content in the zeolite was evaluated to be 19.6%[26]according to the XRD results. The characteristic comparison of the synthesized binderless and the commercial binder-containing samples is summarized in Table 1. The model oil was prepared by mixing n-paraffin with cyclohexane. A straightrun naphtha obtained from the Sinopec Shanghai Petrochemical Co., Ltd (Shanghai, China) was used as the raw material, with its group composition as well as the n-paraffin distribution listed in Tables 2 and 3, respectively.
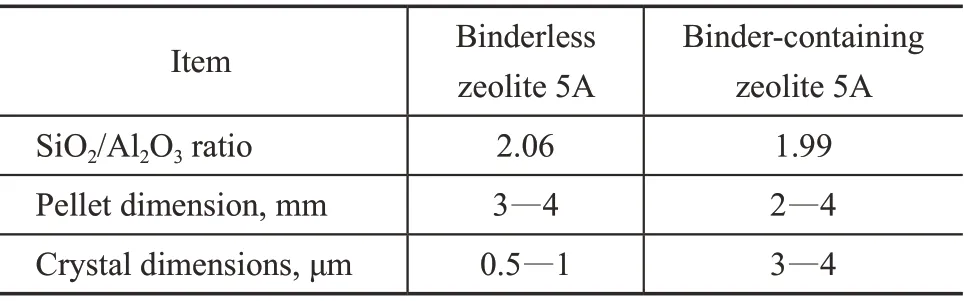
Table 1 Physical properties of binderless and bindercontaining zeolite 5A

Table 2 PONA compositions of the naphtha

Table 3 Distributions of n-paraffins in naphtha
2.2 Characterization
The compositions of n-paraffin/cyclohexane solution, original naphtha, raffinate and desorption oil samples were determined by using a GC-920 gas chromatograph (Shanghai Haixin Chromatography Analysis Co., Ltd., Shanghai, China) equipped with a hydrogen flame ionization detector (FID) and a PONA capillary column (50 m × 0.2 mm × 0.5 μm). All GC analyses were carried out by preheating the sample from 323 K (which should be maintained for 5 min) to 473 K (which should be maintained for 5 min) at a temperature increase rate of 5 K/min.
The powder X-ray diffraction (XRD) patterns were collected at room temperature using a Rigaku D/max 2550 diffractometer (Rigaku Industrial Corp., Japan) with Cu Kα radiation operated at 40 kV and 100 mA. The data were recorded with the scanning angle (2θ) ranging from 5° to 60° at step sizes of 0.02 with a scanning speed of 10 (°)/min.The crystal morphology images of zeolite 5A sample were obtained by using a Nova NanoSEM 450 scanning electron microscope (SEM)(FEI, USA) with an accelerating voltage of 15 kV and a beam current of 10 nA. The elemental analysis was carried out on a Falcon energy-dispersive spectrometer (EDS) (EDAX Inc., USA), which was attached to the SEM. A sputter coating with a thin layer of platinum was performed to avoid charging. Five to eight points were measured at various positions to obtain an average elemental composition.
2.3 Adsorption experiments
The adsorption kinetics curves of n-hexane on the binderless and the binder-containing zeolite 5A samples were obtained by determining the uptake adsorption capacity at different adsorption time under an adsortion temperature of 298 K in a n-hexane/cyclohexane solution.The adsorption breakthough curves of n-paraffins on the binderless and the binder-containing zeolite 5A samples were determined in a fixed-bed adsorber (with a height of 0.2 m, an inner diameter of 10 mm, and a zeolite loading amount of around 9 g) under the operating conditions covering a liquid space velocity of 1.91 h-1and an adsorption temperature of 313 K.
In addition, the optimized utilization performance of naphtha via the adsorption process based on the synthesized binderless zeolite 5A was evaluated in a fixed-bed adsorber (with a height of 1.2 m, an inner diameter of 50 mm, and a zeolite loading amount of around 2 kg). Naphtha was completely vaporized when passing through the zeolite 5A bed in the process of adsorption, and the desorption was implemented using a N2countercurrent flow at a desorption temperature of 673 K. The raffinate and desorption oil were condensed and collected for composition analyses. The detailed process can be found in the previous publication[16].
2.4 Adsorption kinetics model
The adsorption kinetics curves of n-hexane on the binderless and the binder-containing zeolite 5A samples can be explained by using Equation (1)[27].
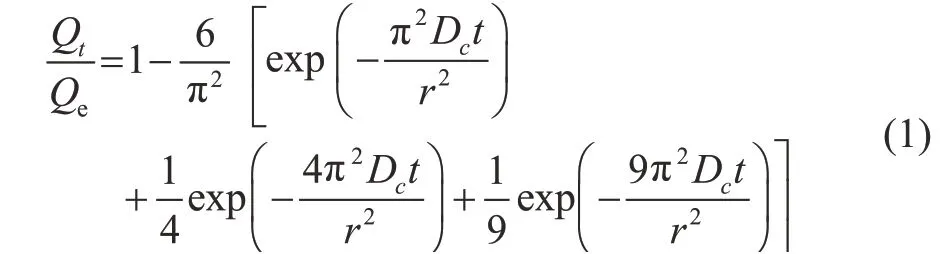
where Qt(mg/g-zeolite) and Qe(mg/g-zeolite) represent the adsorption capacity at an adsorption time of t (s) and the equilibrium adsorption capacity, respectively. Dc(cm2/s) refers to the apparent diffusion coefficient and r (cm) is the radius of the zeolite spheres.
2.5 Adsorption breakthrough model
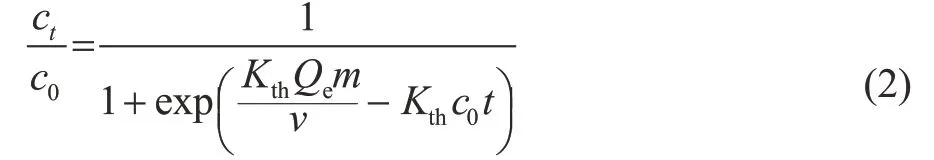
The adsorption breakthrough curves can be explained using the Thomas model[28], which is expressed as Equation (2).where Kth(mL/(min·mg)) is the rate constant of Thomas, c0(mg/L) and ct(mg/L) represent the initial concentration of n-paraffins and concentration of n-paraffins at the adsorption time of t (min), respectively, m (g) is the weight of zeolite sample in the fixed-bed, and ν (mL/min) refers to the feed flow rate. In this research, the adsorption breakthrough and adsorption saturation are indicated when the total content of n-paraffins in the raffinate reaches 5% and 95%, respectively.
2.6 Calculation of research octane number
The research octane number (RON) of oil can be calculated using the method proposed by Gorana, et al.[29](see Equation (3))
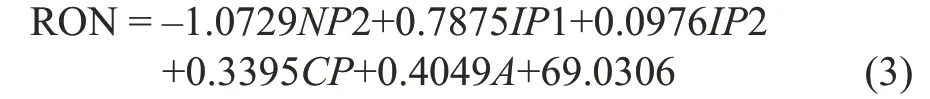
The parameters involved are defined as follows:
NP = the total mass fraction of all n-paraffins
So off they went, driving the country roads of northern Rhode Island on the kind of day only mid-May can produce:sparkling sunshine, unclouded azure4 skies and vibrant5 newness of the green growing all around. They went past small villages and burgeoning6 housing developments, past abandoned apple orchards7, back to where trees and brush have devoured8 old homesteads.
NP1 = the total mass fraction of n-C4and n-C5
NP2 = NP-NP1
IP = the total mass fraction of all i-paraffins
IP1 = the total mass fraction of i-C4, i-C5, 2,2-diMeC4, 2,3-diMeC4, 2,2-diMeC5, 2,4-diMeC5, 2,2,3-triMeC4, 3,3-diMeC5and 2,3-diMeC5
IP2 = IP-IP1
CP = the total mass fraction of naphthenes
A = the total mass fraction of aromatics
2.7 Calculation of potential aromatic content
The potential aromatic content (Ar) can be calculated by Equation (4).
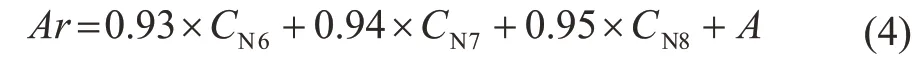
where CN6, CN7and CN8represent cycloalkanes with six, seven, and eight carbon atoms in the molecule, respectively, which can be converted into benzene, toluene and xylene(%), respectively. A is the total content of benzene, toluene and xylene in the original naphtha (%).
2.8 Calculation of olefin yields of cracking process
Under the experimental steam cracking conditions covering an outlet temperature of 1113 K, a residence time of 0.4 s, and a steam/oil ratio of 0.6, which were close to those used by a commercial unit, the yields of ethylene, propylene and butadiene could be calculated using the previously observed expressions (5), (6) and (7), respectively[30].

where xnis the mass fraction of n-paraffins in naphtha. ye,yp, and ybrepresent the yield of ethylene, propylene, and butadiene, respectively.
3 Results and Discussion
3.1 Characterization of zeolite 5A samples
The elemental compositions obtained from the EDS analyses of the synthesized zeolite 5A and the commercial sample were 3.5Na:12.6Ca:19.0Al:20.3Si: 44.6O and 5.4Na:7.0Ca:19.5Al:20.1Si:46.2O:0.5K: 0.5Mg:0.8P, respectively. Accordingly, the Si/Al molar ratio and Ca2+exchange degree were 1.03 and 80.5% for the binderless zeolite and 0.99 and 54.7% for the bindercontaining sample, respectively. The SEM images are presented in Figure 1 and the cubic crystals of zeolite A can be clearly recognized in both samples. For the binder-containing zeolite sample, the crystal surface is associated with a great deal of binder that can largely affect the porosity. The pore structure analyses of the binderless and the binder-containing zeolite 5A samples are shown in Table 4. As compared to the bindercontaining sample, the binderless sample displays larger specific surface area and macropore diameter, which are favorable to the adsorption as well as diffusion of n-paraffin molecules.

Figure 1 SEM images of : (a) binderless zeolite 5A and (b) binder-containing zeolite 5A
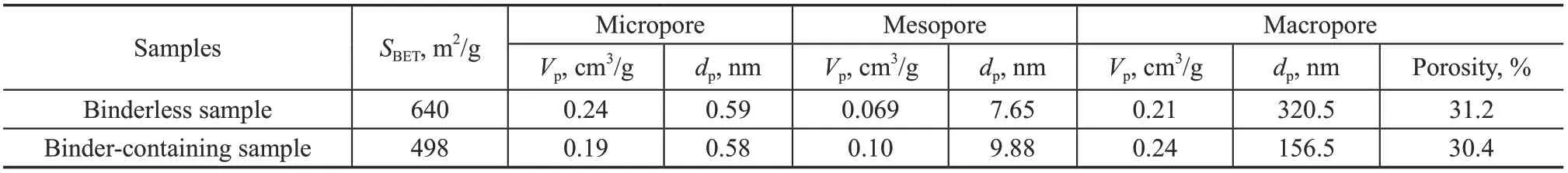
Table 4 Pore structure analysis results of binderless and binder-containing zeolite 5A
3.2 Adsorption kinetics of n-hexane on zeolite 5A samples
The adsorption rate curves of n-hexane on the binderless and the binder-containing zeolite samples are shown in Figure 2. The adsorption kinetics data could be well correlated by using Equation (1), with the kinetics parameters extracted and listed in Table 5. The adsorption capacities of n-hexane were observed to be 116 and 90 mg/g-zeolite on the binderless zeolite 5A and the binder-containing zeolite 5A samples, respectively. In addition, as shown in Table 5, the apparent diffusion coefficient of n-hexane on the binderless zeolite 5A sample was 2.65×10-6cm2/s, which was by 2.5 times higher than that on the binder-containing sample. These results indicate that the synthesized binderless zeolite 5A has larger adsorption capacity and higher adsorption rate of n-hexane as compared to those of the binder-containing zeolite product, leading to a more efficient separation of n-paraffins from naphtha.

Figure 2 Adsorption of n-hexane on zeolite 5A samples at 298 K

Table 5 Kinetics parameters of n-hexane adsorption on zeolite 5A samples at 298 K
3.3 Dynamic adsorption of n-paraffins on zeolite 5A samples
The adsorption breakthrough curves for n-paraffins in the fixed-bed adsorber filled with the binderless and the bindercontaining zeolite 5A pellets are shown in Figure 3. The content of n-paraffins in the raffinate on both zeolites could be maintained very low at the beginning of the adsorption. However, with the increase of adsorption time, the n-paraffin content in the raffinate increased rapidly after the adsorption curve reached the breakthrough point. In Figure 3, with an increasing length of carbon chain, n-paraffins show a gradually increasing breakthrough time, indicating that the carbon number of n-paraffin exerts a significant influence on adsorption as well as difussion in the zeolite. It can be explained that the interaction energy between n-paraffin molecules and zeolite 5A adsorption sites increases with the increment of carbon number[31]. By comparing Figure 3(a) with Figure 3(b), the adsorption breakthrough times are 25 min and 10 min for the fixed-bed adsorber filled with the binderless and the binder-containing zeolite 5A samples, respectively. Therefore, the synthesized binderless zeolite can be expected to achieve more efficient separation of n-paraffins.Moreover, the experimental adsorption breakthrough curves were correlated by applying the Thomas model, and the fitting parameters were derived, with the data listed in Table 6. Additionally, Qb(mg/g-zeolite) which is defined as the adsorption capacity at breakthrough point and LMTZ(cm) which is the length of mass-transfer zone were calculated, with the data listed in Table 6. The binderless zeolite has significantly higher adsorption capacities for n-hexane, n-heptane, and n-octane than the binder-containing zeolite sample. As compared to the binder-containing sample, the synthesized binderless zeolite 5A exhibits by 25%-35% higher adsorption capacity, which is consistent with the results of static adsorption. In other words, the loading amount and the adsorber volume can be reduced by replacing the binder-containing zeolite with the binderless product. Moreover, the binderless zeolite sample shows higher Thomas constants than the binder-containing zeolite sample (0.00578 vs. 0.00190 mL/(min·mg) for n-C6, 0.00457 vs. 0.00173 mL/(min·mg) for n-C7, and 0.00352 vs. 0.00133 mL/(min·mg) for n-C8).

Figure 3 Adsorption breakthrough curves of n-paraffins, at 313 K
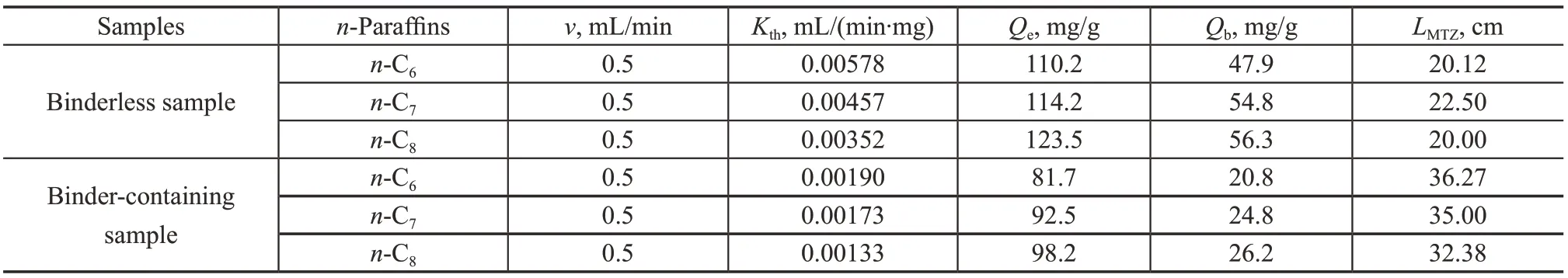
Table 6 Thomas model parameters of n-paraffins adsorption breakthrough curves on the binderless and the binder-containing zeolite 5A samples at 313 K
Futhermore, the diffusion property of n-paraffins in the fixed-bed adsorber could be well evaluated by the MTZ length. There is no mass transfer resistance for an ideal fixed-bed adsorber, and the length of MTZ is 0. However, in the actual adsorber, the n-paraffins molecules need to overcome the mass transfer resistance to diffuse into the adsorption sites. The MTZ length of the n-paraffins in the binder-containing zeolite 5A laden fixed-bed adsorber is longer than that of the binderless zeolite bed (36.27 cm vs. 20.12 cm for n-C6, 35.00 cm vs. 22.50 cm for n-C7and 32.28 cm vs. 20.00 cm for n-C8), indicating that the adsorption on the binderless zeolite 5A bed has less mass transfer resistance than the binder-containing zeolite bed. Such results suggest that the binderless zeolite 5A is beneficial to the economic separation of naphtha.
3.4 Optimized utilization performance of naphtha
Adsorptive separation was performed according to the aforementioned experimental procedure (see Section 2.3) under the operating conditions covering a feed space velocity of 90 h-1and an adsorption temperature of 573 K. Based on the composition obtained from GC analysis, the RON of naphtha is evaluated to be 60.64. Furthermore, the PONA compositions and RON of the raffinate are shown in Table 7. The total content of n-paraffins in the raffinate is reduced to 0.80% and the raffinate has a high RON rating of 94.37, indicating an excellent blending component for high RON gasoline. The compositions and potential aromatic contents (Ar) of the original naphtha and the raffinate are presented in Table 8. The calculated Ar of the raffinate increases by 10% as compared to the original naphtha. The desorption efficiency of n-paraffins is 85.3% after 30 min of N2desorption, and the n-paraffins concentration in the desorption oil reaches 89.3%.

Table 7 PONA compositions and RON of the raffinate
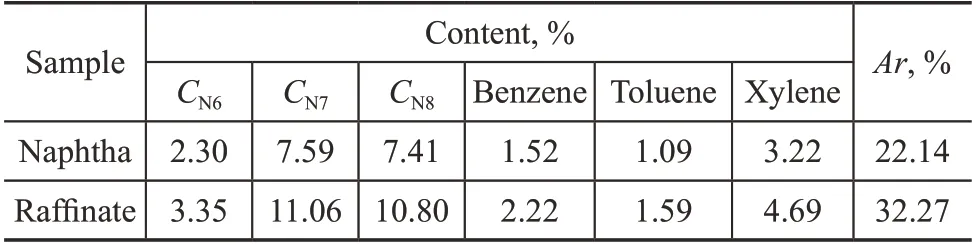
Table 8 Potential aromatic contents of the original naphtha and raffinate
The yields of olefins with respect to the original naphtha and the desorption oil are evaluated, with the results shown in Table 9. The desorption oil demonstrates by 13.3% higher ethylene yield and by 11.7% higher total yield of ethylene, propylene, and butadiene, indicating that the consumption of steam cracking feedstock could be decreased by 25% at the same ethylene production capacity.

Table 9 Olefins yields with naphtha and desorption oil as steam cracking feedstock
4 Conclusions
We demonstrate the efficient adsorptive separation of naphtha via using the binderless zeolite 5A as the adsorbent. Compared to the binder-containing zeolite, the binderless zeolite exhibits by 25%—35% higher adsorption capacity, and has by 115%—130% higher adsorption capacity at breakthrough point and much shorter length of mass-transfer zone. The adsorptive separation of n-paraffins from naphtha was carried out in a fixed-bed adsorber containing the synthesized binderless zeolite 5A under the operating conditions covering a feed space velocity of 90 h-1and an adsorption temperature of 573 K. As compared to the original naphtha, the raffinate shows by 34 units more research octane number and by around 10 percentages higher potential aromatic content while the desorption oil exhibits by 13.3% higher ethylene yield and by 11.7% more total olefins yield.
Acknowledgements:This work is financially supported by the Natural Science Foundation of Shanghai (Grant 16ZR1408100), the National Natural Science Foundation of China (Grant 91634112 and 21878097) and the Open Project of State Key Laboratory of Chemical Engineering (SKL-ChE-16C01).
杂志排行
中国炼油与石油化工的其它文章
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Effects of Blending Long-Chain Alcohols with Jet Fuel on the Macroscopic Spray Characteristics
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
