Interface microstructure and properties of submerged arc brazing tin-based babbit
2019-10-22ZhouFangmingLiJingXuDonghaoShiMingxiao
Zhou Fangming , Li Jing , Xu Donghao , Shi Mingxiao
周方明,李敬,徐冬豪,石铭霄
1.Jiangsu Province Key Laboratory of Advanced Welding Technology,Jiangsu University of Science and Technology,Zhenjiang 212003,China;
2.School of Materials Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang 212003,China
Abstract The submerged arc brazing method was used to connect the tin-based babbit alloy with the steel matrix.The microstructure of the submerged arc brazed Babbitt interface layer on the surface of Q235B steel was analyzed by OM,SEM and EDS and the hardness properties of the joint interface layer were tested by MH-5 microhardness tester.the result of research shows that a layer of canine-shaped intermetallic compound with uneven thickness is formed at the interface,and the thickness is 10 -20 μm.The interface layer includes two kinds of compound layers,namely the FeSn layer near the side of the steel substrate and FeSn2layer near the side of the babbit.During the interfacial reaction process,Fe atoms in the steel matrix dissolve into the liquid babbit alloy and form a certain concentration gradient at the interface.The farther from the interface,the lower the Fe atom concentration.The growth of Gibbs free energy of FeSn is lower when the temperature is above 780.15 K,and the temperature during the welding process is much higher than 780.15 K,moreover the precipitation temperature of FeSn is higher.Therefore,in the subsequent cooling process,FeSn is first precipitated from the interface near the side of steel matrix and then FeSn2is precipitated from the interface near the side of babbit alloy.Microhardness test showed that the intermetallic compound at the interface layer significantly improved the hardness properties.
Key words tin-based babbitt alloy,submerged arc brazing,interfacial structure,intermetallic compound,hardness
0 Introduction
With the rapid development of the manufacturing industry,the development of mechanical equipment in the direction of heavy load,high speed and large scale in recent years has put forward higher requirements for the quality of the surface of the sliding bearing.As bearing alloys,such as Cu-based alloys[1-3],aluminum-based alloys[4-5],graphite[6-7],and tin-based babbit[8-10].Among these bearing alloys,tinbased babbit are widely used as antifriction layers for plain bearings due to their good wear resistance,seizure resistance and embedding properties.The connection of tin-based babbit and iron matrix materials is considered to be relatively effective measures for preparing babbit layers,but for the current preparation method of babbit layer,it is difficult to achieve green manufacturing and high-efficiency production at the same time.The reason is that traditional casting and arc spraying require hanging tin before production and must remove about 5 mm thick babbit after casting,nevertheless the joint strength is low,resulting in waste of resources and low production efficiency[9];welding fumes and splashing for MIG method[11]is more serious,polluting the environment;TIG arc brazing[12]preparation of the babbit layer is thinner,and the production efficiency is low,the search for a new method for preparing a babbit layer has important research significance.
The iron matrix material and the tin-based babbit alloy are incompatible metals because of their large differences in thermophysical properties,such as melting point (iron is 1 538 °C,tin-based babbit is 230 °C).For these dissimilar metal connections,their interfacial bonding strength depends on the interface microstructure,which has attracted the attention of researchers in recent years[8,13-14].At present,submerged arc brazing is a new type of surface treatment technology,that is a submerged arc welding in which an arc is burned under a brazing flux and achieve the effect of brazing.The essence removes the surface oxide film by brazing agent to promote the wetting and spreading of the liquid babbit alloy,and combines with the solid steel through the interfacial reaction.However,during the process of submerged arc brazing of babbit,the thermal movement of the Fe element in the matrix is intensified and migrates into the alloy layer,which easily reacts with the alloying elements to form a brittle intermetallic compound layer,and during the dynamic heating of the arc,the metal phase composition of the compound is difficult to control,which makes it difficult to guarantee the bonding properties between the babbit layer and the substrate;this is an urgent technical problem to be solved for engineering equipment requiring high performance stability.However,there is still a lack of research on the microstructure characteristics of the interface layer between the babbit layer and the matrix and the growth behavior of intermetallic compounds.In this paper,the Babbit layer was prepared by submerged arc brazing method.The microstructure of the interfacial layer,the phase composition and growth behavior of the compound layer were studied in depth.
1 Experiment
The test piece is a Q235B steel plate with a size of 40 mm × 40 mm × 10 mm.The chemical composition is shown in Table 1.The test brazing material is ZChSnSb11-6 type babbit alloy,and 1.0 g is selected to make a round cake.The chemical composition is shown in Table 2.The test brazing flux is a special flux,and the chemical composition is shown in Table 3.Before welding,a thin steel wire brush and sandpaper are needed to remove the oxide layer on the surface of the steel substrate,and soft solder then the surface oil is cleaned with acetone,and the test filler is ultrasonically cleaned in an anhydrous ethanol solution.The workpiece was fixed by a clamp,and the welding was carried out by using the MAGIC WAVE 2600 AC-DC pulsed tungsten argon arc welding machine manufactured by Fronius,Austria.The schematic diagram of the fixed-point submerged arc brazing is shown in Fig.1.
After welding,the workpiece is cut along the vertical direction of the weld,and is molded into a sample by resinmolding.The water-grinded metallographic sandpaper is pre-ground in the order of 400,600 and 1 000,and then polished with a 1 μm diamond polishing agent.After polishing,4% nitric acid alcohol is used for corrosion with 3 -5 s.The microscopic characteristics of the interface layer of the babbit alloy were analyzed by optical microscopy (OM) and scanning electron microscopy (SEM).The chemical composition of the interface layer was analyzed by energy dispersive spectroscopy (EDS).The microhardness of the joint was tested by MH-5 microhardness tester,load 0.25 N,loading time 10 s.

Table 1 Chemical composition of the Q235B(wt%)
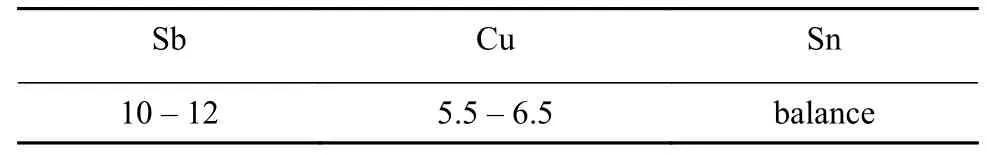
Table 2 Chemical composition of the brazing filler metal(wt%)
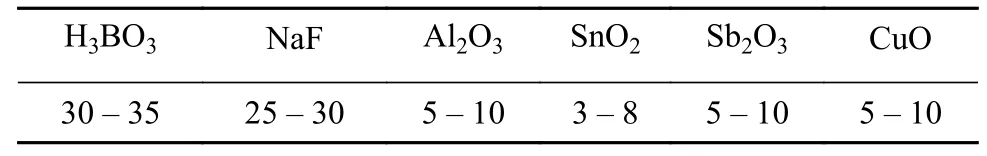
Table 3 Composition of the brazing flux (wt%)

Fig.1 Schematic digram of fixed-point submerged arc brazing
2 Results and discussion
2.1 Microstructure and genesis of interface layer
The thickness,microstructure and regularities of distribution of the interface layer of the submerged arc brazing of Q235B determine the mechanical properties of the babbit alloy interfacing layer.The microstructure of the interface layer is shown in Fig.2.A canine-shaped intermetallic compound layer with an uneven thickness is formed at the interface and its thickness is 10 -20 μm.The analysis shows that the heating temperature is high under the condition of submerged arc.The Fe atom at the interface of Q235B steel is excited by the activity of the hot atom to diffuse into the molten babbit metal.It undergoes a severe metallurgical reaction with the Sn element of the babbit alloy,and the thickness of the interface layer is uneven.Forming an intermetallic compound layer having a non-uniform thickness at the interface;the interface layer is distributed in the substrate and the babbit layer due to the migration of the Fe element into the interface layer.The microstructure of the babbit interface layer is still a typical as-cast structure,that is,the soft phase matrix (α solid solution) is distributed with the hard phase compound β phase (star-shaped Cu6Sn5) and the ε phase (block-shaped SnSb).

Fig.2 Microstructure of the babbit interface layer
2.2 EDS characteristics and analysis of interface layer
The element distribution of the interface layer and the composition of the compound layer play an extremely important role in the phase composition of the interface.First,perform element line scan on the interface layer.In the welding process,the interaction between atoms is maily the interaction between Fe atoms and Sn atoms,so only the distribution of Fe atoms and Sn atoms is observed.It can be seen from Fig.3 that the Fe atoms are mainly distributed in the substrate,and the peaks are gradually reduced in the interface layer;the Sn atoms are mainly distributed in the interface layer,and there is almost no in the matrix.In the process of submerged arc brazing,the babbit alloy is liquid,the steel matrix is solid,the distance between atoms in the liquid babbit alloy is larger than that in the solid matrix,and the Fe atom penetrates into the weld to dissolve and diffuse.A small amount of Fe atoms entered the depth of the liquid babbit alloy under the agitation of the arc force,and the Sn atom failed to penetrate the interface and enter the workpiece.
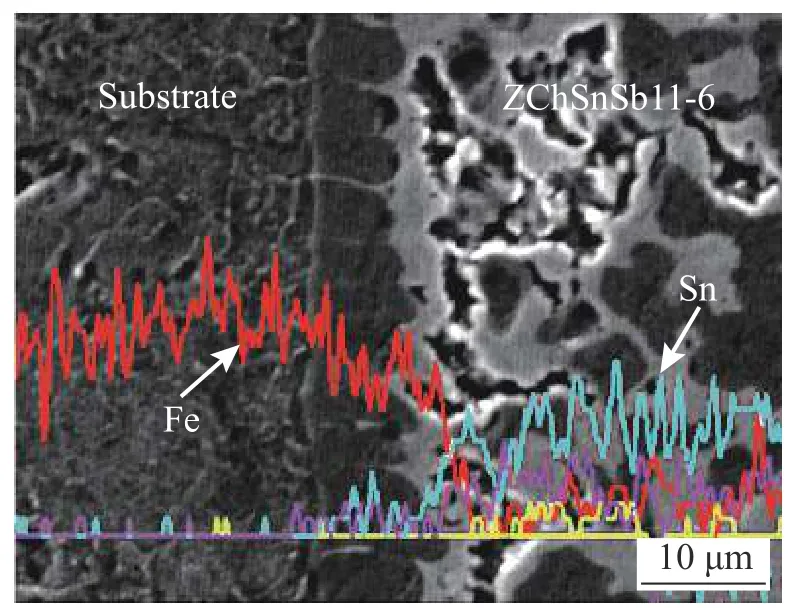
Fig.3 EDS line scan pattern of interface layer
Characteristic point elemental analysis was then performed on the different interface layers in Fig.4,and the results are shown in Table 4.The results show that the interface product at the position of the spot spectrum 1 is dark gray,in which the content of Fe atom and Sn atom is equivalent.The interface product at the position of the spot spectrum 2 is light gray Sn-rich phase,and the content ratio of Fe atom to Sn atom is 1:2.And the change of the content of each chemical element in the feature point is consistent with the line distribution result of the corresponding element in Fig.3.According to the analysis,the intermetallic compound generated at the position of the spot spectrum 1 is FeSn,and the compound formed at the position of the spot spectrum 2 is FeSn2.In the initial stage of the reaction,a large amount of Fe atoms are dissolved in the weld,and FeN is formed by combining with the Sn atom.When the iron atom concentration reaches saturation,the iron atom further diffuses into the babbit alloy,and iron atoms react with tin atoms to form FeSn2with low iron content.Through the analysis of the characteristic points in the interface layer,a series of chemical reactions occur between the Fe atom entering the interface layer and the Sn atom in the interface layer to form FeSn and FeSn2compounds.
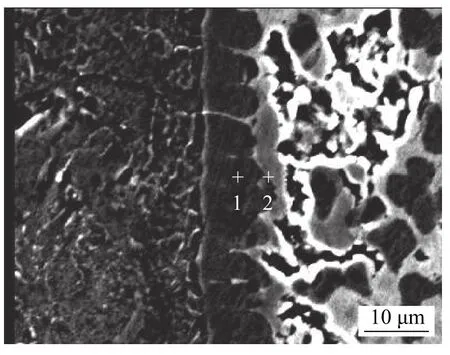
Fig.4 EDS point scan pattern of babbit interface layer
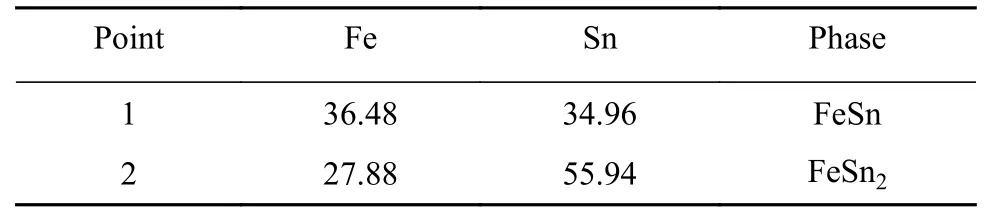
Table 4 EDS results of characteristic point (at%)
2.3 Thermodynamic analysis of compound growth in interface layer
The growth of the compound is determined by its growth Gibbs free energy ΔG.By calculating the Gibbs free energy ΔGof the compound,the possibility of various compounds at the interface can be predicted.The growth Gibbs free energy of Fe-Sn compound can be calculated using the classical thermodynamic formula:


According to the thermodynamic data of the Fe-Snbased compound[9-10],the growth Gibbs free energy of each compound can be obtained:

Referring to Fig.5,the interface reaction product should be FeSn,FeSn2and Fe3Sn2with lower growth Gibbs free energy in equilibrium state,wherein Fe3Sn2has the lowest growth free energy ΔG0,but Fe3Sn2is extremely unstable,only in equilibrium or slow cooling can be precipitated under conditions.The Q235B surface submerged arc brazing babbit process has a high heating temperature and a fast cooling rate,and it is difficult to form Fe3Sn2under such conditions.During the submerged arc brazing interface reaction,Fe atoms in the steel matrix dissolve into the liquid babbit alloy and form a certain concentration gradient.The farther from the interface,the lower the Fe atom concentration,and at the same time,the growth Gibbs free energy of FeSn is low above 780.15 K,but the temperature during the welding process is much higher than 780.15 K and the precipitation temperature of FeSn is higher which is about 880.15 K,nevertheless the precipitation temperature of FeSn2is about 780.15 K,so in the subsequent cooling process,FeSn is precipitated on the side of the interface near the steel substrate and then Sn-rich FeSn2is precipitated on the side close to the babbit alloy.
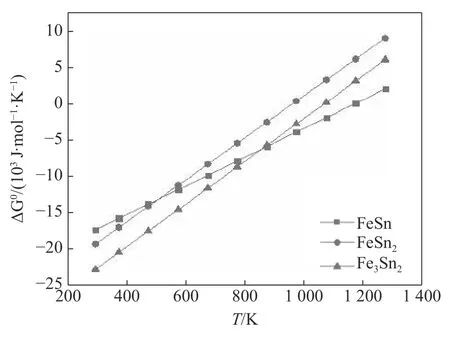
Fig.5 The relationship of several Fe-Sn compound growth Gibbs free energy and temperature
2.4 Hardness performance analysis of surfacing interface layer
The microhardness test of the submerged arc brazing babbit brazing layer on Q235B surface was carried out and the hardness distribution in the vertical interface direction is shown in Fig.6.It can be seen from Fig.6 that the microhardness of the weld is much lower than that of the base metal and the heat affected zone.The heat affected zone has obvious hardening phenomenon and the highest hardness can reach 376.69 HV.Then,it gradually decreases toward the base metal and finally reaches the average hardness of the base material of 239.65 HV.The microscopic average hardness of the babbit brazing interface layer is about 100 HV,then the hardness decreases toward the inner direction of the babbit brazing layer and the final average hardness is about 27.62 HV.This is due to the presence of a large amount of Fe-Sn compounds at the interface layer of the babbit brazing.These intermetallic compounds have high hardness and poor plasticity,resulting in a hardness significantly greater than that in the brazing layer.

Fig.6 Microhardness distribution of babbit surfacing interface
3 Conclusions
(1) The babbit layer was prepared by submerged arc brazing method.Under the condition of submerged arc,the Fe element on the surface of the workpiece diffused into the babbit alloy surfacing layer under the action of dynamic arc and formed an uneven thickness of the canine-like intermetallic compound layer at the interface.
(2) The babbit alloy surfacing interface layer consists of two compounds,namely FeSn on the steel substrate side and FeSn2on the babbit side.During the interfacial reaction,the Fe atoms in the steel matrix dissolve into the liquid babbit alloy and form a certain concentration gradient at the interface.The farther from the interface,the lower the Fe atom concentration.
(3) FeSn has a low growth free energy when the temperature is above 780.15 K,and the temperature during the welding process is much higher than 780.15 K,and the precipitation temperature of FeSn is higher,so FeSn is first precipitated on the side of the steel substrace in the subsequent cooling process.FeSn is precipitated on one side of the substrate,and then FeSn2is precipitated on the side of the babbit.
(4) The Fe-Sn compound significantly increases the hardness of the interface layer of the babbit alloy surfacing,up to about 100 HV.The hardness in the surfacing layer decreases with the decrease of Fe content,and the average hardness is about 27.62 HV.
猜你喜欢
杂志排行
China Welding的其它文章
- Effect of heat treatment on microstructure and properties of single crystal copper cold-welded joints
- Extrusion process and property of AZ31 magnesium alloy
- Lap joining Al5052 to Ti6Al4V by GTAW with AlSi5 filler wire
- Performance study of a complex thermal barrier functional coating with an electro-spark deposited burn-resistant layer
- The microscopic mechanical performance for nonuniform welded joint of nickel-based alloy with nanoindentation
- Welding deviation detection method based on weld pool image contour features
