Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
2020-01-13ZhaoYutongZongMengyaFanCunzhengChenKunWangDanhongZhangMinghui
Zhao Yutong; Zong Mengya; Fan Cunzheng; Chen Kun; Wang Danhong, ; Zhang Minghui
(1. TKL of Metal and Molecule Based Material Chemistry, National Institute for Adνanced Materials, School of Materials Science and Engineering, Nankai Uniνersity, Tianjin 300350; 2. Key Laboratory of Adνanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai Uniνersity, Tianjin 300071)
Abstract: VO2 (M) doped with different metals (Ti, Ce, Nb, Zr, W, and Mo) was successfully synthesized by the hydrothermal reduction process using oxalic acid as the reductant. The results showed that doping metals in the VO2 (M) lattice could enhance the oxidative desulfurization (ODS) activity. The DSC and Raman spectra revealed that VO2 (M) retained its monoclinic structure after doping. Mechanism for the improvement of ODS activity by doping was investigated. Electron transfer to V (V) atom occurs upon doping, and then V (V) changes into V (IV), which can promote the ODS reaction. This explanation can be confirmed by the fact that doping can promote the proportion of V (Ⅳ) after doping as revealed by the XPS analysis.
Key words: doped VO2; hydrothermal reduction method; oxidative desulfurization; mechanism; electron transfer
1 Introduction
Many stringent regulations have been enacted to strictly limit the sulfur content in petroleum products to an extremely low level (<10 μg/g)[1-2]. Traditional hydrodesulfurization (HDS) process is difficult to remove refractory dibenzothiophene (DBT) and 4,6-dimethydibenzothiophene (4,6-DMDBT) affected by their steric hindrance[3-4]. Oxidative desulfurization (ODS) reaction can remove these sulfur compounds efficiently at room temperature and atmospheric pressure and is able to produce a sulfur-free liquid fuel[5]. In recent decades, many studies have focused on finding superior ODS catalysts[6-8]. Currently, many publications have reported that V2O5catalysts[9-12]show high activity in ODS.

Figure 1 Unit cell of VO2 (M) and VO2 (R) (marked by purple line)[13]
In our previous work[13], we applied monoclinic VO2(M) and rutile VO2(R) in ODS reactions. It is generally acknowledged that a phase transition occurred from VO2(M) to VO2(R) at 68 °C (Figure 1). We have proved through experiments that VO2(R) showed lower ODS reaction activation energy (Ea) than VO2(M). This fact can be explained by the energy band structure of metallic VO2(R) and semiconductive VO2(M)[14].As suggested by Goodenough (Figure 2)[15], when the temperature is below the phase transition temperature, the monoclinic structure VO2(M) distorts and increases the orbital hybridization of V 3d and O 2p, leading to π* band up and band down (as the repulsive force becomes larger, making the band gap between π* and π become larger). At the same time, V atoms move and form closer V-V bond in pairs along the CRaxis (called V-V dimerization). Therefore, along the CRaxis the long and short folded V-V chains are formed, and the symmetry is reduced. V-V dimerization causes the dⅡorbit that is parallel to the CRaxis to be overlapped with each other, resulting in splitting of the dⅡenergy band into two upper and lower dⅡbands, with one being filled with electrons and the other being empty. The π* band is lower than the empty dⅡband, so the π* band acts as the conduction band, while the full band dⅡacts as the valence band. A 0.67 eV gap is formed between the π* band and the full dⅡband, indicating its semiconductive nature. Above the phase transition temperature, the monoclinic structure VO2(M) turns into the quadrangular rutile structure VO2(R), and the antibonding orbital π* band and the bonding orbital π band are formed by the hybridization of the 3dπorbit of V atom and the 2pπorbit of O atom. Another 3d orbit of V atom forms a dⅡband which is parallel to CR, partially filled with electrons as the conduction band and overlapped with the π* band. At this point, the Fermi level (EF) falls between the dⅡband and the π* band, showing the metallic property[15]. The low-temperature VO2(M) phase with the monoclinic P21/c space group acts as a semiconductor. Besides, the transmission of near-infrared light is high. However, the high temperature VO2(R) phase with the tetragonal P42/mnm space group is metallic in nature which is highly reflective to the near-infrared light.
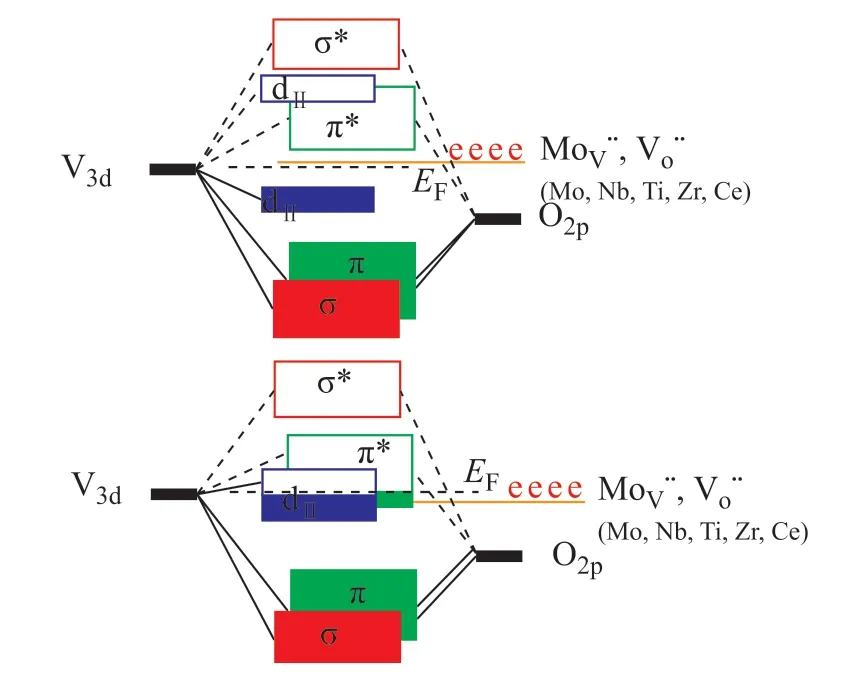
Figure 2 The band structure of the semiconductive VO2(M) (the left one) and the metallic VO2(R) (the right one)
This unique characteristic of VO2allows it to contribute to a variety of technological applications. So far, a plenty of articles have been published about vanadium dioxide VO2(M). The vast majority of these publications have focused on the applications such as smart windows[16-17], resonators[18], sensors[19-20], etc.
In this work, we doped transition metals in the lattice of VO2by substituting V4+to form electrons expressed in Equation (1). The doped VO2catalysts show high ODS activity compared with that of pure VO2. The XPS results denote the high V4+/V5+molar ratios for the doped catalysts, implying the formation of electrons after doping. Therefore, high ODS activity can be related to the electrons formed according to the band structure as shown in Figure 2.
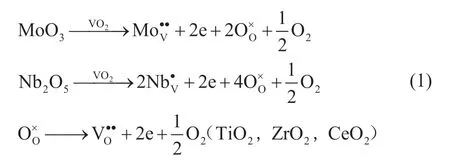
2 Experimental
2.1 Catalysts preparation
VO2(M) powder was obtained by the hydrothermal reduction method. In the aqueous solution, oxalic acid, V2O5, and 3% of transition metals [Ti(SO4)2, Ce2(SO4)3, Nb2O5, ZrO2, (NH4)6Mo7O24·4H2O, etc.] were mixed fully at 70 °C. The solution was transferred into a stainless-steel autoclave lined with Teflon. Then it was sealed and kept at 200 °C for 24 h. When the autoclave was cooled down to room temperature, the solution was filtered to separate the precipitate. After the precipitate was washed with ethanol and deionized water, it was dried in air for one night. The precursor is put into a tubular resistance furnace and calcined at 500 °C for 2 h in nitrogen atmosphere.
2.2 Characterization of catalyst
X-ray diffraction (XRD) spectra of the catalysts were obtained in a Bruker D8 FOCUS diffractometer using Cu Kα radiation (λ= 0.154178 nm, tube voltage= 40 kV and tube current= 100 mA) and a scanning angle ranging from 3° to 80° (2θ) at a scanning rate of 6 (°)/min.
The differential scanning calorimetry (DSC) was applied on a TA SDT Q600 instrument. We heated all catalysts from room temperature to 100 °C at a temperature increase rate of 5 °C/min.
The in-situ Raman spectra of the composite samples were obtained by a Raman microscope using a 532 nm laser source with an input power of 1 mW in a RTS-HiR-AM.The X-ray photoelectron spectroscopy (XPS) was operated on a Thermo Scientific ESCALAB 250Xi XPS system.
2.3 Catalytic tests
The VO2(M) was ground to be used as the catalyst. The method for preparation of the simulated model oil was as follows: The 500 μg/g of DBT solution was obtained by mixing 0.0505 g of DBT and 100 g of decalin. Subsequently, 0.1141 g of t-butyl hydroperoxide (TBHP) was added to the above solution. During the ODS reaction, a 50-mL round-bottomed flask was preheated to 80 °C, then 10 g of model oil were added into it under stirring. The reaction with the catalyst lasted about 2 hours. The ODS products were analyzed with a GC2060 chromatograph equipped with a flame ionization detector (FID).
3 Results and Discussion
3.1 Structure of VO2 (M) and doped VO2 (M)
According to the XRD results of these doped samples, most of the diffraction peaks belong to VO2(M) (JCPDS 43-1051, a = 5.752, b = 4.538, c = 5.383, β = 122.64◦, d011= 3.207Å), except the XRD peak at 2θ=25°, which corresponds to the main peak of VO2(B) (JCPDS 65-7960)[21]. It supports the idea denoting that the crystal structures of the doped samples have not changed, and they are almost the same as pure VO2(M).
The structure and property of VO2(M) samples were also characterized by DSC and Raman spectrometry. In Figure 4(a), the DSC curves can clearly detect the phase transition behavior of VO2(M) samples doped with Nb/Zr/Ce. A structural phase transition from monoclinic VO2(M) to tetragonal VO2(R) is evidenced by the endothermic peak[22]. The phase transition temperatures for the doped VO2(M) were slightly increased as compared with that for pure VO2(M) (65 °C). In addition, the Raman spectra shown in Figure 4(b) further confirmed that the structure of VO2sample did not change before and after doping. It shows the characteristics of VO2pattern[23]. The frequency peaks of VO2(M) at 192 cm-1, 220 cm-1, and 627 cm-1belong to the V-V paired vibration, V-V oblique vibration, and V-O vibration, respectively.
3.2 Oxidative desulfurization on doped VO2 (M) catalysts
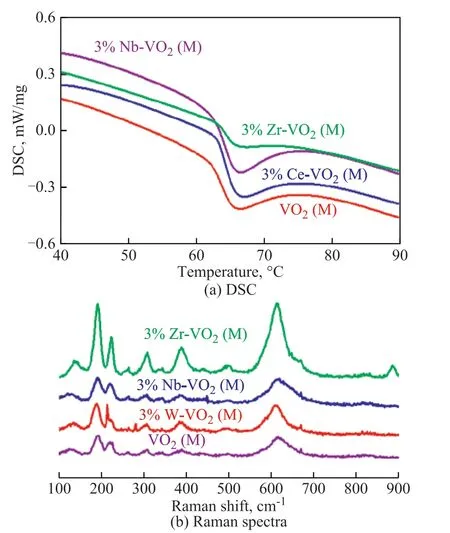
Figure 4 DSC results for VO2 (M) and 3%Nb/Zr/Ce-VO2 (M)and Raman spectra for VO2(M) and 3%W/Nb/Zr-VO2 (M)
The doped VO2samples were tested as ODS catalysts at 80 °C (above the phase transition temperature), with the catalytic performance results shown in Figure 5. Under the reaction conditions the catalysts functioned as the VO2(R) phase. The conversion of DBT over pure VO2reached 70% in 120 min. In comparison to the performance of pure VO2, the conversion of DBT over VO2-Mo, VO2-Nb, VO2-Ce, and VO2-Zr catalysts could reach 80%. The above results could be totally reproduced in three trials. The doped VO2catalysts demonstrated better performance than pure VO2. This outcome reveals a trend that the doping effect can facilitate the ODS reaction.
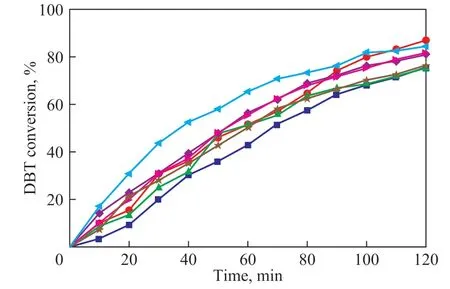
Figure 5 Conversion of DBT over VO2 and doped VO2 catalysts conducted at 80 °C
3.3 Mechanism for the improvement of ODS activity by doping
Surface structures of the catalysts doped with different metals were analyzed by XPS. The XPS spectra and peak fitting results are shown in Figure 6 and Figure 7. The fitting peaks show the variation in the chemical state of O and V depending on the type of doping elementss. It can be seen from Figure 6 that the catalysts have a strong O1s peak at 530.5 eV, which can be attributed to the lattice oxygen in VO2. Moreover, the peak of oxygen vacancy is identified at around 532 eV. In Figure 7, the XPS results of V2p can be fitted to three peaks, which can be assigned to 2p1/2, 2p3/2-V (Ⅳ), and 2p3/2-V(Ⅴ), respectively. According to the principle of XPS, the peak of the low valence state lies at a lower binding energy. By calculating the peak area ratio, the proportion of V (Ⅳ) increases after doping. It can be explained by the fact that when metals (such as Ce, Nb, Ti, Mo, etc.) are doped into VO2, electrons captured by impurity ions or oxygen vacancy are formed as shown in Eq. 1, and the electrons can easily transfer to V (Ⅴ) to generate V (Ⅳ).
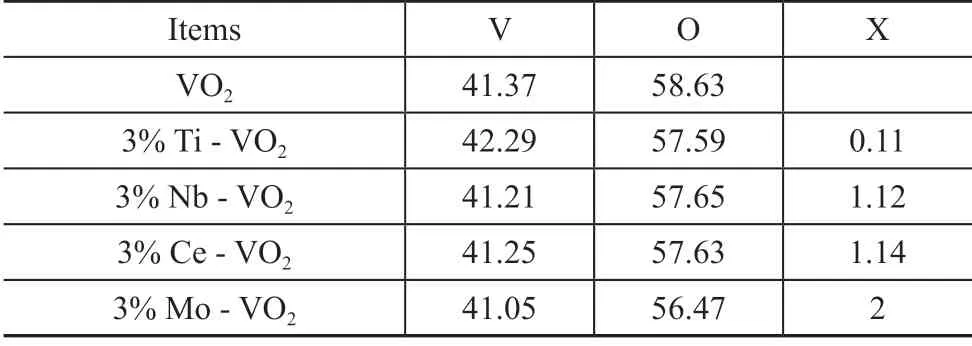
Table 1 Element contents according to XPS results %

Figure 6 XPS spectra (O1s) and peak fitting results of pure and doped VO2 catalysts
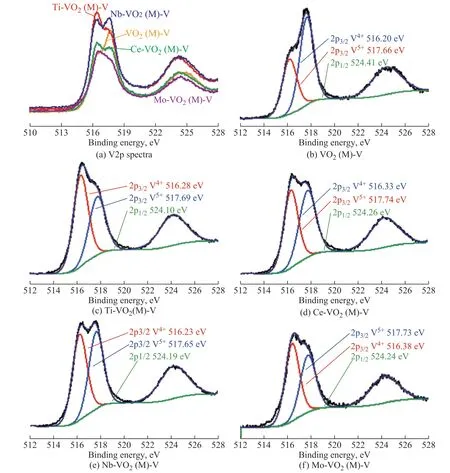
Figure 7 XPS spectra (V2p) and peak fitting results of pure and doped VO2 catalysts
The semi-quantitative XPS analysis (Table 1) showed that the metal elements were successfully doped into the VO2structure. The XRD results showed that the structure of VO2had not changed after doping, and the doping process could be expressed by Eq. (1). When the metal oxide is doped in VO2, oxygen vacancies and electrons are produced, which is consistent with the peak fitting results of XPS. In general, transfer of electrons from the doping element to the V (V) occurs, and then V (V) catches the electrons prior to being transformed into V (IV), which could promote the reaction[13]. When the activity of catalyst is studied, the reaction temperature is 80 °C. It can be seen from the energy band structure and the phase change information that at this temperature VO2is transformed into the R-phase to assume the metallic function. The ODS results show that the doped VO2catalysts can improve the catalytic activity for converting DBT to different extent. As our previous work suggested[24], the electrons around V transfer to the peroxy O atom in TBHP to readily form the hydroxyl radicals (·OH). Hydroxyl radicals (·OH) form the main active species in ODS reaction. The metallic VO2(R) can facilitate the electron transfer. Since the electrons released during the doping process are therefore involved in the catalytic reaction, the catalytic activity of the doped catalyst for DBT conversion is improved.
4 Conclusions
The ODS activity of VO2(M) catalysts doped with different metals are enhanced thanks to doping. According to the characterization results, the doping metals in VO2(M) can produce electrons, which can promote the ODS activity. This work gives a new method to improve activity of the catalyst for ODS reaction. The design of phase transition catalysts doped with metals is prospected. More detailed work remains to be done to figure out the explicit reaction mechanism.
Acknowledgments:This work was supported by the Natural Science Foundation of Tianjin (17JCYBJC20000).
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effects of Blending Long-Chain Alcohols with Jet Fuel on the Macroscopic Spray Characteristics
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
