基于柔性双(甲基苯并咪唑)和羧酸配体的Cd(Ⅱ)/Co(Ⅱ)/Zn(Ⅱ)配合物的合成和表征
2019-09-09张兆沛VlasenkoVolodymyrAnatoliyovych刘润强张裕平
张兆沛 Vlasenko Volodymyr Anatoliyovych 刘润强 杨 里 刘 露*, 张裕平
(1河南科技学院生命科技学院,河南科技学院现代生物育种河南省协同创新中心,新乡 453003)
(2Agrotechnologies and Environmental Use Department,Sumy National Agrarian University,Sumy,40021,Ukraine)
(3河南科技学院资源与环境学院,新乡 453003)
(4河南科技学院化学化工学院,新乡 453003)
The thriving regions of crystal engineering have offered a sane junction between aesthetics of crystalline architectures and their potential applications,of which the eventual destination is to triumphantly acquire target materials that own tailored structures and physicochemical properties[1-10].So far,although lots of 0D/1D/2D/3D frameworks have been reported,the construction of novel-innovative architectures and a systematic study still require a long-term challenge[11-13].Flexible N-donors ligands are highly attractive,because their free conformation allows for greater structural diversity.Carboxylates serving as co-ligands have also been extensively adopted to build new complexes[14-17].Furthermore,the two-ligand assembly system could provid more variability to fabricate complicated structures.Taking this idea into consideration,in this paper,we choose 1,1′-(1,6-hexane)bis-(2-methylbenzimidazole)(hbmb)as the flexible N-donor ligand and three disparate O-donor carboxylates,homophthalic acid(H2pta),1,4-benzenedicarboxylic acid(1,4-H2bdc)and 3,3′,4,4′-benzophenone tetracarboxylic acid(H4bptc),as auxiliary ligands in the synthesis of metalorganic coordination polymer materials.At last,three new complexes,[Cd(hbmb)0.5(pta)]n(1),[Co(hbmb)(1,4-bdc)]n(2)and{[Zn2(hbmb)1.5(bptc)(H2O)]·0.5hbmb·3H2O}n(3)were prepared successfully.The structures of 1~3 have been determined by single-crystal X-ray diffraction analyses and further characterized by infrared spectra,elemental analyses.Besides,the effect of flexible ligand hbmb and three carboxylate co-ligands on the frameworks of 1~3 are debated in detail.
1 Experimental
1.1 Materials and methods
All reagents and solvents were commercially available except for hbmb,which was synthesized according to the literature[18].FT-IR spectra were recorded on a FTIR-7600 spectrophotometer with KBr pellets in 400~4 000 cm-1region.Elemental analyses(C,H and N)were carried out on a FLASH EA 1112 elemental analyzer.
1.2 Synthesis of[Cd(hbmb)0.5(pta)]n(1)
A mixture of Cd(Ac)2·2H2O(0.1 mmol),hbmb(0.1 mmol),H2pta(0.05 mmol),EtOH(5 mL)and H2O(5 mL)was placed in a 25 mL Teflon-lined stainless steel container.The mixture was sealed and heated at 160℃for three days.After the mixture was cooled to ambient temperature at a rate of 5 ℃·h-1,colorless irregular-shaped crystals of 1 were obtained with a yield of 10%(based on Cd).Anal.Calcd.for C21H21Cd N2O4(%):C,52.78;H,4.42;N,5.86.Found(%):C,52.75;H,4.40;N,5.90.IR(KBr,cm-1):3 056(w),3 027(w),2 931(m),2 863(w),1 562(s),1 508(m),1 459(m),1 388(w),1 292(m),1 238(m),1 153(m),1 014(m),941(s),833(w),746(s),673(s).
1.3 Synthesis of[Co(hbmb)(1,4-bdc)]n(2)
A mixture of CoCl2·6H2O(0.1 mmol),hbmb(0.1 mmol),1,4-H2bdc(0.2 mmol),H2O(8 mL)was placed in a 25 mL Teflon-lined stainless steel container at 120℃for three days.After the mixture was cooled to room temperature at a rate of 5 ℃·h-1,purple irregularshaped crystals suitable for X-ray diffraction were obtained with a yield of 15%(based on Co).Anal.Calcd.for C30H30CoN4O4(%):C,63.26;H,5.30;N,9.83.Found(%):C,63.31;H,5.31;N,9.80.IR(KBr,cm-1):2 937(m),2 865(m),1 814(vs),1 461(s),1 334(vs),1 292(w),1 137(m),1 081(m),1 016(s),927(m),887(m),811(vs),744(vs),595(s).
1.4 Synthesis of{[Zn2(hbmb)1.5(bptc)(H2O)]·0.5hbmb·3H2O}n(3)
A mixture of ZnCO3(0.1 mmol),hbmb(0.1 mmol),H4bptc(0.05 mmol),H2O(7 mL)was placed in a 25 mL Teflon-lined stainless steel container.The mixture was sealed and heated at 160℃for three days.After the mixture was cooled to ambient temperature at a rate of 5 ℃·h-1,yellow block-shaped crystals of 3 were obtained with a yield of 5%(based on Zn).Anal.Calcd.for C61H66Zn2N8O13(%):C,58.61;H,5.32;N,8.96.Found(%):C,58.65;H,5.29;N,8.99.IR(KBr,cm-1):3 427(s),2 036(w),1 634(m),1 493(vw),1 395(vw),1 319(vw),1 254(vw),1 188(m),1 112(m),797(s),623(w).
1.5 Crystal structural determination
The data of the 1~3 were collected on a Rigaku Saturn 724 CCD diffractomer(Mo Kα,λ=0.071 073 nm)at temperature of(20±1)℃.Absorption corrections were applied by using multi-scan program.The data were modified for Lorentz and polarization effects.The structures were solved by direct methods and then refined by means of F2with a full-matrix least-squares technique that adopted the SHELX-97 crystallographic software package[19].The hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters.Crystallographic crystal data and structure processing parameters for 1~3 are summarized in Table 1.Selected bond lengths and bond angles of 1~3 are listed in Table 2.
CCDC:1885275,1;1885276,2;1885277,3.
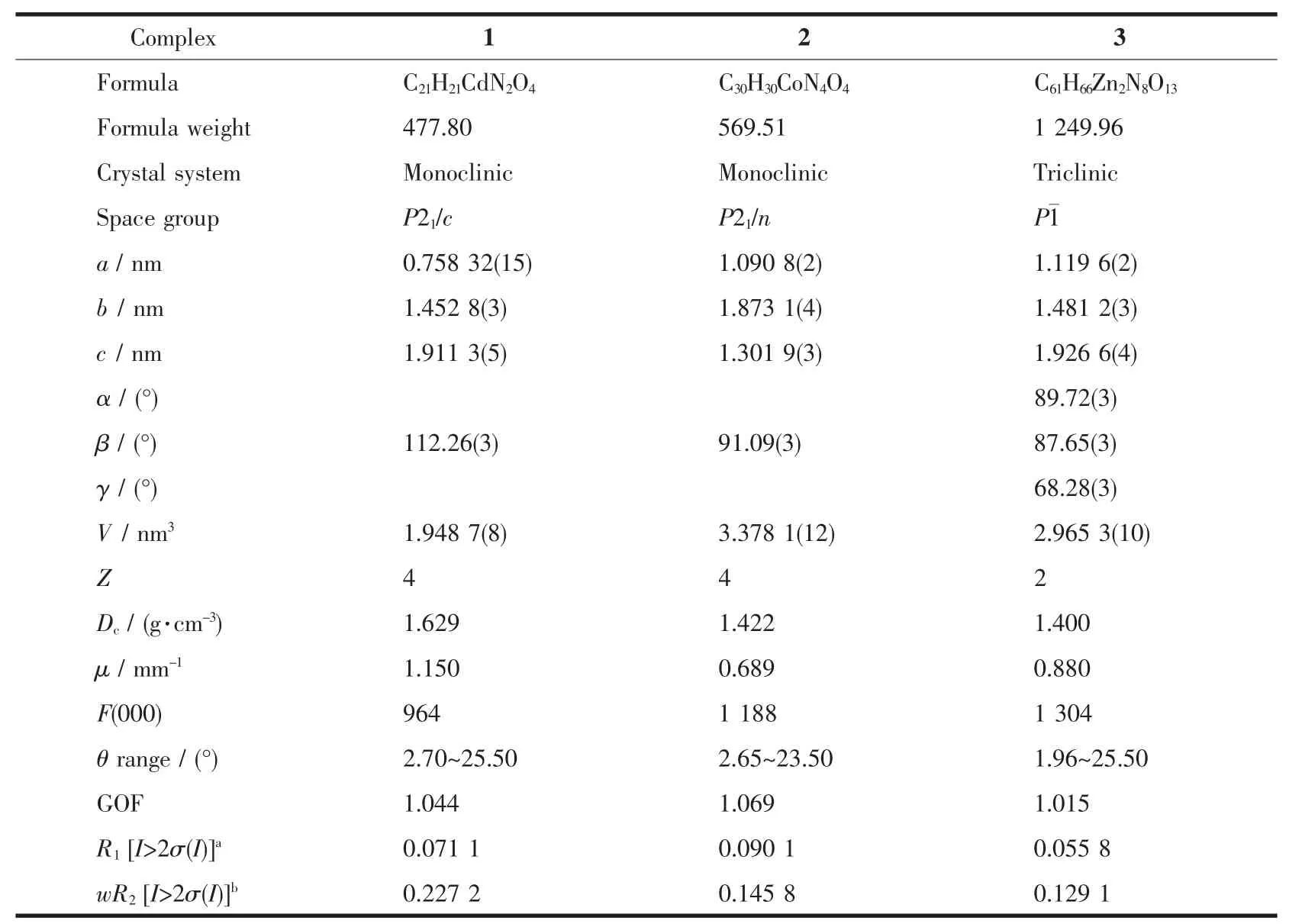
Table 1 Crystal data and structure refinement details for complexes 1~3
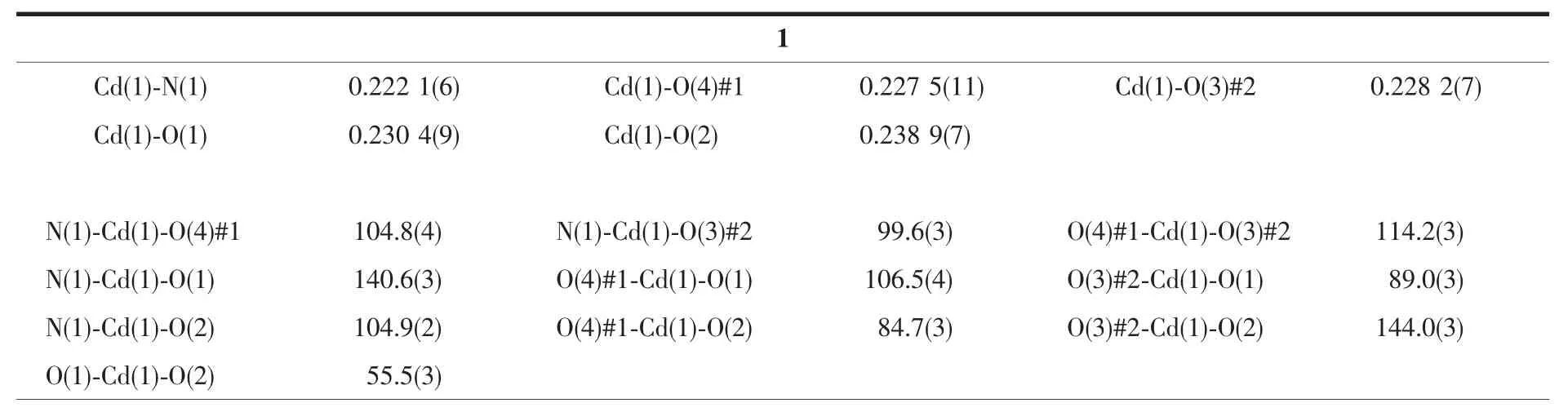
Table 2 Selected bond lengths(nm)and bond angles(°)for 1~3
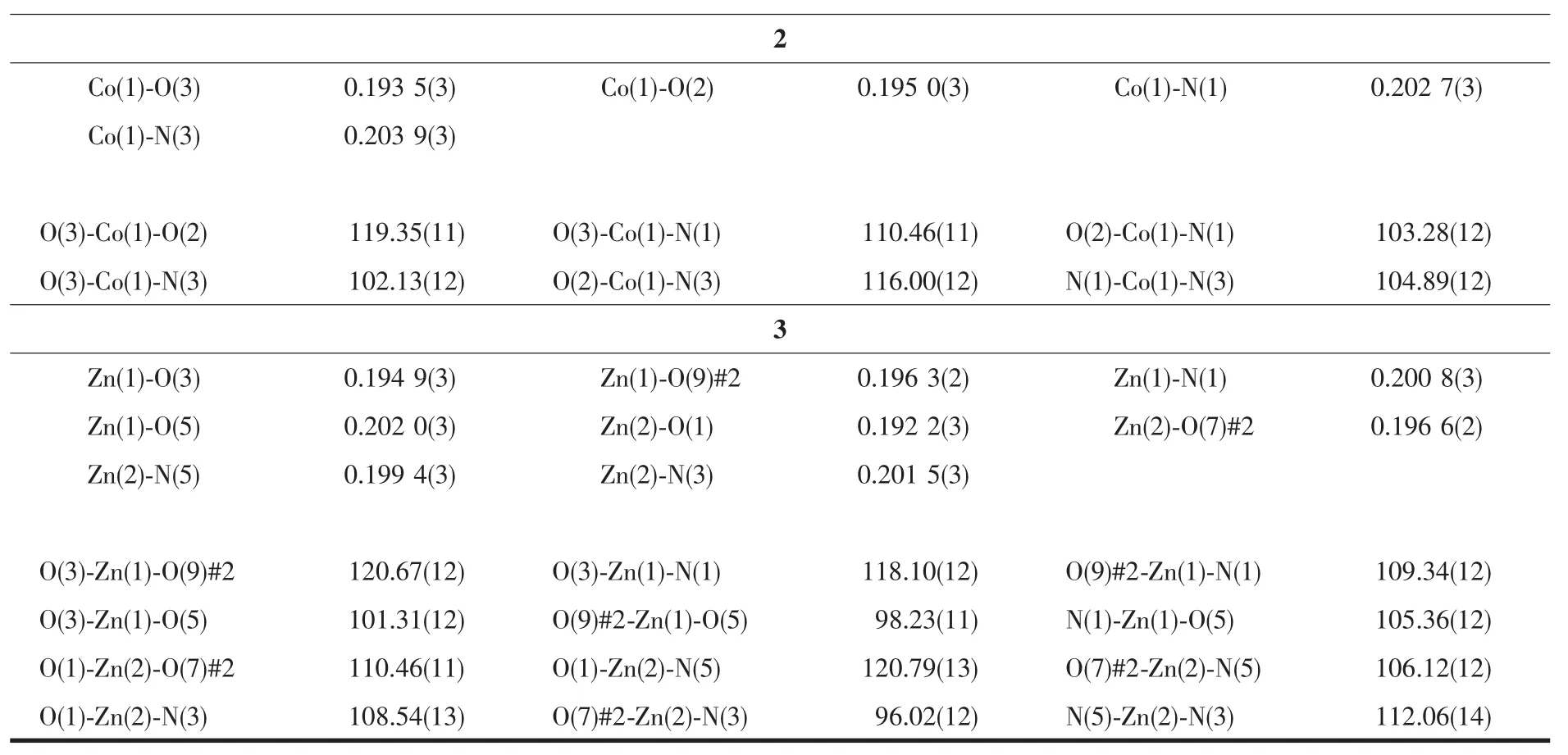
Continued Table 2
2 Results and discussion
2.1 Crystal structure of[Cd(hbmb)0.5(pta)]n(1)
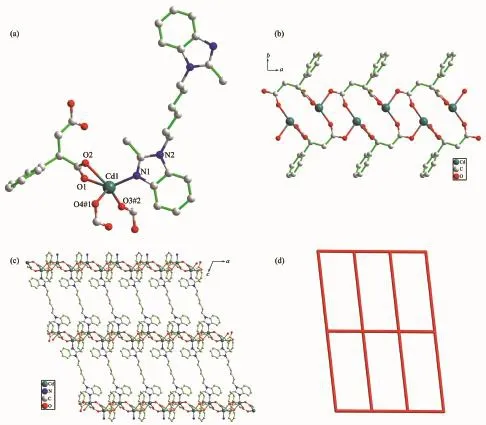
Fig.1 (a)Coordination environment of Cd(Ⅱ) ion in 1 with hydrogen atoms omitted for clarity;(b)One-dimensional(1D)chain generated by Cd(Ⅱ) ions and pta2-linkers in 1;(c)Schematic view of the 2D net built by 1D Cd(Ⅱ)/pta2-chain and hbmb in 1;(d)Schematic representation of 44·62 topology of complex 1
Structural analysis indicates that complex 1 is a 2D structure with point symbol of(44·62)and crystallizes in the monoclinic P21/c space group.The asymmetric unit of 1 is composed of one crystallographically independent Cd(Ⅱ) ion,half a hbmb ligand,and one pta2-ligand.As shown in Fig.1a,the Cd(Ⅱ) ion exhibits a five-coordinated environment,which is provided by one nitrogen atom (N1)from one separate hbmb and four oxygen atoms(O1,O2,O3#2,O4#1)from three independent pta2-anions.The structural index parameter(τ)[20]is close to 0.00,indicating that the geometry around Cd(Ⅱ)can be described as an ideal squarepyramidal geometry.The Cd-O distances are in a range of 0.227 5(11)~0.238 9(7)nm,while the Cd1-N1 bond length is 0.222 1(6)nm.hbmb shows the symmetric trans-conformation with the Ndonor…N-Csp3groups of pta2-ligand take an inconsistent coordination mode.One carboxylate group of pta2-ligand adopts a chelating bidentate mode,and the other is in a bridging mono-bidentate mode.Each pta2-anion bridges three Cd(Ⅱ)ions in this way to give rise to a 1D infinite chain(Cd(Ⅱ)/pta2-chain)along the c-axis,containing a binuclear unit[Cd2(CO2)2]with the Cd…Cd separation of 0.424 00 nm (Fig.1b).The Cd(Ⅱ)ions of two neighboring 1D Cd(Ⅱ)/pta2-chains were connected by the trans-conformational hbmb ligands generating the 2D sheet of 1 (Fig.1c).The bridged Cd…Cd distance along μ-hbmb is 1.643 36 nm.Topological analysis was performed on 1.As depicted in Fig.1d,if the binuclear unit[Cd2(CO2)2]is taken as a 2-connector and hbmb is defined as linkers,the 2D framework of 1 can be described as(44·62)topology.
2.2 Crystal structure of[Co(hbmb)(1,4-bdc)]n(2)
Complex 2 crystallizes in the monoclinic space group P21/n and features a 2D network motif.The asymmetric unit consists of one crystallographically unique Co(Ⅱ) ions,one deprotonated 1,4-bdc2-anions,and one hbmb ligand.As illustrated in Fig.2a,the Co(Ⅱ) ion displays distorted tetrahedral geometry,defined by two carboxyl oxygen atoms(O2,O3)from two 1,4-bdc2-anions and two nitrogen atoms (N1,N3)from two hbmb ligands.The τ4parameters introduced by Houser[21]of Co1 are 0.88.The Co1-O2 and Co1-O3 bond distances are 0.195 0(3)and 0.193 5(3)nm,respectively,while the Co1-N1 and Co1-N3 bond lengths are 0.202 7(3)and 0.203 9(3)nm,respectively.The hbmb ligand adopts asymmetric trans-conformation73.233°and 88.281°.This kind of hbmb connect the adjacent Co(Ⅱ) ions giving rise to a 1D Co(Ⅱ)/hbmb chain along the b-axis with a Co…Co separation of 1.090 80 nm (Fig.2b).Interestingly,two carboxylate groups of 1,4-bdc2-both adopt bi-monodentate coor-dination mode.The nearby Co(Ⅱ)ions are bridged by 1,4-bdc2-anions to product a 1D Co(Ⅱ)/1,4-bdc2-chain along the a-axis with a Co…Co separation of 1.105 50 nm(Fig.2c).The interlaced connection of the 1D Co(Ⅱ)/hbmb chain and 1D Co(Ⅱ)/1,4-bdc2-chain constructs the corrugated 2D network by sharing Co(Ⅱ)ions(Fig.2d).Topological analysis was performed on 2.As depicted in Fig.2e,each Co(Ⅱ) ion is encircled by four organic ligands (two hbmb ligands and two 1,4-bdc2-ligands).If the Co(Ⅱ)ion is taken as a 4-connector,the 1,4-bdc2-and hbmb can be defined as linkers,the 2D framework of 2 can be described as(42·6)(42·63·8)topology.
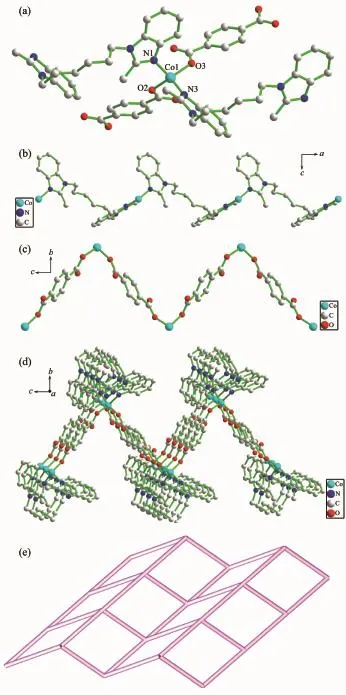
Fig.2 (a)Coordination environment of Co(Ⅱ)ion in 2 with hydrogen atoms omitted for clarity;(b)1D chain along b-axis alternately generated by ligand hbmb bridging two adjacent Co(Ⅱ) ions;(c)1D chain resulting from 1,4-bdc2-anions connectting two adjacent Co(Ⅱ) ions along a-axis;(d)Undulated 2D network bridged by hbmb and 1,4-bdc2-in 2;(e)Schematic description of a 4-connected 2D network with(43·62·8)topology for 2
2.3 Crystal structure of{[Zn2(hbmb)1.5(bptc)(H2O)]·0.5hbmb·3H2O}n(3)
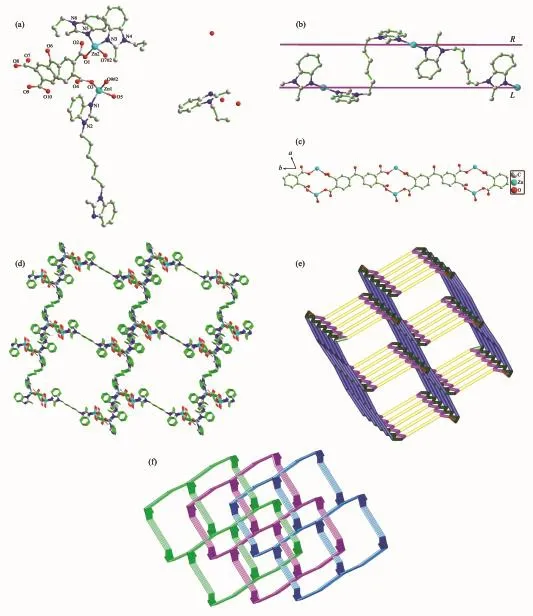
Fig.3 (a)Coordination environment of Zn (Ⅱ) ion in 3 with hydrogen atoms omitted for clarity;(b)Pseudo meso-helix alternately generated by hbmb-Ⅰ and hbmb-Ⅱ bridging two adjacent Zn(Ⅱ) ions;(c)1D chains built though Zn(Ⅱ) ions and bptc4-linkers in 3;(d)Perspective view of 3D coordination framework fabricated by 1D Zn(Ⅱ)/bptc4-chains and 1D Zn(Ⅱ)/hbmb chains;(e)Single 3D topology network;(f)Schematic representation of 3-fold interpenetrated topology nets for 3
Complex 3 exhibits a beautiful 3-fold interpenetrating 3D network,which crystallizes in the triclinic system,space group P1.Each asymmetric unit of 3 consists of two crystallographically independent Zn(Ⅱ)ions,one and a half hbmb ligands,one bptc4-,one coordinated water molecule,half a dissociative hbmb and three guest water molecules.Fig.3a illustrates the coordination environment of the Zn(Ⅱ) ions.The distorted tetrahedral coordination geometry of the central Zn1 ion is completed by one N atom (N1)of one hbmb ligand,two O atoms(O3 and O9#2)of two bptc4-anions and one O atom(O5)of one coordinated water molecule.Zn2 ion also shows a distorted tetrahedral coordination geometry,which is defined by two oxygen atoms (O1 and O7#2)from two bptc4-anions,and two nitrogen atoms(N3 and N5)from two hbmb ligands.The distortion of tetrahedron can be indicated by the calculated value of the τ4parameter introduced by Houser(τ4≈0.85 for Zn1 and τ4≈0.90 for Zn2)[21-22].The distances of Zn-O/N bond are in a range of 0.192 2(3)~0.202 0(3)nm,which are in the normal range[23-24].There are four independent hbmb ligands in complex 3,all of which exhibit symmetrical75.848°.The two types of hbmb(hbmb-Ⅰ and hbmb-Ⅱ)act as bidentate mode to link two adjacent Zn2 ions alternately to generate a pseudo meso-helix(Fig.3b).The Zn2…Zn2 distances across the hbmb bridges are 1.181 11(hbmb-Ⅰ)and 1.333 29(hbmb-Ⅱ)nm,separately.hbmb-Ⅲonly connect two Zn1 ions and the Zn1…Zn1 distance across the hbmb-Ⅲ bridges is 1.630 76 nm.In 3,the bptc4-anions connects four Zn(Ⅱ) cations,and four carboxylate group all takes bridging monodentate coordination mode.The nearby Zn1 and Zn2 ions are linked together by the carboxylate O atoms of the bptc4-to form a 1D Zn(Ⅱ)/bptc4-chain along the c-axis containing the 14-membered rings (Fig.3c).In this ring,the Zn1…Zn1 distance is 0.504 89 nm.The combination of 1D chains of Zn(Ⅱ)/bptc4-and Zn(Ⅱ)/hbmb produces the 3D structure of 3(Fig.3d).From a topological perspective,each Zn1 ion is surrounded by four organic ligands(two hbmb ligands and two bptc4-ligands),each Zn2 ion is surrounded by three organic ligands (one hbmb ligand and two bptc4-ligands),and each bptc4-ligand is connected to four Zn ions.Hence,the Zn1 ions can be regarded as 4-connected nodes,the Zn2 ions can be regarded as 3-connected nodes,the bptc4-anions are defined as 4-connected nodes and hbmb ligands are considered as linkers,respectively.The 3D framework of 3 can be represented as a(3,4,4)-connected 3-fold interpenetrating network with a vertex symbol of(42·62·82)(4·62)(4·64·8)(Fig.3e and 3f).
2.4 Effect of hbmb and carboxylate co-ligands on the structures of 1~3
According to the above description,we find that the flexible hbmb ligand presents symmetric transconformation in 1 and 3,asymmetric trans-conformationdifferent in 1~3.The free conformations of-(CH2)-spacer greatly enrich structures of complexes 1~3.In 1,hbmb connects two neighboring 1D Cd(Ⅱ)/pta2-chains by sharing the Cd(Ⅱ)ions to build a 2D sheet.In 2,hbmb links the adjacent Co(Ⅱ) ions,resulting in a 1D Co(Ⅱ)/hbmb chain.In 3,hbmb-Ⅰ and hbmb-Ⅱjoin two adjacent Zn2 ions alternately to produce a pseudo Zn(Ⅱ)/hbmb meso-helix,while hbmb-Ⅲ only connect two Zn1 ions.Owing to the flexible nature of the -(CH2)-spacers,two bis(methylbenzimidazole)groups of hbmb can rotate freely when coordinating with metal centers,which can greatly beautify the structure of complexes 1~3.On the other side,the carboxylate anions also have significant influence on the constructing of resultant structures of 1~3.In synthesis process of 1 ~3,we have selected three carboxylates as auxiliary ligands.In 1,each pta2-anion bridges three Cd(Ⅱ)ions to generate a 1D infinite Cd(Ⅱ)/pta2-chain containing a binuclear unit[Cd2(CO2)2].Under the connection of hbmb,1 shows a flat 2D framework with the point symbol of(44·62).In 2,we select 1,4-H2bdc with a rigid spacer and an angle of 180°between the two carboxylic groups as a coligand.1,4-bdc2-anions bridge the nearby Co(Ⅱ) ions,forming a 1D Co(Ⅱ)/1,4-bdc2-chain.By sharing Co(Ⅱ)ions,the interlaced connection of the 1D Co(Ⅱ)/hbmb chain and 1D Co(Ⅱ)/1,4-bdc2-chain induce the corrugated 2D framework with the point symbol of(42·6)(42·63·8)of 2.By introducing tetracarboxylic acid ligand H4bptc,3D 3-fold interpenetrating(3,4,4)-connected (42·62·82)(4·62)(4·64·8)networks 3 are obtained.The bptc4-link the nearby Zn1 and Zn2 ions to form a 1D Zn(Ⅱ)/bptc4-chain containing a 14-membered rings.The above discussions indicate the configuration of hbmb and diverse carboxylate coligands play a critical role in the construction resultant complexes.The three different structures also show that more complex carboxylic acid co-ligands will lead to the production of higher dimensional complexes.
2.5 Photoluminescence properties
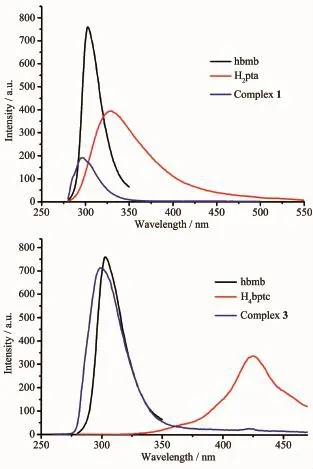
Fig.4 Photoluminescent spectra of free hbmb ligand,free carboxylate ligands and complexes 1 and 3
Currently,the researchers have great interest in fluorescence complexes by reason of their potential applications.Hence,the photoluminescent spectra of complexes 1 and 3,the free hbmb ligand and two carboxylic acids were investigated at ambient temperature.As depicted in Fig.4,the photoluminescent spectra of the free ligand hbmb,H2pta and H4bptc showed different emission peak at 302 nm(λex=286 nm),330 nm(λex=260 nm)and 425 nm(λex=330 nm),severally.Complexes 1 and 3 exhibited the emission maxima at 297 nm(λex=245 nm)for 1,299 nm(λex=245 nm)for 3,severally.For 1,the emission band was blue-shifted(5 and 33 nm)compared with that of the ligand hbmb and H2pta.The emission spectrum for 3 also presented blue shifts (3 and 126 nm)with respect to the ligand hbmb and H4bptc.The hypsochromic shifts of the emission peaks of 1 and 3 indicate that the coordination of the ligand to Cd(Ⅱ)/Zn(Ⅱ) ions increases the rigidity,leading to less vibrations of the skeleton and reducing the loss of energy by radiationless decay of the intraligand emission excited state[25].
3 Conclusions
In summary,by introducing different carboxylate co-ligands into Cd(Ⅱ)/Co(Ⅱ)/Zn(Ⅱ)-hbmb system,three varied architectures have been constructed and characterized by single-crystal X-ray diffraction analyses,infrared spectra and elemental analyses.Complexes 1~3 exhibit enthralling 2D/3D architectures with versatile topologies.The results of the above work manifest that distinct metal ions and various carboxylate co-ligands can greatly affect the final structures of complexes.In addition,1 and 3 maybe potential optical materials.
