Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
2020-01-13ZhengHuiSunHongweiHeYifengSuShuoZhuangMinyangLiuXinyang
Zheng Hui; Sun Hongwei; He Yifeng; Su Shuo; Zhuang Minyang; Liu Xinyang
(SINOPEC Research Institute of Petroleum Processing, Beijing 10083)
Abstract: In order to solve the hardening problem of complex calcium lubricating grease, the water absorption test of several calcium salts was carried out, and it was found that calcium 12-hydroxystearate did not absorb water, and calcium acetate, calcium phosphate and calcium borate had different degrees of water absorption. Calcium acetate has the highest water absorption rate, while calcium phosphate and calcium borate show comparable water absorption rates. Upon using the molecular simulation technology, it is found that in the complex calcium grease system, calcium phosphate and calcium borate tend to combine with water, which inhibits the water absorption of calcium acetate and alleviate the hardening problem.
Key words: lubricating grease; complex calcium; hardening
1 Introduction
Calcium lubricating grease is the earliest grease product. It has a long history and excellent performance in all aspects. However, due to its low dropping point, it is only suitable for low-temperature friction parts. In 1940, Carmichael and Hain had discovered that complex calcium lubricating grease with a dropping point of more than 200 °C could be prepared by the addition of a small amount of calcium salt of low molecular acid[1]. In 1950 Schott and Armstrong had found that the use of a high molar ratio of acetic acid to fatty acid could improve the extreme pressure performance of complex calcium lubricating grease[2]. This type of complex calcium lubricating grease has been widely used in industry with its high temperature performance, excellent resistance to water, excellent colloidal stability, high extreme-pressure wear resistance, good shear stability, etc.
Although complex calcium lubricating grease has a lot of outstanding performance, it hardens in the process of storage and under high-temperature conditions. The hardening of complex calcium lubricating grease has three manifestations[3], viz.: surface hardening, overall hardening, and shear hardening. Overall hardening means that the consistency of the grease increases in the process of storage. Shear hardening refers to the phenomenon that the consistency of the grease increases after being sheared. Surface hardening is the most important and common hardening phenomenon, which is related to the degree of grease surface exposure, atmospheric humidity and temperature. In the actual application of lubricating grease, these three hardening phenomena often occur at the same time. In the process of storage the hardening of complex calcium lubricating grease includes surface hardening and overall hardening. And in the process of work, shear hardening and overall hardening may occur at the same time. Upon lubricating the running bearing with grease, due to mechanical periodic startup and shutdown, the hardening process of complex calcium lubricating grease is more complicated. There may be three types of hardening phenomena, but the mechanism of hardening may vary.
Many scholars[4-9]have done a lot of research work on how to alleviate the hardening problem of complex calcium lubricating grease. The research work mainly focuses on formulas, production processes and additives. Schott[10]used phosphoric acid instead of some acetic acid, which can alleviate the hardening problem of complex calcium lubricating grease in the process of storage. Sproule[11]used boric acid instead of acetic acid to prepare complex calcium lubricating grease. Although the dropping point of the prepared product is not high, it is stable at higher temperature. Zheng Keshu[12]discussed the influence of borate on the performance of complex calcium lubricating grease. Thomas[13]and Laurance[14]used lactic acid to replace a part of acetic acid. Yan Jun[15]used high, medium and low molecular acids to prepare complex calcium lubricating grease and made some adjustments to the production process, which alleviated the hardening phenomenon to some extent. In summary, domestic and foreign scholars have found that the addition of phosphoric acid and boric acid can effectively alleviate the hardening problem of complex calcium lubricating grease, but its mechanism is still unclear.
2 Experimental
2.1 Materials
Hydrogenated white oil 500N (with a kinematic viscosity of 100 cSt (at 40 °C), a flash point of 250 °C, a pour point of -12 °C); 12-hydroxystearic acid, industrial products (manufactured by the Tongliao Tonghua Ramie Chemical Company); acetic acid, analytically pure (manufactured by the Beijing Chemical Industry Co.); boric acid, analytically pure (manufactured by the Beijing Chemical Industry Co.); phosphoric acid, analytically pure (manufactured by the Beijing Chemical Industry Co.); calcium hydroxide, analytically pure (manufactured by the Beijing Chemical Industry Co.).
2.2 Grease manufacturing
The complex calcium lubricating grease was manufactured according to the following procedures: At first a certain amount of the base oil was mixed with a calcium hydroxide emulsion, to which the acetic acid aqueous solution was added at room temperature, and then this mixture was preheated to 85—95 °C prior to being mixed with 12-hydroxystearic acid, boric acid, and phosphoric acid. The temperature of the mixture was then increased to a top temperature of 200 °C. The acids and alkali used in the reaction were according to their stoichiometric ratios, with a 3% excess amount of Ca(OH)2used. The cooling process was performed using the rest oil. The sample was obtained via kneading twice with a three-roller mill.
3 Results and Discussion
3.1 Effect of boric acid on hardening of complex calcium lubricating grease
The grease consists of two components: soap and base oil. The base oil was a hydrogenated white oil 500N. The soap content was 25%, which was synthesized by reactions between 12-hydroxystearic acid, acetic acid, boric acid, and calcium hydroxide. The molar ratio of 12-hydroxystearic acid to acetic acid is 1:5. The complex calcium lubricating grease was prepared by adding boric acid in different proportions. The prepared sample was subjected to hardening test, and its dropping point and cone penetration changes before and after hardening were measured. The results are shown in Table 1.
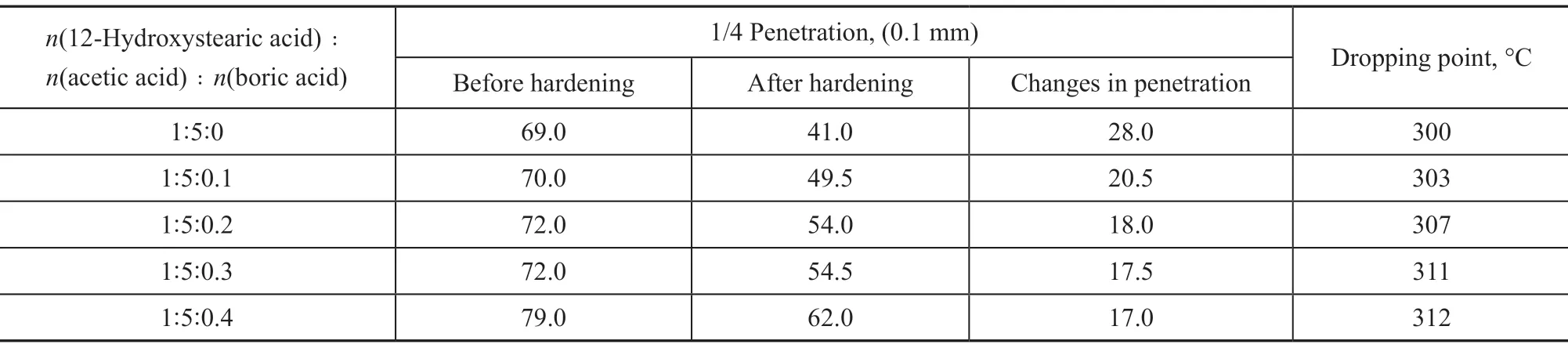
Table 1 Results of the effect of boric acid on hardening of complex calcium lubricating grease
It can be seen from Table 1 that by adding different proportions of boric acid to the traditional complex calcium lubricating grease, the dropping point of the grease is increased. As the proportion of boric acid increases, the consistency of the grease gradually decreases, and the penetration gradually increases, but the hardening problem is gradually alleviated.
3.2 Effect of phosphoric acid on hardening of complex calcium lubricating grease
The grease consists of two components, viz.: soap and base oil. The base oil was hydrogenated white oil 500N. The soap content was 25%, which was synthesized by reacting 12-hydroxystearic acid, acetic acid, and phosphoric acid with calcium hydroxide. The molar ratio of 12-hydroxystearic acid to acetic acid was 1:5. The complex calcium lubricating grease was prepared by adding phosphoric acid in different proportions. The prepared sample was subjected to hardening test, and its dropping point and cone penetration changes before and after hardening were measured. The results are shown in Table 2.It can be seen from Table 2 that by adding different proportions of phosphoric acid to the traditional complex calcium lubricating grease, the dropping point of the grease is increased. As the proportion of phosphoric acid increases, the consistency of the grease gradually decreases, and the penetration gradually increases, but the hardening problem is gradually alleviated.
3.3 Discussion on the mechanism of boric acid and phosphoric acid for relieving hardening
Through the discussion of the factors affecting the hardening of complex calcium lubricating grease, it is believed that acetic acid which absorbs water from the air contributes a lot to the hardening problem[16-18].
3.3.1 Water absorption test of calcium salts
Calcium 12-hydroxystearate, calcium acetate, calcium phosphate, and calcium borate were prepared according to the stoichiometric ratio. These calcium salts were placed in an oven at 100 °C for 2 h to remove excess water. After drying, a certain amount of the sample was weighed and placed in a 25 mm × 25 mm weighing bottle. The weighing bottle filled with calcium salt was placed in a desiccator filled with distilled water at the bottom, and weighed at regular intervals to compare the water absorption of different calcium salts, with the results presented in Table 3.It can be seen from Table 3 that calcium 12-hydroxystearate does not absorb water substantially, and calcium borate, calcium phosphate and calcium acetate show different degrees of water absorption. Among them, calcium acetate has the highest water absorption rate, and calcium borate and calcium phosphate have comparable water absorption rate.
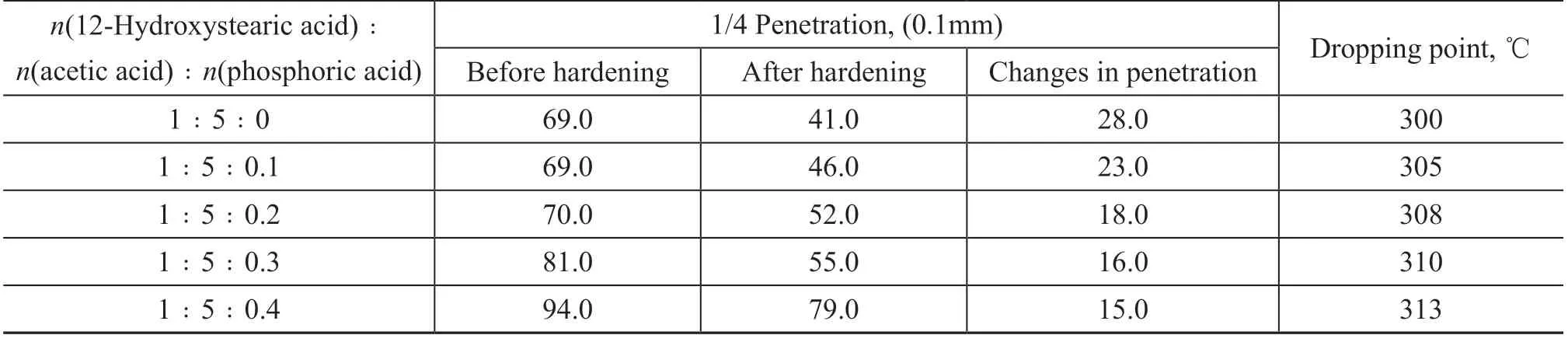
Table 2 Results of the effect of phosphoric acid on hardening of complex calcium lubricating grease

Table 3 Experimental results on water absorption of different calcium salts
3.3.2 Molecular simulation of the binding force between calcium salt and water
Calcium borate, calcium phosphate, and calcium acetate all absorb water. It can be seen from Sections 3.1 and 3.2 that the addition of boric acid and phosphoric acid can effectively alleviate the hardening problem of complex calcium lubricating grease. Therefore, in the grease system, the pattern by which the three calcium salts are combined with water and what is the strength of the combination will be discussed below.
3.3.2.1 Calculation method
The DMol3module of quantum mechanics in the molecular simulation software Materials Studio 6.0 is adopted to carry out the theoretical calculation of this part. The DMol3module is a unique density functional (DFT) quantum mechanical program that is the only commercial quantum program, which can simulate the properties and behavior of gases, solutions, surfaces and solids, and can simultaneously consider both periodic and aperiodic solvation effects. It is used in many fields such as chemistry, materials, chemicals, and solid physics.
In the high-precision DFT calculation, the PW91 functional method based on generalized gradient approximation (GGA) is used, and the DND basis set is selected for full electronic calculation. The convergence criteria of energy, force, and displacement are 2×10-5Ha, 0.04 Ha/nm, and 5×10-4nm, respectively. The threshold for self-consistent field (SCF) iteration convergence is set at 1×10-5Ha. In the Dmol3module, the van der Waals radii of H, C, O, and Ca atoms are: 0.130 nm, 0.200 nm, 0.172 nm, and 0.195 nm, respectively.
The formula for calculation of binding energy between calcium salt and water molecule is:
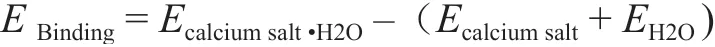
3.3.2.2 Calculation results
(1) Optimal conformation of three calcium salts Figure 1 shows the optimal conformation of the calcium borate, calcium phosphate, and calcium acetate. The green atom is a calcium atom, the red atom is an oxygen atom, the gray atom is a carbon atom, the white atom is a hydrogen atom, the pink atom is a boron atom, and the purple atom is a phosphorus atom.
(2) Electronic structure properties of water molecule and calcium salt molecule
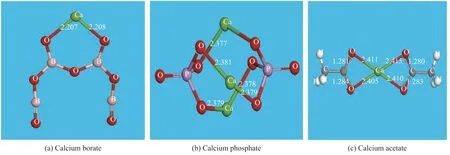
Figure 1 Optimal conformation of calcium salts
Upon taking calcium borate as an example, Figures 2 and 3 are charge distribution diagrams of water molecule and calcium borate molecule, respectively.
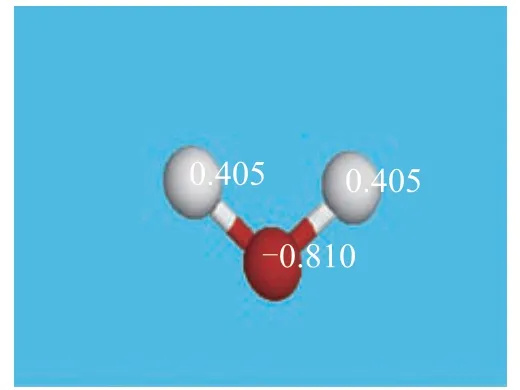
Figure 2 Charge distribution of water
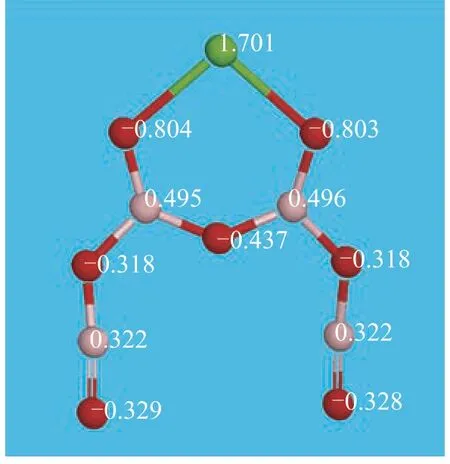
Figure 3 Charge distribution of calcium borate

Figure 4 Lowest vacant orbital diagram of calcium borate
Figure 4 shows the lowest vacant orbital diagram of calcium borate. Calcium ions in calcium borate have an empty valence orbital, and the number of positive charges is 1.701. The oxygen atoms in water molecule have 2 pairs of lone electrons with negative charge. This may cause the water molecule to form a Ca...O coordination covalent bond, making the calcium borate molecules bind together. On the other hand, water molecule may also form intermolecular hydrogen bonds with oxygen atom provided with lone pair electrons in the calcium salt molecule. Table 4 shows the positive charge numbers of the calcium atoms in the three calcium salt molecules. It can be seen from Table 4 that all calcium ions in the three kinds of calcium salt molecules may bind with water molecules through coordination covalent bonds.

Table 4 The positive charge number of calcium atoms in the three calcium salts
(3) Optimal conformation of calcium salt combined with water molecule
Figure 5(a) shows the optimal conformation of calcium borate molecule in combination with water molecule. It can be seen that when calcium borate is combined with water molecule, the distance between calcium ions and oxygen atoms in water molecule is 0.2479 nm, which is far less than the sum of the two atoms of the van der Waals radius (0.367 nm), and is very close to the Ca-O. This can further prove theoretically that calcium ions in calcium borate can form coordination covalent bonds with water molecule.
Figure 5(b) shows the optimal conformation of calcium phosphate molecule in combination with water molecule. It can be seen that when calcium phosphate is combined with water molecule, the distance between calcium ions and oxygen atoms of water molecule is 0.2422 nm. This suggests that the calcium ion may form a coordination covalent bond with the water molecule. In addition, the distance between the hydrogen atom in the water molecule and the oxygen atom in the calcium phosphate is 0.1573 nm, and ∠O-H...O is 148.4°, which suggests that in addition to formation of coordination bonds with calcium ions, water molecule can also form O-H...O hydrogen bond with oxygen atoms in calcium phosphate at the same time.
Figure 5(c) shows the optimal conformation of calcium acetate molecule in combination with water molecule. It can be seen that when calcium acetate is combined with water molecule, the distance between calcium ions and oxygen atoms of water molecule is 0.2454 nm. This suggests that the calcium ion may form a coordination covalent bond with the water molecule. In addition, the distance between the hydrogen atom in the water molecule and the oxygen atom in the calcium acetate molecule is 0.1953 nm, and ∠O-H...O is 125.9°, which suggests that in addition to formation of coordination bonds with calcium ions, water molecule can also form O-H...O hydrogen bond with oxygen atoms in calcium acetate at the same time.

Figure 5 Optimal conformation of calcium salts in combination with water
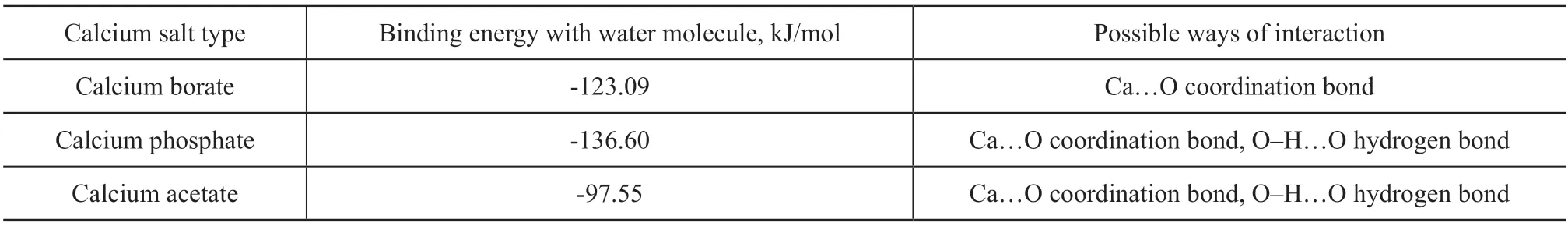
Table 5 Calculation results of binding energy between three calcium salts and water molecule
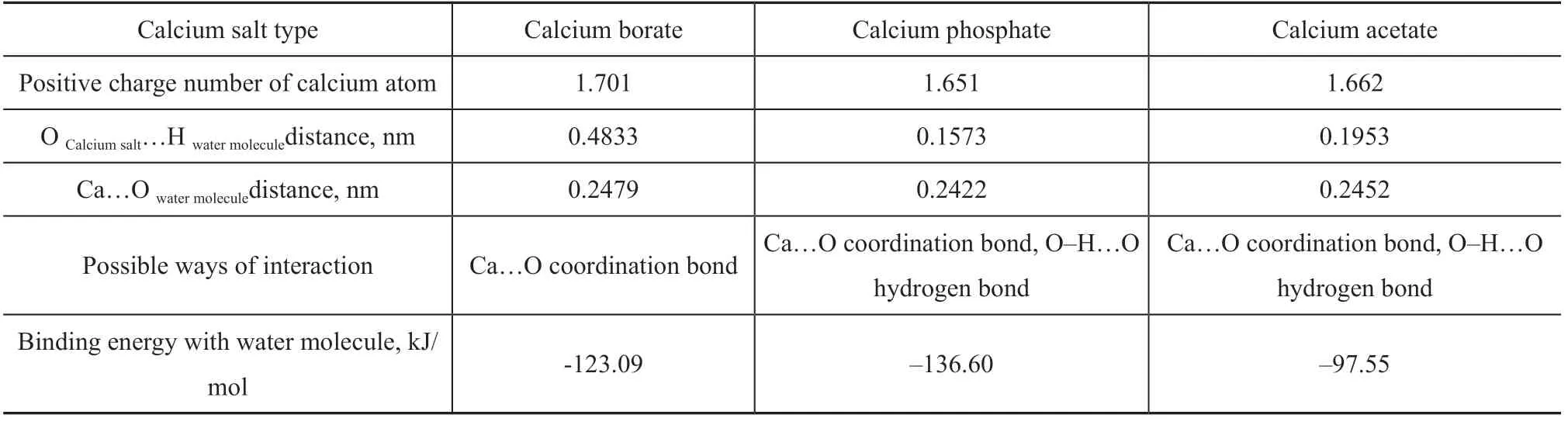
Table 6 Molecular simulation calculation data synthesis
(4) Calculation of binding energy between calcium salt and water molecule
Table 5 shows the calculation results of binding energy between three calcium salts and water molecule. The higher the absolute value of binding energy, the stronger the binding between calcium salts and water molecules would be. The calculation formula of binding energy between calcium salt and water molecule is given in the section 3.3.2.1.
It can be seen from Table 6 that the binding ability of calcium phosphate and calcium borate to water molecule is significantly greater than that of calcium acetate. Therefore, calcium phosphate and calcium borate are superior to calcium acetate in combination with water.
In summary, after adding phosphoric acid and boric acid to the traditional complex calcium lubricating grease, their calcium salts and calcium acetate have a competitive relationship when combining with water. Calcium phosphoric and calcium boric tend to combine with water which can inhibit the water absorption of calcium acetate and alleviate the hardening problem.
4 Conclusions
In the complex calcium lubricating grease system, calcium 12-hydroxystearate does not absorb water substantially, while calcium borate, calcium phosphate, and calcium acetate show different degrees of water absorption. Calcium phosphoric and calcium boric tend to combine with water which can inhibit the water absorption of calcium acetate and alleviate the hardening problem.
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Comparison of Mass Transfer Characteristics between Countercurrent-Flow and Crosscurrent-Flow Rotating Packed Bed
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
