氰基桥联的Fe2Ni双之字链的合成与磁性
2019-09-09贺艳丽孟银杉孙慧莹姜文静矫成奇
贺艳丽 孟银杉 孙慧莹 姜文静 矫成奇 刘 涛
(大连理工大学精细化工国家重点实验室,大连 116024)
0 Introduction
Single-chain magnets(SCMs),which can exhibit slow magnetic relaxation and magnetic hysteresis below blocking temperature,have attracted considerable attention and great interests because of their potential application in high-density information storage and spintronic devices[1-8]. This conception was firstly proposed by Glauber in 1960s that Ising magnetic chain was expected to exhibits low magnetic relaxation behavior[9].However,it was not until 2001 Gatteschi and co-workers reported the first single-chain magnet[Co(hfac)2(NITPHOMe)][10].In recent decades,considerable SCMs with chirality,porosity,spin-crossover,photo-switchable properties have been reported[10-16].In order to obtain high-performance SCMs,two challenges have to be solved:(1)constructing magnetic chains with strong intrachain ferromagnetic interactions so as to meet the Ising chain requirement;(2)minimizing the interchain interaction to avoid the long-range magnetic ordering.Utilizing metallocyanate building blocks as multidentate ligands is an effective approach for constructing SCMs for that it is helpful to form the one-dimensional structure and transmit the intrachain magnetic interactions[14,17-19].Particularly,tetracyanometallate building block facilitates the construction of one-dimensional chain structure,wherein two of the four cyano groups can coordinate with appropriate metal ions to form a double-zigzag chain,and the other two cyano groups can form hydrogen bonding interactions with solvent molecules.The recent study have also dedicated the important role of tetracyanometallate building blocks in the construction of photo-switchable single-chain magnets[20-21].
In order to obtain the SCMs,we plan to use tetracyanometallate building block to link spin carriers such as Co2+,Ni2+,Mn3+and Fe2+into a ferromagnetic chain.The interchain magnetic interaction should be minimized to avoid the long-range ordering.Therefore,suitable diamagnetic auxiliary ligands should be carefully selected to make chains magnetically wellisolated and ensure the uniaxial anisotropy of the transition metal ions[11,13,22-25].In this work,we selected Li[Fe(Ⅲ)(bpy)(CN)4](bpy=2,2′-bipyridine) as the building block to react with Ni(Ⅱ) ions.The Ni(Ⅱ) ion was chosen because the interactions between low-spin Fe(Ⅲ) and high-spin Ni(Ⅱ) are generally ferromagnetic.Three auxiliary ligands 4-phenylpyridine (bp),4-(phenyldiazenyl)pyridine(papy),and 1,2-di(pyridin-4-yl)diazene (azp)were applied,forming three cyanobridged FeⅢ2NiⅡdouble-zigzag chain complexes:{[Fe(bpy)(CN)4]2[Ni(bp)2]·2H2O}n(1),{[Fe(bpy)(CN)4]2[Ni(papy)2]·H2O}n(2)and{[Fe(bpy)(CN)4]2[Ni(azp)]·4H2O}n(3).Herein,we reported the synthesis,crystal structures and magnetic properties of compounds 1~3.
1 Experimental
1.1 Materials and methods
All organic reagents were commercially obtained and used without further purification.Li[Fe(bpy)(CN)4]and the ancillary ligands L(L=bp,papy and azp)were synthesized according to the literature methods[26].Elemental analyses(C,H and N)were performed on an ElementarVario ELⅢanalyzer.Magnetic measurements of the samples were performed on a Quantum Design SQUID(MPMS XL-7)magnetometer.Data were corrected for the diamagnetic contribution calculated from Pascal constants.
1.2 Synthesis of{[Fe(bpy)(CN)4]2[Ni(bp)2]·2H2O}n(1)
A 1.0 mL aqueous solution of Ni(BF4)2·6H2O(0.005 mmol)was placed at the bottom of a test tube,a mixture of methanol and water(1:4,V/V,2 mL)was gently layered on the top of the solution,and then a 1.0 mL methanol solution of Li[Fe(bpy)(CN)4](0.01 mmol)and 4-phenylpyridine(0.01 mmol)was carefully added as the third layer.After four weeks,red crystals of 1 were obtained and collected after washed with water and air dried.Yield:57% based on Ni(BF4)2·6H2O.Anal.Calcd.for C50H38Fe2N14NiO2(%):C 57.84,H 3.66,N 18.89;Found(%):C 57.76,H 3.62,N 18.72.
1.3 Synthesis of{[Fe(bpy)(CN)4]2[Ni(papy)2]·H2O}n(2)
Red crystals of compound 2 were obtained and collected in the same way as for compound 1,except using 4-(phenyldiazenyl)pyridine(0.01 mmol)to replace 4-phenylpyridine(0.01 mmol).Red crystals appeared after four weeks.Yield:61%based on Ni(BF4)2·6H2O.Anal.Calcd.for C50H36Fe2N18NiO(%):C 55.79,H 3.35,N 23.43;Found(%):C 55.68,H 3.40,N 23.39.
1.4 Synthesis of{[Fe(bpy)(CN)4]2[Ni(azp)]·4H2O}n(3)
One milliliter aqueous solution of Ni(ClO4)2·6H2O(0.005 mmol)was placed at the bottom of a test tube.A mixture of methanol and water(1∶2,V/V,2 mL)was gently layered on the top of the solution,and then 1.0 mL methanol solution of Li[Fe(bpy)(CN)4](0.01 mmol)and 1,2-di(pyridin-4-yl)diazene(0.01 mmol)was carefully added as the third layer.After few weeks,red crystals of 3 were obtained and collected after washed with water and air dried.Yield:54%based on Ni(ClO4)2·6H2O.Anal.Calcd.for C38H32Fe2N16NiO4(%):C 48.14,H 3.38,N 23.65;Found(%):C 48.20,H 3.32,N 23.58.
1.5 X-ray crystallography
The data were collected on a Bruker Smart APEXⅡX-diffractometer equipped with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)using the SMART and SAINT[27]programs at 298 K for compounds 1~3.Final unit cell parameters were based on all observed reflections from integration of all frame data.The structures were solved in the space group by direct method and refined by the full-matrix least-squares using SHELXTL-97 fitting on F2[28].For compounds 1~3,all non-hydrogen atoms were refined anisotropically.The hydrogen atoms of organic ligands were located geometrically and fixed isotropic thermal parameters.The crystal data and details of the structure refinement of compounds 1~3 are summarized in Table 1.Selected bond distances and angles of compounds 1~3 are listed in Table 2.
CCDC:1904149,1;1904150,2;1904151,3.

Table 1 Crystal data and structure refinements for compounds 1~3
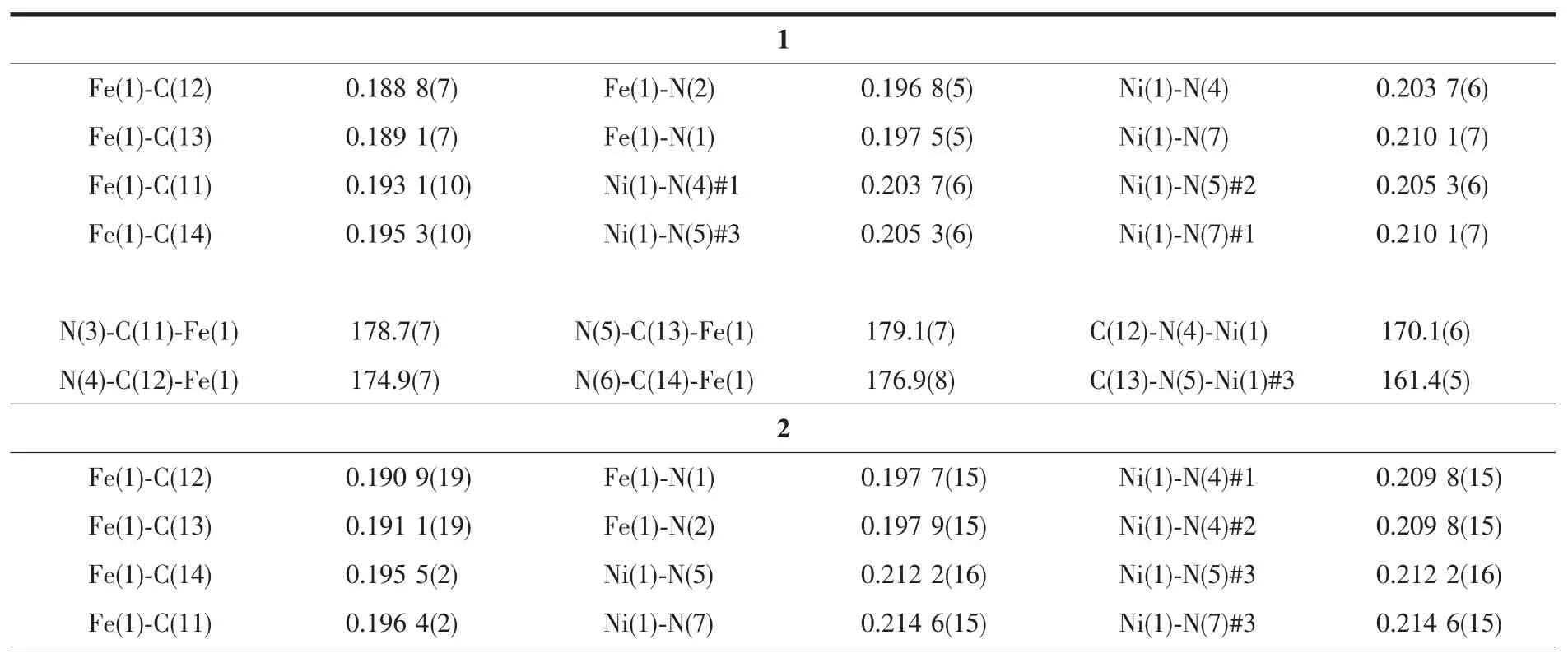
Table 2 Selected bond lengths(nm)and angles(°)for compounds 1~3
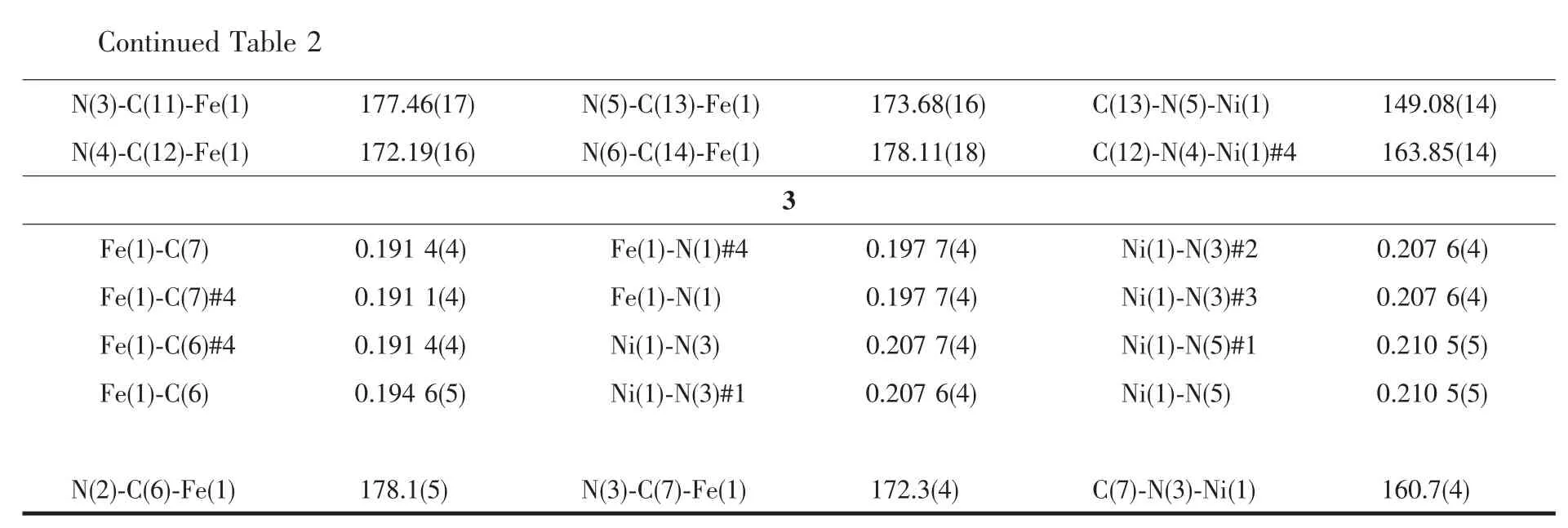
Symmetry transformations used to generate equivalent atoms:#1:-x+2,-y+1,-z+2;#2:x,-y+1,z+1/2;#3:x+2,y,-z+3/2 for 1;#1:-x+1,-y+1,-z+1;#2:x-1,y,z;#3:-x,-y+1,-z+1;#4:x+1,y,z for 2;#1:-x,-y,-z;#2:x,-y,z;#3:-x,y,-z;#4:-x+1,y,-z for 3.
2 Results and discussion
2.1 Structure characterization
Single-crystal X-ray diffraction analysis revealed that 1 crystallizes in the monoclinic space group C2/c,2 in the triclinic space group P1,and 3 in the monoclinic space group I2/m,respectively(Table 1).All of them show a similar skeleton,constructed by the cyano-bridged FeⅢ2NiⅡdouble-zigzag chains.Uncoordinated water molecules are located between the chains.Each repeating unit comprises of neutral[Fe(bpy)(CN)4]2Ni(L)2,in which each nickel ion is coordinated with two ligands L along the apical direction.Different from compounds 1 and 2,the neutral layer of compound 3 is further linked by the bidentate ligands along the apical direction of the Ni(Ⅱ)centers.Within the repeating unit,the nickel ion is coordinated by two nitrogen atoms from the ligands L and four cyanide nitrogen atoms from two contiguous[Fe(bpy)(CN)4]-portions.Each iron ion is located in an octahedral environment,comprising four carbon atoms from the cyanide groups and two nitrogen atoms from the bidentate ligand bpy.
For compound 1,the Fe-N and Fe-C bond lengths are 0.197(5)~0.198(5)nm and 0.189(7)~0.195(10)nm,respectively,which are characteristic of the LS Fe(Ⅲ)ions.The Ni-N bond distances are in a range of 0.204(6)~0.210(7)nm,which are in good agreement with those of high-spin Ni(Ⅱ)compounds.The Fe-C≡N angles are almost linear with bond angles of 174.9(7)°~179.1(7)°.The values of the C≡N-Ni angles are 161.4(5)°~170.1(6)°.Meanwhile,the π…π (0.376 1 nm)stacking interactions exist between the pyridine rings of adjacent bpy ligands and aromatic rings of the adjacent 4-phenylpyridine ligands. The nearest distance between the adjacent pyridine rings is 0.376 1(2)nm,and the nearest distance of aromatic rings of the 4-phenylpyridine ligands is 0.384 8(2)nm.The shortest intrachain Fe…Fe,Fe…Ni and Ni…Ni distances are 0.707 8(3),0.502 9(2)and 0.675 9(3)nm,respectively.The nearest interchain Ni…Ni distance is 1.454 8(6)nm,which indicates that interchain magnetic interactions should be weak.
For compound 2,the Fe-N and Fe-C bond lengths are in a range of 0.197 7(15)~0.197 9(15)nm and 0.190 9(19)~0.196 4(2)nm,respectively.The Ni-N distances range from 0.209 8(15)to 0.214 6(15)nm.The Fe-C≡N linkages are close to linearity with bond angles of 172.2(16)°~178.1(18)°.The bond lengths and bond angles of compound 2 confirm that the Fe(Ⅲ)is in the low-spin state.In comparison with compound 1,the C≡N-Ni angles fall in a range of 149.08(14)°~163.85(14)°,which depart significantly from linearity.The shortest intrachain Fe…Fe,Fe…Ni and Ni…Ni distances are 0.646 1(20),0.507 0(34)and 0.646 1(47)nm,respectively.The nearest interchain Ni…Ni distance is 1.403 2(41)nm.
For compound 3,the Fe-N(0.197 7(4)nm)and Fe-C(0.191 1(4)~0.194 6(5)nm)bond lengths are in good agreement with the reported LS Fe(Ⅲ)compounds.The Ni-N bond distances are 0.207 6(4)~0.210 5(5)nm.The Fe-C≡N angles deviate slightly from linearity,which are in a range of 172.3(4)°~178.1(5)°.The values of the C≡N-Ni angles are 160.7(4)°.The shortest intrachain Fe…Fe,Fe…Ni and Ni…Ni distances are 0.662 2(5),0.500 9(7)and 0.662 2(5)nm,respectively.The nearest interchain Ni…Ni distance is 1.322 9(8)nm.Compared with compounds 2 and 3,compound 1 exhibits longer interchain distances,which may diminish the interchain magnetic interactions and benefit for slow magnetic relaxation of SCMs.To further elucidate the differences of them,we applied geometry analysis to see how the ancillary ligands influence the coordination environment of Ni(Ⅱ)ion(Table 3).One can note that coordination environments of Ni(Ⅱ) in compounds 1~3 are all octahedron type and the CShM value of Ni for compound 1 is the highest.This indicates that the Ni(Ⅱ)ion in 1 locates in a more distorted octahedron environment.

Table 3 SHAPE analysis of Ni six-coordinated geometry in compound 1~3
2.2 Magnetic characterizations

Fig.3 Temperature-dependent magnetic susceptibilities of 1(a),2(b)and 3(c)in a temperature range of 2~300 K under an applied field of 1 000 Oe and field-dependent magnetizations of 1(d),2(e)and 3(f)
Temperature-dependent magnetic susceptibilities data of 1~3 were collected under a DC field of 1 000 Oe in a temperature range of 2~300 K(Fig.3).The χT values for 1,2 and 3 were 2.90,2.74 and 3.06 cm3·mol-1·K at 300 K,respectively,which are approximatively in a range of 2.48~2.80 cm3·mol-1·K expected for two LS Fe(Ⅲ) (S=1/2,g=2.6~2.8)and one HS Ni(Ⅱ)(S=1,g=2.2~2.3).When the temperature went down,χT values of 1~3 first increased smoothly and then increased rapidly at 80 ,60 and 50 K,respectively,reaching the maximum values of 52.16,35.68 and 14.59 cm3·mol-1·K at 4.2,3.9 and 2.4 K.The χT vs T plots indicate the typical ferromagnetic interaction between Fe(Ⅲ) and Ni(Ⅱ) ions within the chain.When the temperature decreased further to 2 K,the χT values of 1 and 2 decreased and reached the values of 33.1 and 25.50 cm3·mol-1·K,respectively.It is probably caused by the zero-field splitting of Ni(Ⅱ)ions and/or weak interchain antiferromagnetic interactions.In the temperature range of 30~300 K,the magnetic susceptibility data of 1~3 were fitted with the Curie-Weiss law,giving Curie constant C of 2.78,2.65 and 2.55 cm3·mol-1·K and Weiss constant θ of 11.27,9.29 and 3.05 K,respectively.The positive Weiss constants of 1~3 further confirm the ferromagnetic coupling interactions between Fe(Ⅲ) and Ni(Ⅱ) ions.Meanwhile,the field-dependent magnetization at 2 K also confirms the ferromagnetic behavior.The isothermal magnetization of 1~3 first increased linearly and then increased gradually,reaching a maximum value of 4.35Nβ,4.20Nβ and 4.52Nβ at 50 kOe,respectively.The values are close to the saturation value for two magnetically isolated low-spin Fe(Ⅲ)ions and one magnetically isolated high-spin Ni(Ⅱ)ion.

Fig.4 Frequency dependence of ac magnetic signals of compounds 2(a)and 3(b)at H ac=3.5 Oe and H dc=0 Oe;Frequency dependence of ac magnetic signals of compound 1(c,d)
To further probe the dynamics of the magnetization of the three compounds,the alternating current(ac)magnetic susceptibilities were studied.For compounds 2 and 3,no obvious frequency-dependent in-phase (χ′)and out-of-phase signals(χ″)were observed,indicating that compounds 2 and 3 are not single-chain magnets(Fig.4(a)and(b)).For compound 1,temperature-and frequency-dependent in-phase components(χ′)were observed below 2.8 K,as shown in Fig.4(c).Moreover,the plots of field-cooled magnetization(FC)and zero-field-cooled magnetization(ZFC)under a field of 100 Oe of 1 showed no bifurcation(Fig.5),excluding the spontaneous magnetization above 1.8 K.The generalized Debye model was used to extract the energy barrier based on the relationship of ln(χ′/χ″)=ln(ωτ0)+Ea/(kBT).The obtained energy barrier Ea/kBwas 10.9 K and the relaxation time τ0was 7.8×10-4s(Fig.6),which are in the typical range for SCMs.
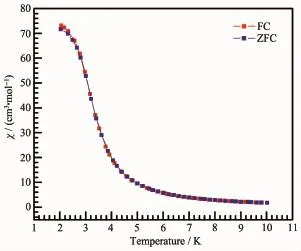
Fig.5 Field-cooled magnetization(FC)and zero fieldcooled magnetization(ZFC)curves of compound 1
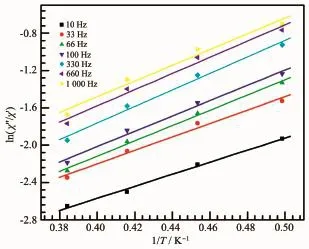
Fig.6 Plots of ln(χ″/χ′)vs 1/T for 1
Three cyano-bridged FeⅢ2NiⅡdouble-zigzag chains show different magnetic behaviors. The neutral ancillary ligands should be the main reason for the different magnetic behaviors of them.For compounds 1~3,ferromagnetic interactions exist between the Fe(Ⅲ)and Ni(Ⅱ) ions,which can be rationalized according to the orthogonality of the magnetic orbitals of the lowspin Fe(Ⅲ) and high-spin Ni(Ⅱ) ions[29-35].Compared to compounds 2 and 3,the Ni-N≡C bending angle of compound 1 is smaller,which presents a stronger ferromagnetic interaction.In addition,when the Ni-N≡C angles further decrease,an antiferromagnetic contribution will arise and the overall magnetic coupling would be weakened[35].Although there exist π…π stacking interactions between the interchain in compound 1,the nearest interchain Ni…Ni distance 1.454 8(6)nm is the largest among the three compounds.Such a large interchain distance will diminish the interchain magnetic interactions and benefit for the SCM behavior.It is worth noting that steric hindrance can enhance the bending of the C≡N-Ni angle and elongate the Ni-N bond lengths.The smaller bending angle of C≡N-Ni and shorter Ni-N bond length should be also responsible for the singlechain magnet behavior of compound 1.For the ligand shape,these results indicate that the introduction of long monodentate ligand plays an important role for obtaining SCMs.
3 Conclusions
In summary,three new cyano-bridged FeⅢ2NiⅡdouble-zigzag chains,{[Fe(bpy)(CN)4]2[Ni(bp)2]·2H2O}n(1),{[Fe(bpy)(CN)4]2[Ni(papy)2]·H2O}n(2)and{[Fe(bpy)(CN)4]2[Ni(azp)]·4H2O}n(3)were synthesized.The magnetic studies demonstrate the existence of ferromagnetic interactions between Fe(Ⅲ) and Ni(Ⅱ) ions and slow magnetic relaxation behavior of compound 1.Compounds 2 and 3 show ferromagnetic behavior but no single-chain magnets property.Our results demonstrate that ancillary ligands play an important role in influencing the intra-and interchain interactions as well as the local coordination environments.This work is useful for the design of new SCMs in the future.
