Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
2020-01-13BianQingminXinMudiXuGuangtongChenShuaiZouKangShiYanqiang
Bian Qingmin; Xin Mudi; Xu Guangtong; Chen Shuai; Zou Kang; Shi Yanqiang
(State Key Laboratory of Catalytic Materials and Reaction Engineering, SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The adsorptive separation of ethylene from ethane exhibits a less energy-intensive-alternative technique with development potential among all processes for separation of ethylene/ ethane currently. In this approach, zeolite 5A with different particle sizes ranging from 3 340 nm to 440 nm was prepared by hydrothermal synthesis. The effect of particle size on the adsorptive separation performance of zeolite 5A was investigated. The results show that the particle size has a significant effect on the ethylene IAST (Ideal Adsorbed Solution Theory) selectivity of zeolite 5A. The zeolite 5A with a particle size of 710 nm demonstrated the highest ethylene selectivity (5.6). The relatively high crystallinity of zeolite 5A is in favor of massive adsorption capacities of ethylene and ethane.
Key words: zeolite 5A; particle sizes; adsorptive separation; ethylene; ethane
1 Introduction
Ethylene is one of the most important and fundamental feedstocks that can be used to manufacture a wide range of essential chemicals, such as polyethylene[1]. Owing to the coexistence of C2hydrocarbons[2], the separation of ethane and ethylene is a crucial stage in the further synthesis of ethylene-based polymers. However, the separation is exceedingly challenging[3], due to the close physical and chemical properties of ethylene and ethane[4]. At present, the industrial separation of ethylene and ethane is mainly accomplished by cryogenic separation, which is not only harsh in operating conditions[3], but also extremely energy intensive[5-7]. The pressure swing adsorption (PSA) process using zeolites as adsorbents, which can perform the adsorption-desorption process of ethylene and ethane at room temperature[8-11], is proposed to be a favorable energy-saving alternative in ethylene/ethane separation. However, due to the physical adsorption of ethylene and ethane by the zeolite, the adsorption capacity and selectivity of most zeolite-based adsorbents are not satisfactory. In the past two decades, many efforts have been dedicated to improve the adsorption capacity and selectivity of zeolites. Among them, selective adsorption of ion-exchanged zeolites has been extensively studied by far[12-15]. However, the variation of the selective adsorptive separation performance, which is caused by the alteration in particle size of zeolites, is not thoroughly studied. In this approach, a series of zeolite 5A with different particle sizes were synthesized to thoroughly investigate its influence on adsorptive separation of ethylene and ethane. During this process, zeolite 5A with a suitable particle size (~710 nm) exhibited the highest ethylene selectivity, which may due to the different degree of influence on the ethylene adsorption properties of zeolite 5A with various particle sizes by moisture. The zeolite 5A with fewer defects will facilitate the higher capacity for adsorption of both ethylene and ethane simultaneously.
2 Experimental
2.1 Materials.
Sasol’s high-purity boehmite (AlO(OH), CR), sodium silicate (Na2SiO3·9H2O, AR), sodium hydroxide (NaOH, AR), calcium chloride (CaCl2, AR), ethylene (C2H4, 99.999%), ethane (C2H6, 99.95%) were used in the experiments..
2.2 Preparation of zeolite 5A
2.2.1 Preparation of zeolite 5A
In a typical procedure, the solution containing sodium silicate, sodium hydroxide, and boehmite with a molar ratio equating to 1.0Al2O3: 1.92SiO2: 3.16Na2O: 128H2O[16]was subjected to aging under stirring at room temperature in a Teflon-lined stainless-steel autoclave. Then the gel was hydrothermally crystallized at a certain temperature for a certain period. The as-synthesized zeolite NaA was filtered and washed with excessive amount of deionized water until the discharged water showed a pH value of 7—8, and then the product was dried at 353 K overnight. The parameters used in synthesis of zeolite 5A are shown in Table 1. For conducting the ionexchange, 1 g of zeolites NaA was dispersed in 10 mL of 0.9 mol/L calcium chloride aqueous solution. After being subjected to stirring at 343 K for 1 h, the zeolite was filtered, washed with excessive amount of deionized water, dried at 353 K overnight, and subsequently calcined at 823 K for 3 h.
2.2.2 Water absorption experiment
After being dehydrated at 823 K, the sample was quickly transferred to a chamber, in which a constant temperature and humidity were maintained, and then the water adsorption experiment was performed at a temperature of 303 K and a relative humidity of 60 % for 30 minutes.
2.3 Characterization and gas adsorption measurements
Zeolite samples were characterized by X-ray diffraction (XRD) on a Rigaku TTR-III X-ray diffractometer, using Cu Kα radiation (40 kV, 250 mA) in the scanning angle (2θ) range of 5°—50°. The morphology of samples was characterized by Hitachi S-4800 scanning electron microscopy (SEM). A Rigaku ZSX100E X-ray fluorescence (XRF) spectrometer was used to analyze the bulk chemical composition of the zeolite. The N2adsorption-desorption isotherms were measured on a Micromeritics ASAP 2420 surface area and porosimetry analyzer. Thermogravimetric analysis (TGA) was performed on a TGA Q500 instrument by heating the zeolite 5A at a temperature increase rate of 10 K/min from room temperature to 573 K in a nitrogen atmosphere. C2H6, C2H4and He adsorption isotherms were collected on an Intelligent Gravimetric Adsorber (IGA) of Hiden Isochema.
3 Results and Discussion
3.1 Characterization of LTA Zeolite
The purity and crystallinity of the synthesized LTA zeolite were characterized by XRD. As shown in Figure 1, all the six samples exhibited typical LTA structure with sharp and intense diffraction peaks, confirming that zeolite 5A was successfully synthesized with a high crystallinity. The relative crystallinity of other samples were deteriorated subtly, when considering the crystallinity of S1 as 100%, which could be attributed to the partial destruction of long range ordered structure from decreasing particle sizes, as shown in Figure 1.
To further investigate the morphology and particle size of synthesized zeolite 5A, SEM images were presented in Figure 2. Zeolite 5A particles exhibited the cube morphology with a uniform and regular geometry, indicating a highly crystalline structure during the formation process, which was consistent with the XRD results. The particle size distribution was statistically measured, as shown in Figure 3. The average particle size is shown in Table 1. The results confirm the successful synthesis of zeolite 5A with different particle sizes ranging from 3340 nm to 440 nm.
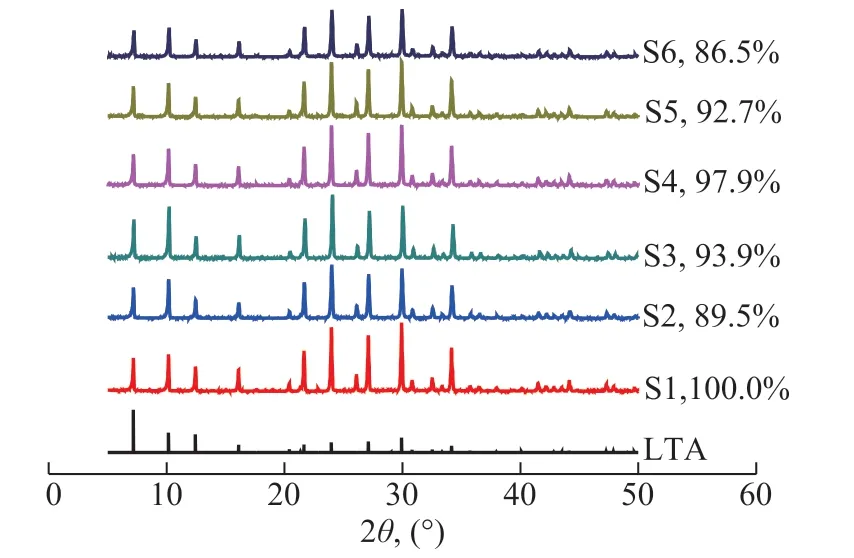
Figure 1 XRD patterns of the synthesized zeolite 5A samples
The Ca2+exchange degree of zeolite 5A was confirmed by XRF. As illustrated in Table 1, all samples achieved a similar Ca2+exchange degree (> 86%) with a low content of remaining Na+. The BET surface areas and pore volume of the zeolite 5A were determined by N2adsorption measurement at 77 K, as depicted in Table 1. According to the N2adsorption results, the BET surface area and pore volume of all the zeolite 5A samples were close to previously reported values[16](599 m2/g and 0.288 cm3/g), indicating that the structure of the material was not destroyed when the synthesis conditions were altered, which was consistent with the XRD and SEM results. In addition, the variation of the BET surface area of zeolite 5A was substantially consistent with the change of crystallinity.

Figure 2 SEM images of synthesized zeolite 5A samples

Figure 3 Particle size distribution of synthesized zeolite 5A samples
3.2 C2H4 and C2H6 adsorption capacity on zeolite 5A
The adsorption isotherms data of C2H4and C2H6on zeolite 5A are presented in Figure 4. Clearly, the adsorption behavior of C2H4on all zeolite samples is quite similar, as does C2H6. As shown in Table 2, the adsorption capacities of all zeolite 5A samples for ethylene and ethane are greater than that reported in the literature[12]. The capacities of S1 at 303 K, 100 kPa for adsorption of ethylene and ethane reach 3.49 mmol/g and 2.71 mmol/g, respectively, which are by 42% and 58% higher than that reported in the literature, respectively. The outstanding adsorption properties of synthesized zeolite 5A may be attributed to the low silicon/aluminum ratio, which can result in more exchangeable cation sites. The Ca2+sites, as the adsorption center[17], can induce the polarization of ethylene and ethane, leading to the enhancement of adsorption capacities. In addition, there are still some differences in the adsorption capacities of various samples (as shown in Table 2). The results indicate that the adsorption capacities of zeolite 5A for ethylene and ethane dropped down with its gradually declining crystallinity, which may be stemmed from the emergence of disordered structure.
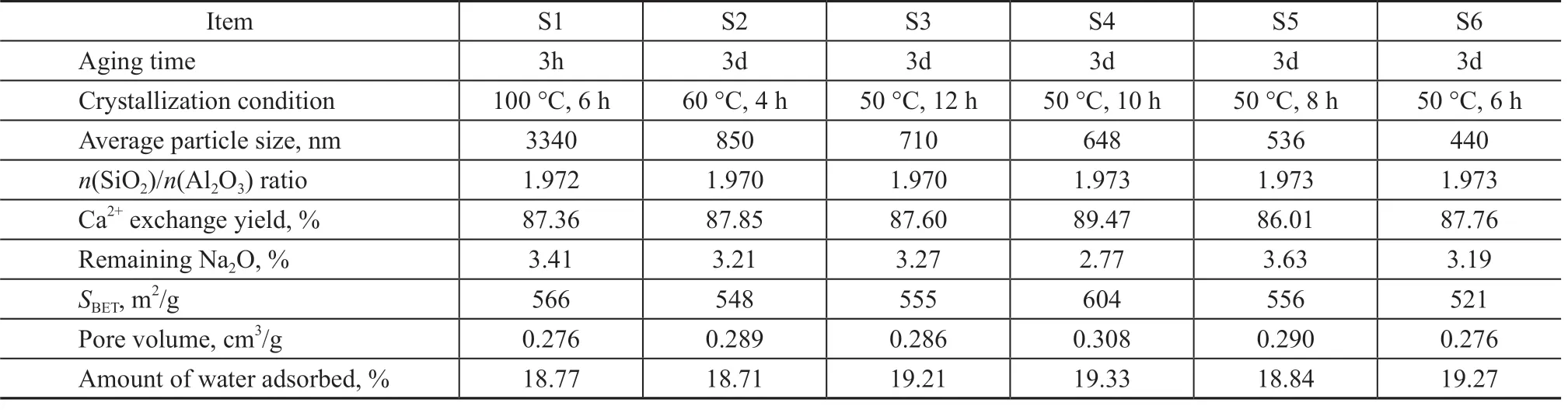
Table 1 Physicochemical properties of synthesized zeolite 5A samples
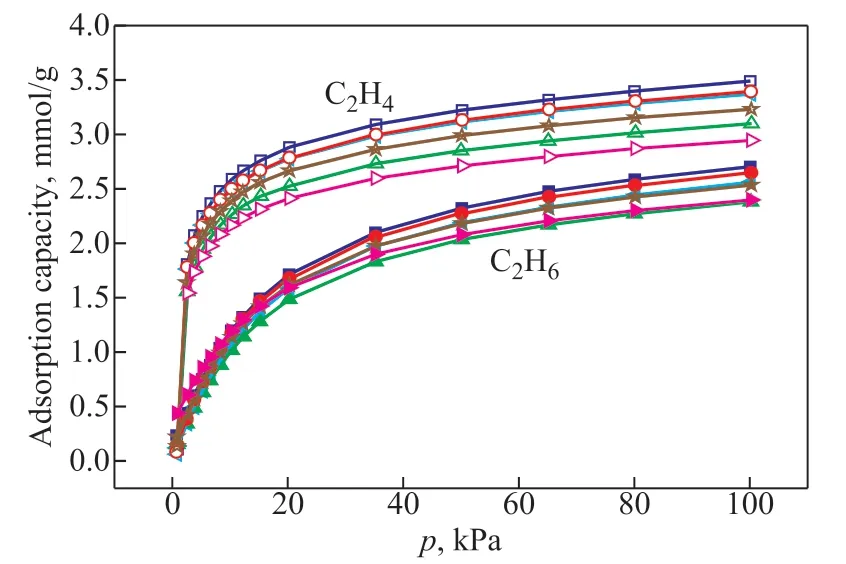
Figure 4 C2H6 and C2H4 adsorption isotherms for zeolite 5A samples at 303 K

Table 2 Adsorption capacities1) and crystallinity of zeolite 5A samples
3.3 C2H4/C2H6 adsorption selectivity in zeolite 5A
To investigate the performance of zeolite 5A for separation of ethylene from ethane, the separation selectivity of C2H4/C2H6binary mixture was calculated using the IAST (ideal adsorbed solution theory) model[19-21]. The measured data of single-component gas adsorption isotherms of ethylene and ethane can be fitted using the DSLF (Dual-Site Langmuir-Freundlich) model[22-23]:
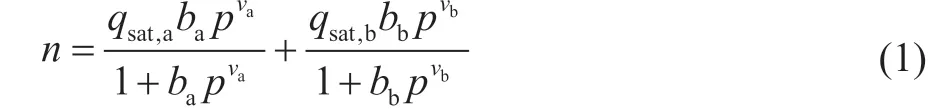
where n (mmol/g) represents the adsorbed amount, qsat(mmol/g) represents saturated adsorption capacity, b (kPa-1) represents the affinity coefficient of the sites, p (kPa) represents the gas pressure, ν indicates the deviations from an ideal homogeneous surface, a and b represent two different adsorption sites. The fitting results show that the adsorption behavior of ethylene and ethane on zeolite 5A can be accurately described by the DSLF model with the correlation coefficient R2>0.999 to meet the requirements (R2>0.99) of this model.
Figure 5 depicts the ethylene selectivity curve of zeolite 5A. Obviously, the ethylene selectivity of all samples increases with the pressure, which is coincident with the results reported in the literature[12]. In the range of 0—10 kPa, the ethylene selectivity exhibited a rapid increase trend. However, when the pressure is above 10 kPa, the ethylene selective grows slowly. More importantly, it is obvious that the ethylene selectivity of zeolite 5A with various particle sizes is different.
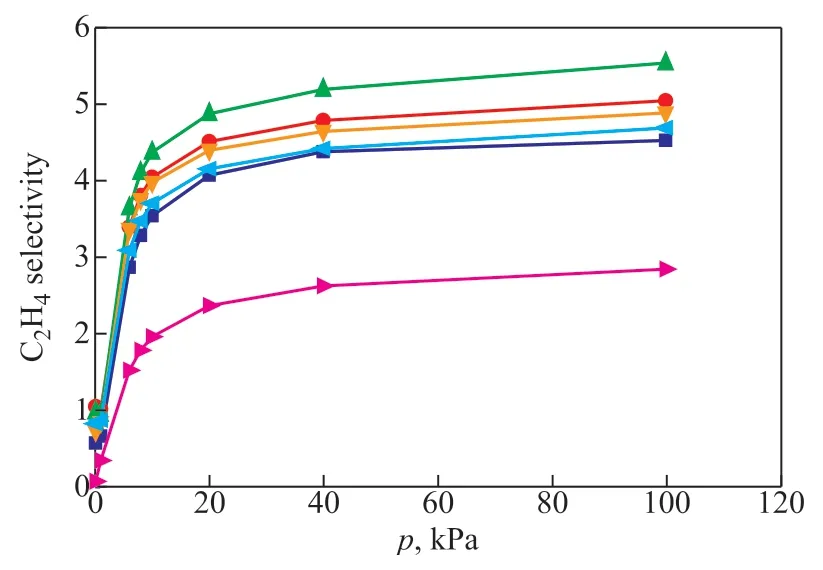
Figure 5 Ethylene/ethane (volume ratio of 1:1) selectivity of zeolite 5A samples at 303 K
On account of a relatively low vacuum environment (10-5—10-6Pa), the samples can hardly be completely dry, even after vacuum degassing at 423 K. Due to the strong affinity between Ca2+on zeolite 5A and polar moisture[18], the absorbed water will occupy the Ca2+adsorption sites of zeolite 5A, resulting in the competitive adsorption of ethylene and water on zeolite 5A during the adsorptive separation procedure. To investigate the effect of water on the adsorption properties of zeolite 5A, the ethylene adsorption performance of water-adsorbed zeolite 5A was executed. From the very beginning, all the zeolite 5A samples with different particle sizes was subject to adsorption of the same amount of water, which could be verified via total weight loss before 573 K by thermogravimetric analysis, as shown in Table 1. Then, the ethylene adsorption experiment was further carried out on the water-adsorbed zeolite 5A. Since the water-adsorbed zeolite 5A was subtly dehydrated by the gas flow during the test, helium was used to do a blank experiment to reduce the error caused by the sample selfdehydration. The adsorption isotherms data of C2H4and He on the water-adsorbed zeolite 5A are presented in Figure 6. As shown in Figure 6, all the water-adsorbed zeolite 5A samples lost weight in both C2H4and He atmosphere, which was caused by the dehydration of the samples during the test. More importantly, it can be observed that the water loss of zeolite 5A with different particle sizes in He atmosphere was similar, indicating that the self-dehydration rate of various samples caused by the influence of gas flow was almost the same. However, in C2H4atmosphere, the weight loss of samples was caused by the self-dehydration and the dehydration caused by the competitive adsorption of ethylene (since one ethylene molecule might displace multiple water molecules). By comparison, it was found that the water loss caused by the competitive adsorption of ethylene (denoted as ΔS, shown in Figure 6) of zeolite 5A with different particle sizes was extremely different, revealing that the diverse extent of the effect of water on the ethylene adsorption properties of zeolite 5A samples with various particle sizes. Among all the zeolite samples, the ΔS of S3-H2O was the largest, indicating that S3 was minimally affected by water and had a strongest adsorption of ethylene under the same experimental conditions.
The C2H4selectivity of zeolite 5A increases at first and then decreases with a reducing particle size, which is consistent with the trend of ΔS with particle size (shown in Figure 7), indicating that the change of C2H4selectivity may be due to the different effect of water on the ethylene adsorption properties of zeolite 5A samples with various particle size. Among all the zeolite samples with particle size ranging from 3340 nm to 440 nm, the zeolite 5A sample with a particle size of 710 nm demonstrated a highest ethylene selectivity (5.6), which may be attributed to its ethylene adsorption performance, which is minimally affected by water and has a stronger adsorption property for ethylene under the same experimental conditions.
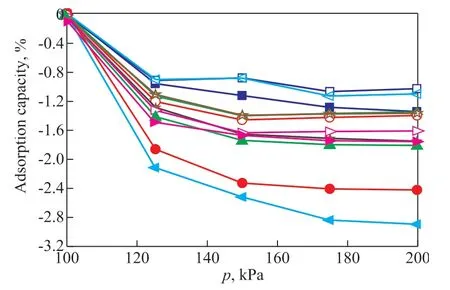
Figure 6 C2H4 and He adsorption isotherms for waterabsorbed zeolite 5A samples at 303 K

Figure 7 C2H4/C2H6 (volume ratio of 1:1) selectivity and the △S of zeolite 5A samples at 303 K, 100 kPa
4 Conclusions
The relationship between ethylene/ ethane selectivity and the particle size of zeolite 5A was investigated by various characterization methods and IAST selectivity calculation. The results indicate that the particle size has a significant effect on its performance for adsorptive separation of ethylene/ ethane. On one side, the adsorption capacities of zeolite 5A for ethylene and ethane dropped down with its gradually declining crystallinity. Zeolite with excessively reduced particle size (smaller than 650 nm) will result in a partially destruction of crystal structure, which will further exert a negative impact on the ethylene and ethane adsorption capacity. On the other side, an appropriately reducing particle size (from 3340 nm to 710 nm) can increase the ethylene selectivity of zeolite 5A. Nevertheless, an excessive reduction in particle size of zeolite 5A will result in a sharply decrease in its ethylene selectivity. The change of ethylene selectivity may cast about for how the water can influence the different ethylene adsorption properties of zeolite 5A samples with various particle sizes.
Acknowledgement:This work was supported by the National Key R&D Program (2016YFB0301601).
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effects of Blending Long-Chain Alcohols with Jet Fuel on the Macroscopic Spray Characteristics
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
