Molecular Simulation of Transesterification of Ethylene Carbonate and Methanol Catalyzed by Ionic Liquids
2020-01-13GuoLiyingWuHaoWangYirongCuiZhongyiChenYanming
Guo Liying; Wu Hao; Wang Yirong; Cui Zhongyi; Chen Yanming
(School of Petrochemical Engineering, Shenyang Uniνersity of Technology, Liaoyang, 111003)
Abstract: Four ionic liquids [BMIM]OH, [BMIM]IM, [BMIM]Br, and [BMIM]PF6 were synthesized and characterized by infrared spectroscopy. Then the effects of ionic liquids (ILs), cocatalysts, and reaction temperature on the catalytic performance for transesterification of ethylene carbonate and methanol were investigated with orthogonal experiments. The influence of cations and anions of ILs on catalytic activity was revealed by the density functional theory (DFT). The reaction mechanism was proposed based on the experimental results and DFT. The results demonstrated that the optimal catalyst was [Bmim]PF6/CaO, which exhibited the advantages of high activity, excellent stability, and easy recycling. Under the optimized conditions covering a catalytic temperature of 130 °C, an ionic liquid/cocatalyst mass ratio of 5:1, and a catalyst dosage of 4.0%, the conversion rate could reach 65.23% with a dimethyl carbonate selectivity of 98.95%. No significant loss of catalyst activity was detected after 7 recycle times.
Key words: ionic liquids; catalyst; transesterification; molecular simulation
1 Introduction
Dimethyl carbonate (DMC) is an important green organic reaction intermediate, which contains functional groups, such as CH3-, CH3O-, -CO-, and CH3O-CO-. As a reagent for carbonylation, methylation, and carboxymethylation, it can be used to replace many toxic compounds in an attempt to manufacture fine chemicals with high added value[1-2]. It can also be used as a gasoline additive to reduce toxic exhaust emissions thanks to its high octane number in gasoline[3-4]. Nowadays, the industrial manufacture of DMC is mainly based on the transesterification and oxidative carbonylation of methanol, because the trans-esterification process requires a mild reaction condition, resulting in an economic development advantage[5-8].
Compared with the traditional catalysts, ILs are applied in many fields due to their lower vapor pressure, zero volatility, and other unique properties[9-11]. In 1986, Boon reported that dialkyl imidazolium chloraluminate ILs could catalyze the Friedel-Crafts acylation[12]. Many experts made a lot of theoretical research on the structure, thermodynamics, and dynamic properties of ILs through DFT[13-18]. Meng and coworkers proved the existence of C-H…F and N-H…F hydrogen bonds in [BMIM]
Talaty and coworkers verified that nucleophilic reaction occurred at C2 site on the imidazolium cation under the Lewis alkaline condition[20]. Song and coworkers reported that asymmetric ring opening of epoxides catalyzed by hydrophobic ILs could lead to more products with better quality[21]. Knifton and coworkers indicated that the intermediate product of the reaction of ethylene carbonate (EC) and CH3OH was hydroxyethyl methyl carbonate (HEMC)[22]. Herein, the synergistic catalytic properties of different ILs and cocatalysts for transesterification of ethylene carbonate and methanol are compared by orthogonal experiments, and the general models for the open-ring reaction of transesterification are established.
2 Experimental
2.1 Reagents
Ethylene carbonate (EC), anhydrous methanol, calcium oxide, anhydrous calcium carbonate, magnesium methoxide, and other reagents (analytic grade) were purchased from the Sinopharm Chemical Reagent Co., Ltd. (China) and were used without further purification.
2.2 Instruments
Chemical structures were described by a MAGNAIR750 infrared spectrometer, and products were analyzed by a SP-2100A gas chromatograph. In addition, a rotary evaporator (SFX-2L), a magnetic stirrer (DF-101S), a vacuum drying oven (DZF-6050) and a rotary vane vacuum pump (2-XZ-4) were also used in the experiments.
2.3 The process of transesterification reaction catalyzed by ILs
The catalytic performance of four ILs which were synthesized according to the literature reports[23-26]was studied. The reaction steps were as follows. Firstly, CH3OH (2.73 mol), ILs and cocatalyst were added to the reaction distillation unit. Then, EC (0.273 mol) was slowly dropped into the reactor through a constant pressure funnel. It was observed that the distillation temperature was maintained at 63.7 °C (the azeotrope boiling point of CH3OH and DMC) with the addition of EC, and the overhead fraction was collected (while keeping the reflux ratio at 3:1). The products were quantitatively analyzed on the gas chromatograph. Finally, the conversion of EC and selectivity of DMC were calculated, and the catalytic mechanism of ILs in transeterification was analyzed based on the literature report[27]. The reaction equation is shown in Figure 1.
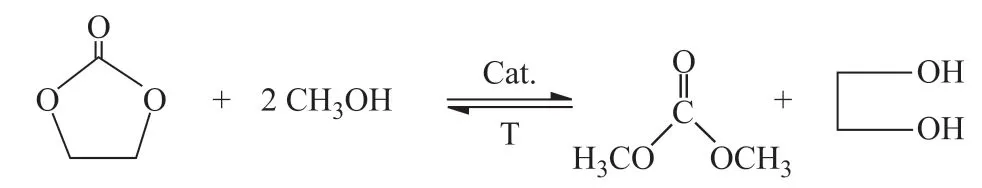
Figure 1 Synthesis of DMC from EC and CH3OH
3 Results and Discussion
3.1 Chemical structure of the catalysts
Figure 2 shows the infrared spectra of ionic liquid [BMIM]Br, alkaline ionic liquids [BMIM]OH and [BMIM]IM, and hydrophobic ionic liquid [BMIM]PF6.
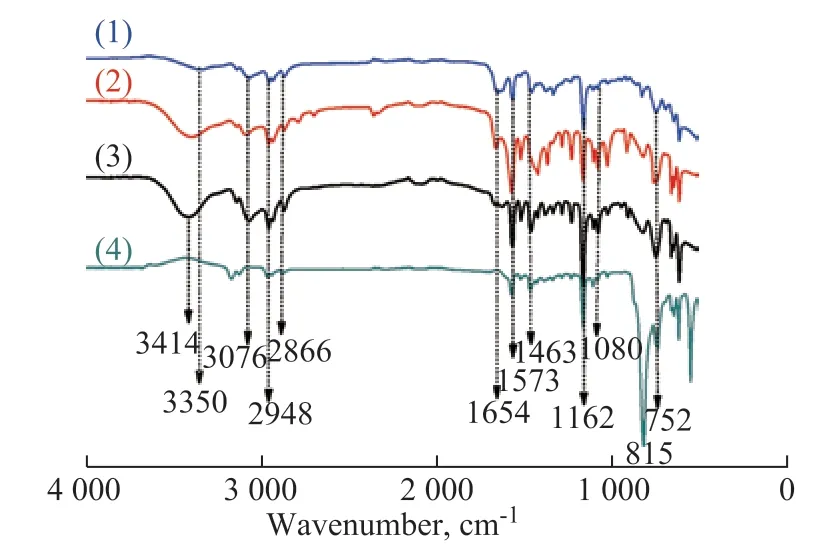
Figure 2 Infrared spectra of different ILs
The stretching vibration peaks of hydroxyl groups are usually located at 3 650—3 200 cm-1. But due to the association of hydrogen bonds, the peaks of hydroxyl groups occur at 3 400—3 200 cm-1with larger peak intensity and wider peak shape. Therefore, the peak at 3 350 cm-1in spectrum (1) is attributed to -OH groups. The peaks of anionic imidazole are observed at 3 076, 2 948, 2 866, 1 573, 1 463, 1 162, 1 107, 1 080, 1 016, and 752 cm-1in spectrum (2). In spectrum (3), the vibration peak of Br-H is identified at 3 414 cm-1, and in spectrum (4), the absorption peak of P-F is found at 815 cm-1. These peaks indicate that Br-, OH-, IM-, and PF6- have been successfully linked to [BMIM]+.
3.2 The influence of ionic liquids, cocatalysts and reaction temperature on catalytic performance
Under the conditions covering a reaction time of 4.0 h, and a dosage of catalytic system equating to 3.0%, the influence of the catalyst system and temperature was investigated. In the following experiments, the same mass ratio (4:1) of ILs to cocatalysts was used, with the corresponding results listed in Table 1.
The test results reveal that the influence of factors studied hereby decreases in the following order: ILs > cocatalysts > temperature, and with regard to EC conversion, the influence of factors decreases in the following order: ILs > temperature > cocatalysts for DMC selectivity. Thus, the optimum catalytic process condition is A2B1C2, which is [BMIM]PF6/CaO at 130 °C. Based on above data, the synergistic effect of cations and anions can affect the catalytic activity directly, and the stronger the base site of the cocatalyst, the better the catalytic performance. In the meantime, enhancing the Lewis base site can improve the conversion, but has little effect on the selectivity. Furthermore, CaO shows the strongest activity, which is ascribed to the dissociation and adsorption of CH3OH on the surface. The hydrogen bond can form between the surface O2-and the methanol hydroxyl Hδ+, which reduces the energy required for bond breaking. Therefore, a stronger nucleophilic ability of catalysts can improve the catalytic activity.
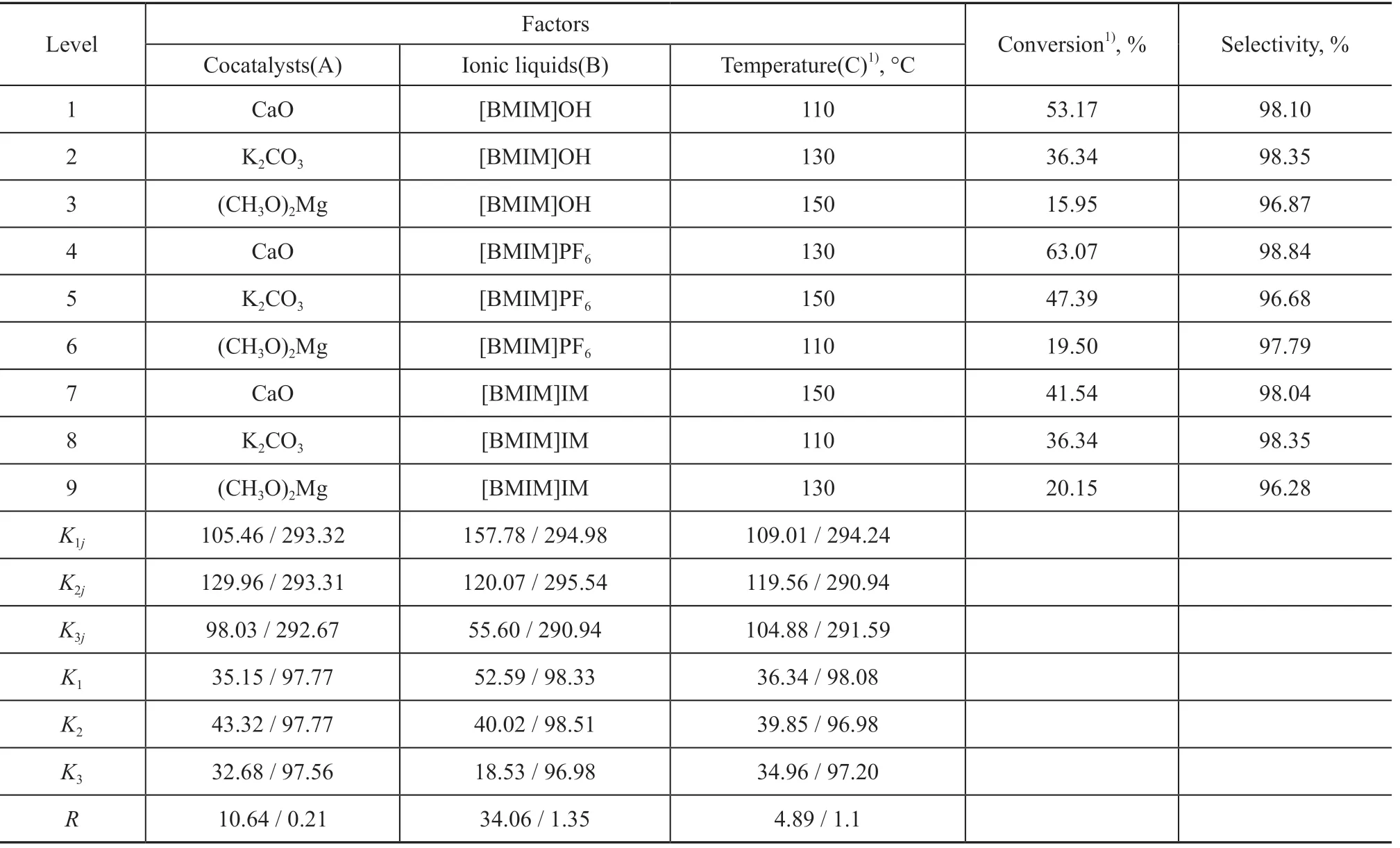
Table 1 Orthogonal experiment results
3.3 The influence of mass ratio of [BMIM]PF6/CaO on catalytic performance
Under the conditions covering a temperature of 130 °C, a catalyst system dosage of 3.0%, the influence of the mass ratio of [BMIM]PF6to CaO on the catalytic performance was investigated, with the results shown in Figure 3.
The conversion of EC improves significantly with the increase of the proportion of [BMIM]PF6to CaO. However, the selectivity of DMC varies less. When the mass ratio of [BMIM]PF6to CaO exceeds 5:1, the EC conversion tends to be stable, because the number of catalytic active sites can basically meet the needs of the reaction. So a [BMIM]PF6/CaO mass ratio of 5:1 is selected as the best ratio.
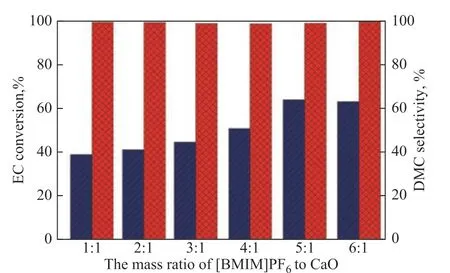
Figure 3 Influence of the mass ratio of [BMIM]PF6 to CaO on catalytic performance
3.4 The influence of catalyst dosage on catalytic performance
Under the conditions covering a temperature at 130 °C, and a [BMIM]PF6to CaO mass ratio of 5:1 in the catalytic system, the influence of catalyst dosage was investigated, with the results shown in Figure 4.

Figure 4 Influence of catalyst dosage on catalytic performance
It can be seen from Figure 4 that the EC conversion is improved obviously with the increase of catalyst dosage, while the DMC selectivity is stable. When catalyst dosage is less than 3.0%, the EC conversion is relatively low. As the catalyst dosage increases to 4.0%, the EC conversion increases to 65.23%. Furthermore, when the catalyst is excessive, the catalyst activity remains almost constant. Conclusively, a catalyst dosage of 4.0% is the most appropriate for the synthesis of DMC in catalytic reaction.
3.5 The influence of recycle times on catalytic performance
Under the condition covering a temperature of 130°C, a [BMIM]PF6to CaO mass ratio of 5:1 in catalytic system, and a catalytic system dosage of 4.0%, the influence of the recycle times on catalytic performance was investigated. The EC conversion and the DMC selectivity in first seven consecutive recycle times are shown in Table 2.

Table 2 The influence of recycle times on catalytic performance
The performance of the catalyst is stable with a slight decline of EC conversion, and the DMC selectivity remains above 96% after 7 recycle times. It can be seen that the activity of catalyst after 7 recycle times is a little bit less than that of the fresh catalyst, and the initial decline of catalytic activity is due to partial loss. Hence the catalyst can be considered as reusable.
4 Simulation of Transesterification Catalyzed by ILs
Using B3LYP/6-31+G(d)[28]implemented in the Gaussian 05 package, all the stationary points are located along the reaction pathways. Full optimization and vibrational analysis are carried out for the stationary points on the reaction profile.
4.1 Transition state analysis in the absence of [BMIM]PF6
The geometrical parameters for the two reactants (R1, R2), the intermediate (HEMC), the transition state (TS1, TS2) and the product (DMC) are given in Figure 5. The relative energy of the transesterification reaction in the absence of [BMIM]PF6is shown in Figure 6. Their IRC (with a step-length of 0.1 amu-1/2Bohr) analysis confirms that TS1 connects HEMC and two reactants; TS2 connects HEMC and DMC. It can be intuitively observed from Figure 6 that trans-esterification reaction consists of two steps: the two reactants (R1, R2) firstly form transition state TS1 with a relative energy of 36.33 kcal/mol, which then forms HEMC with a relative energy of 47.38 kcal/mol. Finally, HEMC forms the product DMC via the transition state TS2 with a relative energy of 36.58 kcal/mol and 48.95 kcal/mol, respectively.
4.2 Transition state analysis with [BMIM]PF6
Based on DFT[29], the structure of [BMIM]PF6was optimized, with the results shown in Figure 7.
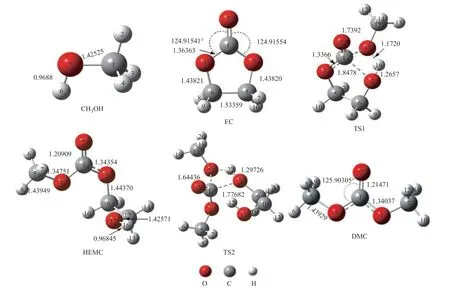
Figure 5 Optimized geometries of the reactants, transition states, reaction intermediates and products for synthesis of DMC
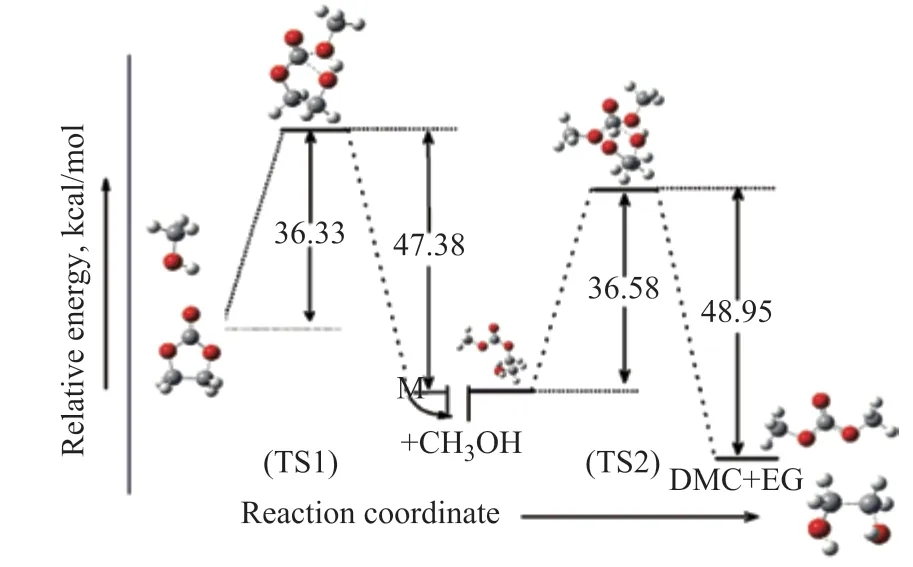
Figure 6 Relative energy of DMC synthesis in the absence of [BMIM]PF6
The geometrical parameters for the transition states (catalyst-TS1, catalyst-TS2) with [BMIM]PF6are given in Figure 8. The relative energy of the transesterification reaction is shown in Figure 9. The unique imaginary frequency of the transition states TS1 and TS2 are -859.08i and -1133.89i, respectively, and the transition states can therefore be confirmed as the real ones. The two reactants (R1, R2) form HEMC via the transition state catalyst-TS1 with a relative energy of 25.85 kcal/mol, and 36.9 kcal/mol, respectively. Then, HEMC forms the product DMC via the transition state catalyst-TS2 with a relative energy of 30.62 kcal/mol, and 42.98 kcal/mol, respectively. It can be seen that the energy of catalyst-TS1 was by 28.85% lower than TS1 and the energy of catalyst-TS2 was by 16.29% lower than TS2. According to the above analysis, the energy of catalyst-TS is lower than that of TS, which shows that the energy needed for trans-esterification is greatly reduced.

Figure 7 Model molecular structure of [BMIM]PF6
4.3 Catalytic mechanism of transesterification
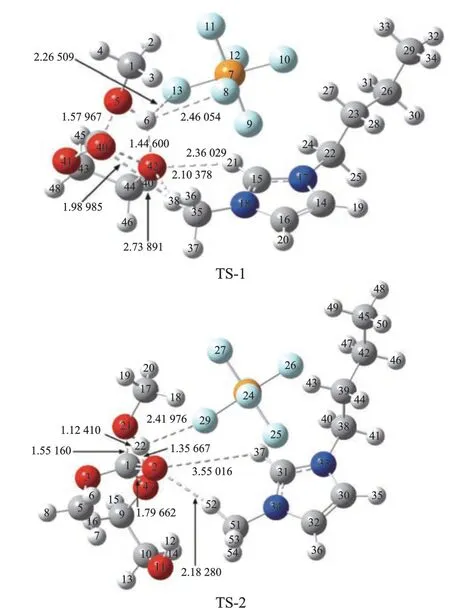
Figure 8 Optimized structure of TS with [BMIM]PF6
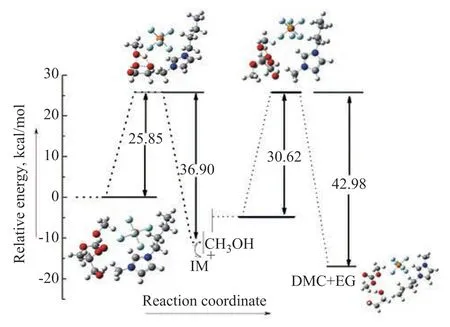
Figure 9 The relative energy of transesterification with [BMIM]PF6
The mechanism of transesterification is revealed according to the DFT (Figure 10). ILs and the substrate through intermolecular hydrogen bonds to form the intermediate (1). CH3O-attacks the carbonyl carbon of EC (1) to form an unstable open-ring activation intermediate (2), which traps H+of CH3OH to form the intermediate (3) (HEMC), which is a monoester product. Subsequently, (3) continues to interact with CH3O-to form (4). Particularly, CH3OH is activated by [BMIM]PF6through strong hydrogen bonding and can more easily form CH3O-to attack the carbonyl carbon of EC.

Figure 10 Reaction mechanism of transesterification
5 Conclusions
In summary, we had successfully synthesized the target ILs and investigated their catalytic performance in the trans-esterification reaction of ethylene carbonate and methanol. In the meantime, the reaction mechanism of the catalysts in the trans-esterification reaction was explored by molecular simulation. The details are presented below:
1) Four kinds of ILs were successfully prepared, and the target ILs were determined by infrared spectrometry.
2) By using the orthogonal experiment and the single variable method, the optimal experimental conditions were obtained, including a temperature of 130°C, an ILs ([BMIM]PF6) to cocatalysts (CaO) mass ratio of 5:1, and a catalyst dosage of 4.0%, leading to an EC conversion of 65.23% and a DMC selectivity of 98.95%. Especially, there was no significant loss of catalytic activity after 7 recycle times of catalyst system.
3) The structure and energy of the intermediates in trans-esterification reaction were analyzed by DFT and the catalytic mechanism of the trans-esterification was revealed. The molecular simulation results showed that ILs could effectively activate CH3OH through hydrogen bond interaction. In addition, the influence of ILs on catalytic activity was revealed by density functional theory.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (NSFC 21706163) and the Foundation from Liaoning Province Department of Education (LQGD2017020).
杂志排行
中国炼油与石油化工的其它文章
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Boosting the Photocatalytic Activity of WO3 by Highly Dispersed CoWO4 or CuWO4
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
