A brief review of brazing diamond in cutting tools
2019-10-22CuiBingTaoShanrenXueHangyanZhongSujuanXuDong
Cui Bing , Tao Shanren , Xue Hangyan , Zhong Sujuan , Xu Dong
崔冰,陶善仁,薛行雁,钟素娟,徐东
1.School of Materials Science and Engineering,Anhui University of Technology,Ma’anshan 243023,China;
2.Zhengzhou Research Institute of Mechanical Engineering,Zhengzhou 450001,China
Abstract Diamond has high hardness and good wear resistance.It is widely used in cutting tools and workpieces.Brazing is an effective method to realize high quality cemented carbide joints in various materials connection technologies.This paper analyzes the research status of diamond brazing in detail.The materials used as brazing filler in diamond brazing are reviewed.Copper base filler and nickel base filler are the most commonly used brazing filler in diamond brazing.The advantages and disadvantages of diamond grinding tools under different production methods are analyzed.In addition,a series of new brazing alloys such as amorphous Ni based brazing filler metals are analyzed.Finally,the development trend of diamond brazing is pointed out.
Key words brazing diamond,manufacturing process,filler alloy,interface microstructure,amorphous Ni-based filler alloy
0 Introduction
Diamond possesses extreme hardness and excellent abrasive performance[1-3].The diamond tools can be divided into drilling tools,sawing tools,grinding tools and other types by different processing methods.The addition which can also be divided into three categories:resin bonded diamond tools,ceramic bonded diamond tools and metal bonded diamond tools (including electroplated and sintered diamond tools) by difference of binders.They are promoted and used on a large scale in industrial production,which significantly improves the overall level[3].
However,the shortcomings are more fully exposed[4].The metal coating in electroplated diamond tools has only mechanical embedding effect on diamond abrasive particles and matrix,which can not provide sufficient binding force for diamond abrasive particles and matrix.As a result,the abrasive particles are easy to fall off,and the abrasive particles are disordered with low exposure and small chip space.The sintered matrix material in sintered diamond tools only has the function of mechanical impregnating and embedding for diamond,and it is difficult to achieve the metallurgical combination between abrasive particles and matrix.The abrasive particles are still easy to fall off after being subjected to force,and it is difficult to be edged and has poor self-sharpening.
Experts and scholars began to devote themselves to the research of brazing diamond tools[5].In order to give full play to the excellent performance of diamond itself and fundamentally change the problem of insufficient control force of the matrix on diamond,it is hoped that the solid connection can be achieved through the chemical metallurgical effect between the filler metal,diamond abrasive and steel matrix. High-temperature brazing diamond tools can achieve the chemical metallurgical combination of diamond abrasive,filler metal and steel matrix by adding filler metal containing strong carbide forming elements (such as Ti,Cr,V,et al.)[6-7],so as to improve the controlling force of the filler metal on diamond,so the diamond abrasive is not easy to fall off in the grinding process.Moreover,the outcroppings of abrasive grains are high,usually up to about 70%or 80% of their height,so that the chip space becomes larger,and the effective utilization rate of diamond abrasive grains is high,and the tool life is long.Diamond tools are prepared by high-temperature brazing instead of electroplating,which can get rid of the burden of environmental pollution caused by electroplating and meet the requirements of green and clean manufacturing in industrial production.
The efficiency of brazing diamond tools in vacuum furnace is higher,but the heating speed and cooling speed of vacuum furnace are slow,which extends the holding time of diamond in brazing process,and it is difficult to effectively control the heating and cooling speed.The defects of high temperature brazing diamond tools are gradually exposed.In order to obtain more excellent machining performance of brazing diamond tools,it need active filler brazing diamond tools brazing mechanism to further improvement of the physical and chemical analysis of brazing interface,and the optimization of diamond brazing process and reduction of the thermal damage of diamond.
1 Processing method
1.1 Physical vapor deposition and chemical vapor deposition
Vapor deposition mainly consists of physical vapor deposition (PVD) and chemical vapor deposition (CVD).Whereas,PVD gasifies the metal into atoms,molecules or ions under vacuum conditions and directly deposits them on the surface of the plated parts,such as vacuum evaporation plating,vacuum sputtering plating and vacuum ion plating.While CVD is the use of gaseous materials in a certain pressure,temperature,time conditions,the surface of the plating,the chemical reaction and the formation of a coating,this process is generally the gaseous compounds are goldplated into the reaction chamber with the plating,through contact with the plating thermal decomposition or chemical reaction and the formation of a coating.With plating,PVD and CVD,need a certain amount of gas phase be plated contacts,that it cannot be applied to the mass of particle diamond,this is due to the method of gas phase is difficult to infiltrate into the internal accumulation of diamond grains and evenly,so as to make the less amount of single plating or lead to uneven coating preparation[8-9].
1.2 Brazing
Brazed diamond technology refers to the technology that can produce chemical reaction with diamond abrasive grains and produce metallurgical filler with steel matrix for brazed connection.Due to the presence of active elements in the filler that can chemically react with diamond,the interface between diamond and filler is chemically metallurgical,so the bonding strength is high and the diamond is not easy to fall off from the matrix[10].This is the biggest difference between brazed diamond tools and other types of diamond tools.The high bonding strength of diamond and matrix leads to a series of advantages of brazed diamond tools.
(1) High exposure of abrasive particles.Due to the high bonding strength,when the thickness of the filler bond layer is maintained at 30% of the height of the abrasive,the control strength of the abrasive can meet the grinding requirements.It’s hard to compare with other tools.
(2) Large chip space.Due to the high exposure,the size of the chip space between the abrasive increases,and the chip or abrasive chip can be successfully discharged from the abrasive particles,which is not easy to cause tool failure due to chip blockage.
(3) Good heat dissipation reduces the possibility of burns.Due to the good chip removal effect,a lot of heat is taken away by the chip,which reduces the temperature of the workpiece surface and also reduces the possibility of the workpiece surface being burned.
Duan et al.[11]brazed diamond with alloy of Cu-Sn-Ti alloy.By analyzing the interface between brazing alloy and diamond,it is found that element diffusion occurs at the interface,and a strong bond is formed between the coating and diamond.It was found that the interfacial bonding strength of composite pitch was high and the grinding effect was better than that of conventional diamond pitch.Wang et al.[12]put forward with large-grained alloying filler vacuum sintering diamond brazing technology vision,and the geometry model was established,the vacuum brazing diamond sintering test and heavy-load grinding test,the result shows that:large-grained nickel-based alloy filler melts completely,and flows good,the diamond has good effect of embedding and climbing.After heavy load grinding,the diamond shows three normal wear states,namely,blade wear,local crushing and grinding,which proves the feasibility of the new process and solves the problems of difficult control of the amount of powdery alloy filler and poor uniformity of filler layer used in diamond brazing and sintering process.
2 Design principles of brazing filler metal
The filler metal for diamond tools must be able to wet the diamond particles well in the first place.The selection of filler should follow the following principles[13]:(1) The fillering temperature is low when the melting point is low,which can greatly reduce or even eliminate the graphitization of diamond.(2) The elements in the filler metal can form compounds with carbon atoms,thus wetting the diamond well without affecting the performance of diamond particles.(3) Diamond tools are required to have better wear resistance and impact resistance in order to extend their service life.(4) The brazing temperature is quite different from the actual operating temperature,so it is easy to generate hot cracks.In order to prevent the occurrence of hot cracks,the linear expansion coefficient of the filler metal and diamond is not significantly different at the same temperature.(5)Under different operating conditions,the diamond tools have different shapes,and the filler metal shall be easily machined into a suitable shape on the basis of convenient machining.(6) In order to reduce the use cost,precious metals should be reduced or not used.On the basis of the above,three active brazing alloys,Ni based,Ag based and Cu based,are commonly used in brazing diamond tools.
2.1 Ag based filler
Ag relative to the based filler metals Ni based filler and Cu based filler,to have a lower melting temperature,wetting and spreading properties is good,one of them.Ag-Cu-Ti alloy filler and Ag-Cu-Cr alloy filler are widely used in practical production.Ag-Cu-Ti alloy has low liquidus temperature which is more reducible for diamond conversion into graphite.Apart from low liquidus temperature,Ag-Cu-Ti alloy has excellent ductility that can be avoided crack formation at diamond-filler interface.Moreover,Ag-Cu-Ti filler has good wettability to diamond,and the active element Ti can react with C in diamond to form TiC.Filler at above 930 ℃ can melt,melting temperature is low.Ag-Cu is a high plastic material,which can release the brazing stress and improve the joint strength.Ag,however,is a precious metal and relatively expensive to use[14].
Prithviraj et al.[15]studied the effects of micro/nano-Al2O3ceramic particles as reinforcement for Ti activated 72Ag-28Cu filler alloy.The results show that the diamond abrasive has good exposure height,the interface between the abrasive and the filler is clear,the crystal shape of abrasive grains is complete after brazing,and there are no obvious impurities,cracks,graphitization and other phenomena on the abrasive surface as demonstrated in Fig.1.
Failure area of a higher magnification image (Fig.2a)reveals the failure mode of ductility and brittleness combination of the filler alloy containing micro-alumina reinforcing agent.As can be seen from the figure,the Al2O3microparticle has brittle fracture in a single sand particle test.The high content of Ag-Cu shows poor toughness.In nano-reinforced alloys,brittle failure occurs near the region of hard brittle intermetallic compounds (Cu3Ti3O).As can be seen from Fig.2b,there are microcracks near the brittle region.This phenomenon mainly occurs in the filler alloy containing 2 wt% nano-alumina reinforced,where the sharp angle near the unreacted nano-alumina particles is the position of the local stress concentrator.
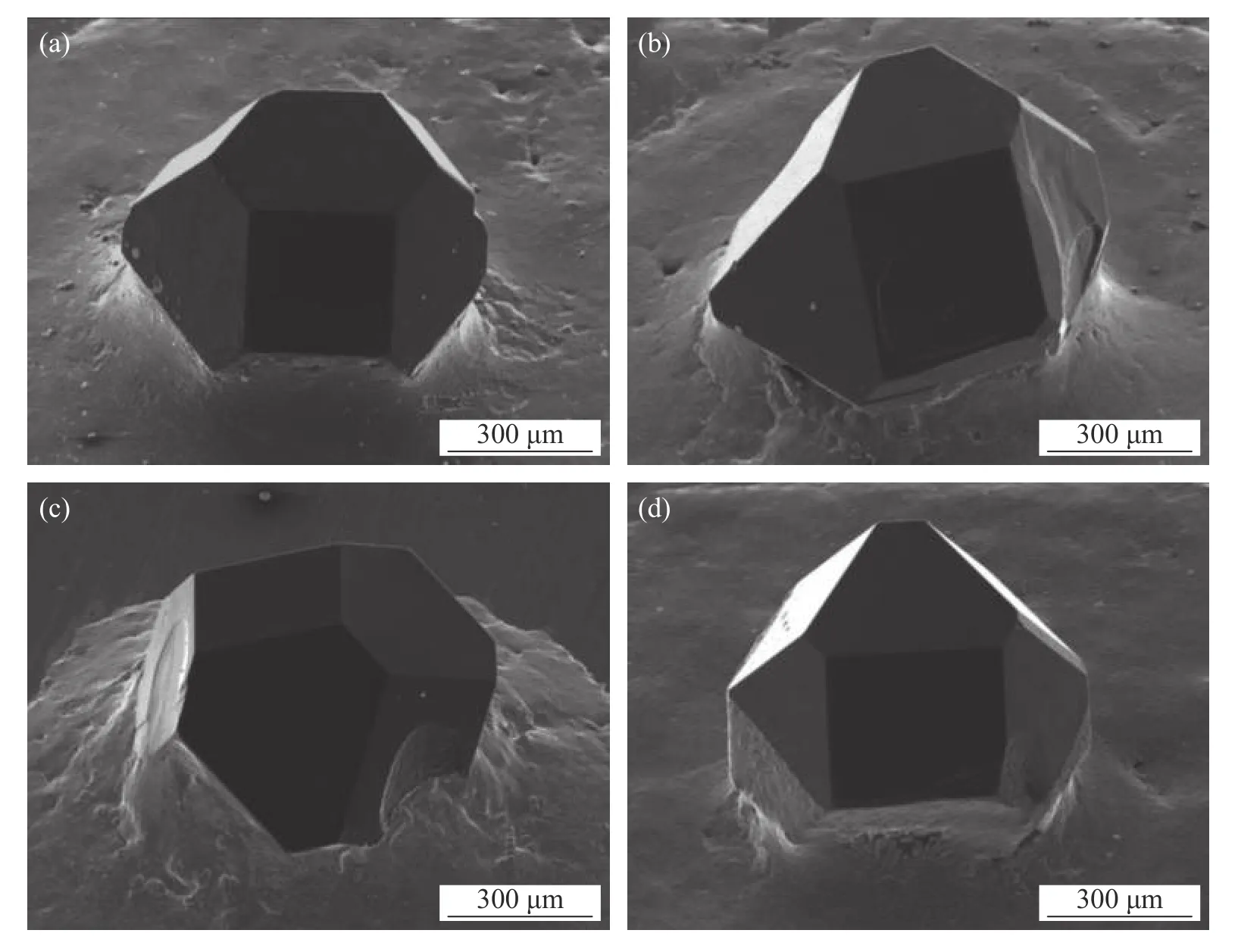
Fig.1 Diamond brazed with Ag-Cu-5Ti alloy reinforced with (a) 1 wt% nano-alumina (b) 2 wt% nano-alumina (c) 1 wt% micro-alumina and (d) 2 wt% micro-alumina

Fig.2 Magnified image of the alloy-reinforcement interface and EDS analyses (a) (72Ag28Cu)-5Ti-1 micro-alumina (b)(72Ag28Cu)-5Ti-2 nano-alumina
Zhang et al.[16]studied the distribution of Ag,Cu,Ti and Fe at the interface as shown in Fig.3,which shows a transition trend,and the concentration gradient changes gently.In the silver-rich region,the content of copper is very low.Correspondingly,in the enrichment area of copper element,the content of silver element is very small,and the two elements are basically complementary.On one side of the steel matrix,there are obviously Ti atoms and Cu atoms on the bias diffusion,while Ti atoms rarely exist in the area far away from the brazing interface,mainly because Ti,Cu and Fe are of the same periodic element,and their atomic radius and properties are similar,so the diffusion phenomenon is very obvious between the filler alloy and the steel matrix,forming a metallurgical bond.
Mukhopadhyay et al.[17]studied the interfacial chemistry and joint strength of synthetic diamond brazed with Ni-Cr-B-Si-Fe and Ti activated Ag-Cu filler alloys and found that Ti-activated Ag-Cu alloy was significantly softer than Ni-Cr alloy and no such crack was detected in the case of Ag-Cu-Ti alloy and the joints failed by ductile fracture of alloy near to the interface,leading to diamond pull out.Fig.3 represents the XRD analyses of the brazed joint and also reveals the stoichiometry of the intermediate compound(s)formed at the interface.For active silver copper based brazed joints,the peaks corresponding to titanium carbide could be detected.The reaction mechanism for the formation of titanium carbide compound is shown below[18]:Gibb's energy formation of this compound at a temperature of 820 °C is approximately -175 kJ/mol.

2.2 Cu-based filler
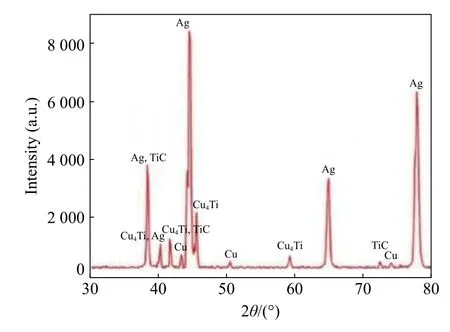
Fig.3 XRD of the interfacial phases of brazed diamond
Cu-based alloy filler metals are mainly divided into Cu-Sn-Ti and Cu-Sn-Zr-Ti.According to the Cu-C phase diagram[19],the solubility of element C in Cu is almost zero,which reduces the erosion of diamond by alloy filler metal.The addition of Sn element to the alloy can reduce the melting point of Cu-based filler and form intermetallic compounds with Cu element to improve the strength of the filler layer.Compared with Ni based filler,filler melting temperature is low,the thermal damage of diamond grits is relatively small,and the active element Ti can realize metallurgical reaction with diamond grains,improving the bonding strength of the diamond particles and filler,but Cu based filler alloy brazing layer with low hardness and poor wear resistance,make the whole of the diamond grains increased risk of loss,eventually lead to a shortened the service life of diamond tools[20].
Buhl et al.[21]studied the influence of brazing process on residual stress,matrix structure and strength of diamond particles was studied.Found between the filler layer and the substrate to generate a large amount of (Cu,Sn) embedded intermetallic phase,when the brazing temperature is 930 ℃,heat preservation for 10 min,after welding diamond particles by the residual compressive stress is 350 MPa.At 880 ℃ and 980 ℃ temperature range,the shear strength decreases with the increase of brazing temperature and heat preservation time.
Diamond grits brazed using a Cu-10Sn-15Ti alloy at 925 ℃ and 1050 ℃ were investigated by the reference [22].The Ti and Sn tended to segregate to the interface between the diamond grit and the brazing alloy.There formed a TiC reaction layer between the diamond grits and the braze matrix.The TiC layer was composed of grains of about 50 nm in size,which could effectively alleviate the interfacial stress associated as shown in Fig.4,which could result in enhanced interfacial bonding strength.
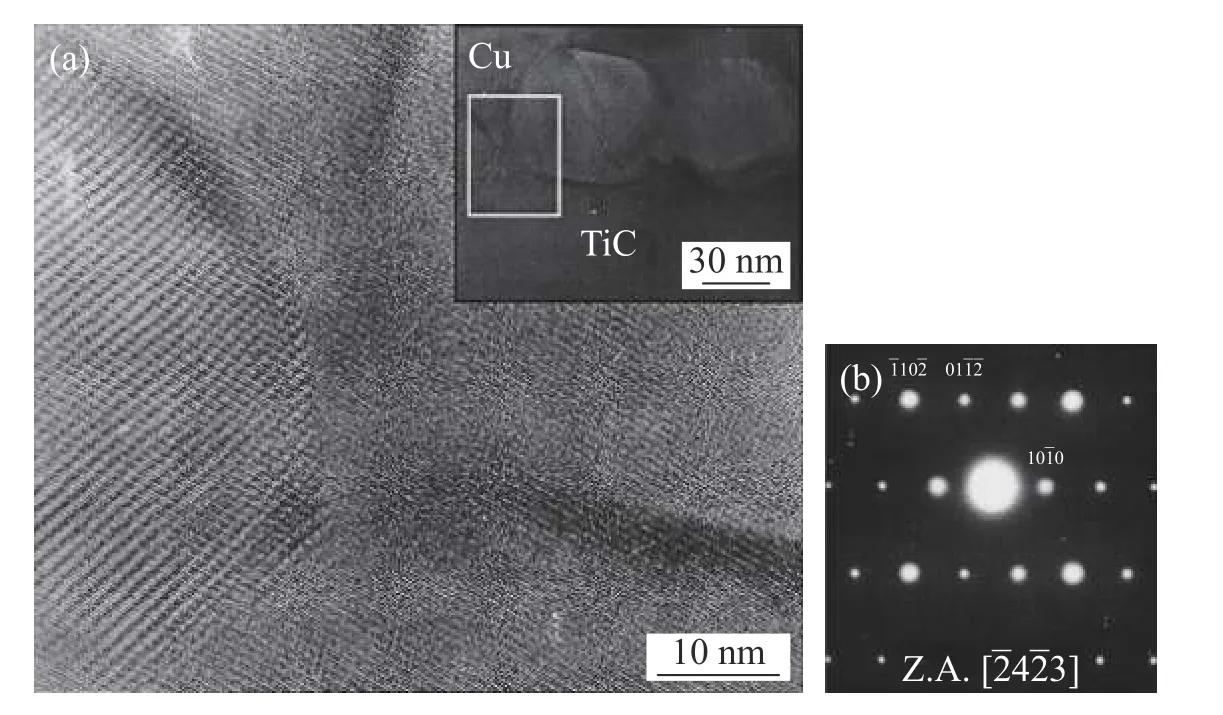
Fig.4 A HRTEM bright-field micrograph (a) The interface between the TiC layer and the braze matrix for a specimen processed at 925 ℃ for 5 min (b) The SAD pattern was taken in a small grain along its zone axis of
Fu et al.[23]carried out the research on the surface of graphite was metallized and brazed by Cu-Sn alloy filler metal with Ti active element.The results show that the wettability of Cu-Sn alloy filler metal containing Ti is better than that of traditional Cu-Sn alloy filler metal.The filler metal and graphite layer have the formation of TiC compound.A lot of Cu-Sn compounds appeared in the filler layer after brazing.
Ma et al.[24]studied the Cu-Sn-Ti alloy was used as the brazing alloy to produce diamond grinding head.Dissolution,diffusion,and chemical reactions occur at the interface between diamond and Cu-based filler metals.The diffusion band width between the Cu based filler metals and the steel matrix is approximately 30 μm,indicating a good metallurgical bond between diamond and Cu based filler metals and between the Cu based filler metals and the steel matrix.
2.3 Ni-based filler
The Ni-based filler has high corrosion resistance,high hardness,wear resistance,high temperature environment adaptability,as a functional brazing layer has been used in aerospace devices and other special fields.With a long history,a high degree of commercialization and much attention,the Ni based filler metal has made great achievements in basic research and applied research.The super high elastic modulus of diamond is around 1100 GPa.Combined with the characteristics of high temperature resistance,high hardness and high strength of Ni-based brazing layer,the Nibased filler metal can be used to make diamond tools for grinding,cutting and drilling under heavy load and bad working conditions[25].
Unlike Ag-Cu-Ti alloy,Ni-Cr based alloys have a superior hardness and abrasion resistance property.As the filler,Ni-Cr-P alloy realizes the solid metallurgical combination of steel matrix and diamond through the high-temperature vacuum brazing process.The brazing tool developed has the characteristics of high exposure of abrasive,little accumulation of filler layer,it was found that Cr,a strong carbide forming element in the filler,diffused to the diamond interface in the brazing process,so as to metallize the abrasive surface and have good wetting,almost no abrading phenomenon in the working process,and its service life is significantly improved compared with that of electroplating[26].
Nickel chrome filler on the surface of diamond is better,and the interface bonding in good condition,the brazing interface is shown in Fig.5a.A white line shown in line scanning analysis,the results are shown in Fig.5b,it can clearly observed that C and Cr distribution curve of the two elements at the interface between abrasive -filler has obvious changes,thus it can be sure these two elements at the interface significant mutual diffusion phenomenon were happened.The Cr elements in the filler metal layer show obvious segregation at the interface,and the Cr concentration distribution near the abrasive surface attachment is much higher than that in the filler metal alloy,indicating that Cr elements are continuously accumulating towards the interface.Correspondingly,element C in the interface layer also exists segregation phenomenon.The distribution curve of element C at the interface has an obvious concentration gradient,and element C spreads from the abrasive surface to the filler layer[27].
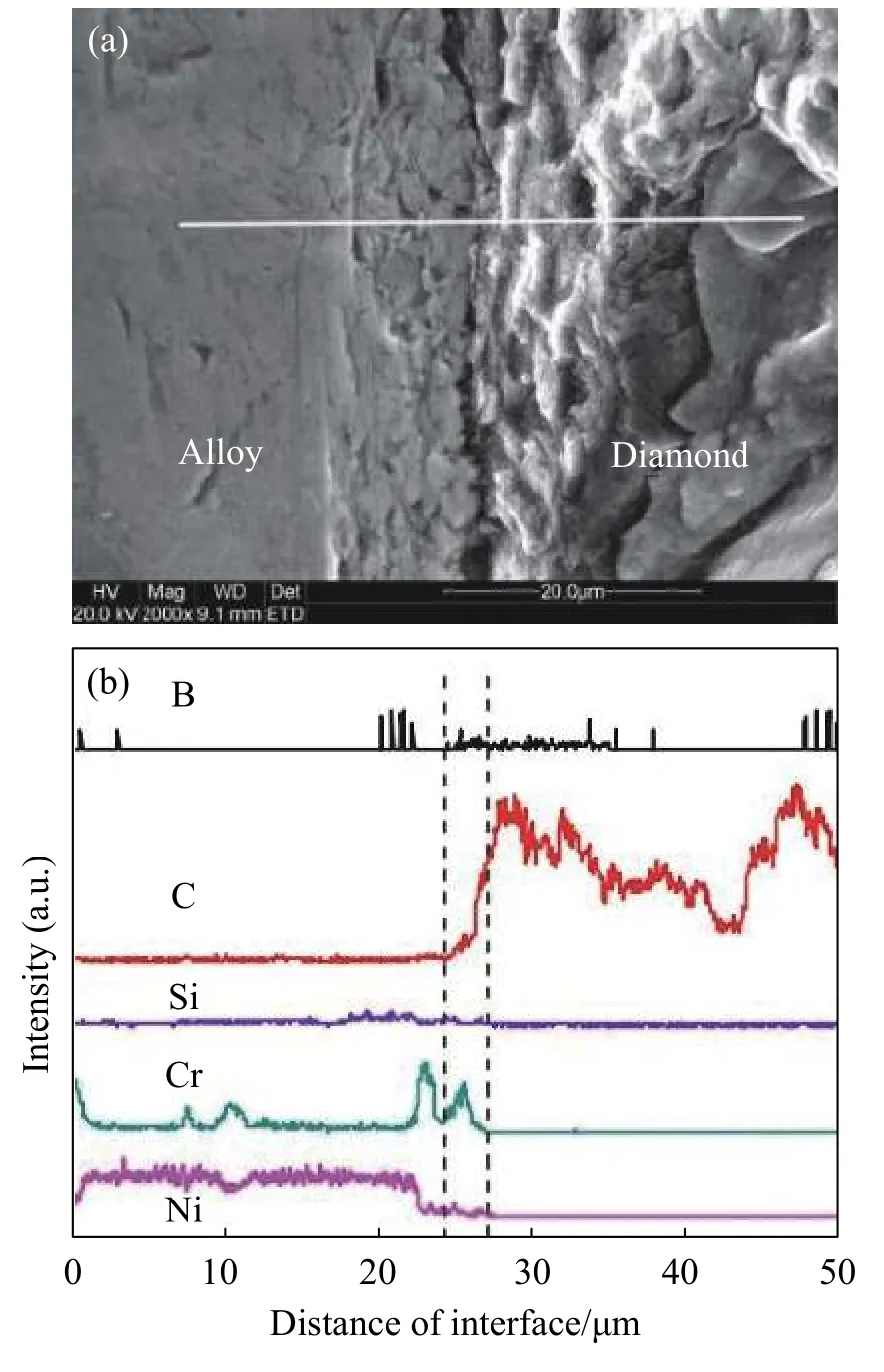
Fig.5 Bonding interface between a diamond abrasive particle and the Ni-Cr filler
As a strong carbide forming element,Cr,the active element in the filler metal,reacts with C element at the abrasive interface at high temperature,and gradually forms stable carbides between the abrasive and filler metal interface.As the reaction continues,Cr elements in the filler metal continue to diffuse to the interface until the equilibrium state,forming a rich Cr layer at the interface.As shown by the interfacial element line scanning distribution curve,the Cr element distribution curve has obvious crest at the grainfiller interface,and this Cr-rich layer region may be the new compound region.In addition,chemical metallurgical bonding occurs at interface junctions to improve their bonding strength[25-28].
Sun et al.[28]studied the brazing alloy of Ni-Cr main phases on the surface of diamond for different brazing time.There are two layers of carbides,the one is Cr3C2and other is Cr7C3by XRD presented,as shown in Fig.6.Fig.7 illustrates the whole process of Cr3C2has a certain orientation relationship with the crystallographic plane of diamond and Cr7C3nucleates on the surface of Cr3C2.With the increase of brazing time,the morphology of carbide Cr3C2turns from linear to lamellar and carbide Cr7C3turns from granule to columnar.After brazing,chemical metallurgical combination is achieved between diamonds and brazing alloy and carbides are the primary reason for the strong bond strength.

Fig.6 XRD patterns of diamond and carbides
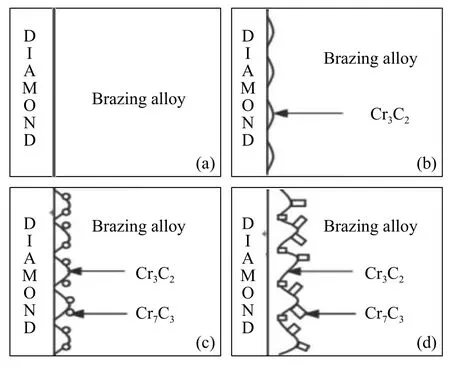
Fig.7 Carbides forming process
2.4 Amorphous Ni-based filler alloy
Amorphous alloys have their own unique disordered structure,and have excellent physical,chemical and mechanical properties,as well as some excellent properties that crystalline alloys lack.Amorphous alloy as filler has great advantages,such as good wetting and fluidity,small brazing gap,uniform structure and composition of brazing joint after welding,which can significantly improve the quality of brazed joint[29-30].
Microsegregation and uniform microstructure with few macrosegregation can be obtained by using amorphous Nibased filler alloys.This helps to form fast melting and diffusion components after heating the filler alloy.It is pointed out that the initial melting point of the amorphous filler alloy is lower than that of the crystalline filler alloy at the same brazing temperature and the superheat of the alloy is higher than that of the crystalline filler alloy.In addition,the molten amorphous filler alloy has good transient fluidity.Therefore,diamond brazing can be carried out in a relatively short time,and the wettability and climbability of the filler alloy to diamond gravel can be fully obtained.Therefore,for induction brazing with short brazing time,the amorphous filler alloy should be selected.
Ma et al.[29]carried out the research on the induction brazing of diamond grits using both amorphous and crystalline Ni-based filler alloy.Fig.8 shows the SEM image of brazed diamond gravel with high frequency induction amorphous filler alloy.It can be seen that the wettability and climbing property of the amorphous filler alloy on diamond particles are obviously better than that of the crystalline filler alloy.The wettability and climbing ability of amorphous nickel base alloy are closely related to its low initial melting temperature.As long as the wetting and climbing of the filler alloy towards the direction of diamond sand is easy,the filler alloy has better mechanical properties for diamond sand.Therefore,on the one hand,diamond particles will not fall off after brazing;On the other hand,it is difficult to crack completely under severe working conditions.The essence of diamond brazing is that in the process of high temperature brazing,the interaction of dissolution,wetting,diffusion and so on between diamond and filler alloy near the interface will occur and combine with each other.These events increase the bonding strength between the diamond particles and the filler alloy.The different composition distribution and melt characteristics of amorphous and crystalline filler alloys inevitably lead to different interfacial microstructure between diamond and filler alloys.Fig.9 shows the brazed diamond gravel eroded by strong acid.Both compounds on brazed diamond particles are Cr3C2,and the compounds on the brazed surface of the amorphous filler alloy brazed diamond particles are finer and more evenly distributed than the corresponding brazed materials.This is due to the uniform composition of the amorphous filler alloy.Therefore,fine compounding is more important than coarse compounding in improving the bonding strength of diamond sand grains and filling alloys.
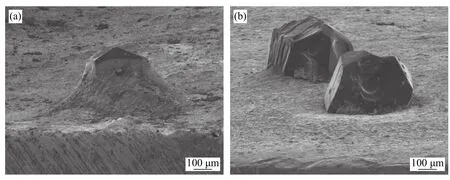
Fig.8 Brazed diamond grits (a) Brazed with amorphous filler alloy (b) Brazed with crystalline filler alloy
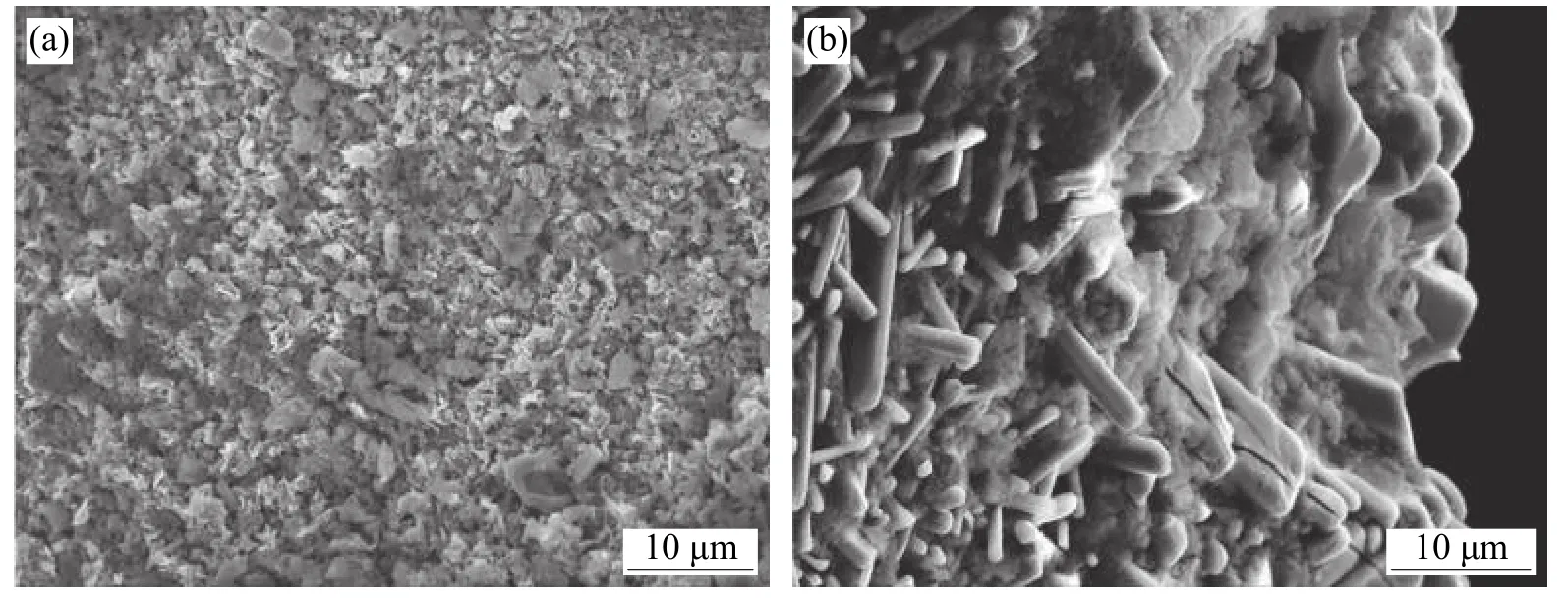
Fig.9 Brazed surface obtained after the diamond bars were eroded using strong acid (a) Brazed with amorphous filler alloy(b) Brazed with crystalline filler alloy
3 Perspectives
Data on the relationship between adhesion work and fracture work can be used for technical purposes.Especially,the effect of a small amount of separated metal on the adhesion and fracture energy of the matrix can be used as an important research topic for fine adjustment of diamond brazing ability.
In addition,it is necessary to reduce the material cost without adding silver filler.The higher melting point of nickel-based brazing filler can greatly increase graphitization brazing diamond at high temperature,which limits the service.In view of the literature review,the development of active filler such as amorphous filler used in diamond brazing needs further research.Compared with the crystalline filler metal,the solid-liquid two-phase zone of the amorphous filler metal is narrower,the melting temperature is lower,the melting speed is faster and the wettability of the matrix is better.Exploring the welding mechanism of amorphous welding materials and developing more excellent new amorphous welding materials have gradually become the focus of research in the field of welding.
4 Conclusions
(1) Starting from the properties of diamond/metal interface,the chemical and physical properties of diamond tools under various production methods and processes are discussed.Depending on their metal's affinity for the diamond,it can lead to graphitization,the formation of carbides,or the failure of the diamond to react.
(2) The metal that forms carbide film on the surface of diamond is usually the first choice,because they can keep the diamond sand grains without damaging it.
(3) Hardness must be proportional to the hardness of the workpiece to be cut,and related to the wear rate of diamond.
(4) The melting point shall be as low as possible to avoid damage to the diamond during sintering or brazing;On this basis,the applications of coated diamond are discussed,and the properties of several metal substrates,such as Ni-Cr,Cu-Sn and Ag-Cu,are described.
(5) Among various newly developed materials,amorphous filler alloy have attracted more and more attentions due to their great potential in brazing diamond.Studies related to the development of active filler metal using in diamond brazing are still required to be performed.
猜你喜欢
杂志排行
China Welding的其它文章
- Principles and application of RES welding technology
- Interface microstructure and properties of submerged arc brazing tin-based babbit
- Welding deviation detection method based on weld pool image contour features
- The microscopic mechanical performance for nonuniform welded joint of nickel-based alloy with nanoindentation
- Performance study of a complex thermal barrier functional coating with an electro-spark deposited burn-resistant layer
- Lap joining Al5052 to Ti6Al4V by GTAW with AlSi5 filler wire
