Parameter Optimization for Improvement in Biomachining Performance
2019-08-01HUANGHuiMAFei
HUANG Hui,MA Fei
Institute of Manufacturing Engineering,Huaqiao University,Xiamen 361021,P.R.China
Abstract: Biomachining utilizing chemolithotrophic microorganisms for material removal is one of the recently developed non-traditional machining methods offering innovative,low cost and environment-friendly benefits.However,its shortcomings are also obvious,low material removal rate(MRR),poor stability and low surface quality. The effects of temperature,shaking rate,pH value and bacteria concentration on the MRR,machining stability(linearity of material removal-time relationship)and surface roughness(Ra)are investigated using Taguchi methodology. The results indicate that the shaking rate is the most crucial parameter for the MRR and stability of MRR;while temperature is the most influential parameter for surface Ra. The optimum process parameters for overall machining property are temperature of 20 ℃,shaking rate of 160 r/min,pH of 1.8 and bacteria concentration of 3.32 × 107 cells/ml.
Key words: biomachining; acidithiobacillus ferrooxidans; material removal rate; surface roughness (Ra); Taguchi methodology
0 Introduction
Biomachining utilizes the biological energy of chemolithotrophic bacteria for metal removal[1-2],with a removal effect similar to chemical machining[3-4]. It is a kind of environmentally-friendly nontraditional machining method with no damage layer left on the machined surface. Bacteria,mainly acidithiobacillus ferrooxidans(A. ferrooxidans),act as a catalyst for continuously oxidizing Fe2+to Fe3+.Fe3+is constantly reduced to Fe2+due to oxidizing metals. Finally,the Fe2+reduced in metal removal reaction is transformed to Fe3+again by the bacteria. Thus,a circulatory system is formed and the bacteria obtain energy from the catalysed oxidation for growth and reproduction.
At the same time,biomachining has some shortcomings that need to be overcome. Because of using living microorganisms as tool,the biomachining properties are obviously influenced by environmental factors which has been investigated by many researchers. Jadhav et al.[5]investigated the effect of parameters such as shaking rate,temperature etc.on copper removal rate and surface roughness(Ra).Xenofontos et al.[3]studied the effect of shaking speed,temperature and inoculation strategy on metal removal amount and surface roughness. Muhammad et al.[1-6]observed the effect of temperature,shaking rate,pH and FeSO4concentration on metal removal rate and selected optimum process parameters for maximizing metal removal rate(MRR)and improving Ra. However,there are still exist limitations in previous studies. The bacteria concentration as an important process parameter has been rarely investigated. Besides,the optimizing targets are only limited to MRR and Ra.
The aim of this paper is to investigate the effects of temperature,shaking rate,pH and bacteria concentration on MRR,stability of instantaneous MRR and surface roughness using Taguchi methodology. At the same time,the optimum biomachining parameter is selected for increasing the comprehensive performance of MRR,stability and reducing surface roughness.
1 Materials and Methods
1.1 Microorganisms and growth procedure
In this study,the microorganisms used for biomachining were A. ferrooxidans,which were cultured in pure form. By using the medium called medium 9K,the pure culture was isolated from acidic pit water containing A. ferrooxidans which was originally withdrawn from an iron mine in China. A scanning electron microscopy(SEM)image of the bacteria is illustrated in Fig.1.

Fig.1 SEM image of A.ferrooxidans
The medium 9K,used in this study,was an aqueous solution of six kinds of inorganic salts,including ferrous sulphate as the energy source for bacteria.The composition of the medium is given in Table 1. The current pH value of the solution was measured using a portable pH meter(resolution 0.01)which was calibrated using standard solutions prior to use. By monitoring the current pH value,the pH of 9K medium was able to adjust to 1.8 by slowly adding 1∶1(volumetric ratio)sulfuric acid and distilled water.
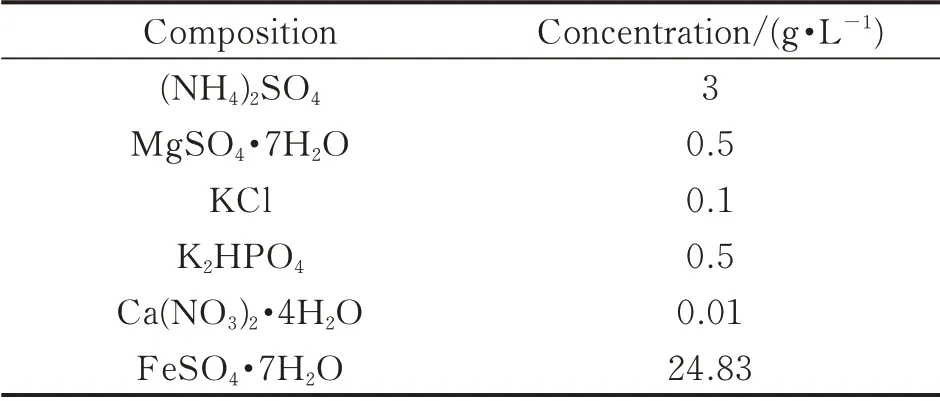
Table 1 9K medium compositions
A. ferrooxidans was cultivated in the liquid 9K medium mentioned above. The process of cultivation is as follows:
First,80 ml sterilized 9K medium in a 250 ml erlenmeyer flask was prepared.
Second,20 ml of the previous generation of A.ferrooxidans culture solution was transferred to the prepared medium.
Third,the flask with inoculated medium was incubated in an incubator shaker at 30 ℃and shaken at 160 r/min.
Finally,the culture solution was incubated in the sterilized 9K medium again as in the previous steps when the ratio of Fe3+concentration to total iron approached 95%. To control the ratio,the Fe3+concentration was measured each hour when the reddish solution appeared.
The A. ferrooxidans solution used as a tool for biomachining was continuously cultured for three periods before the experiments so that a satisfactory growth status was obtained. Thus,the initial concentration of A. ferrooxidans tended to be constant(1.36 × 107cells/ml)in the follow-up biomachining experiments.
1.2 Experimental design
The Taguchi methodology was used for designing of experiment because of high efficiency[7]. And the orthogonal design of four factors and three levels(as shown in Table 2)was taken to optimize the MRR,stability of instantaneous MRR and Ra of machined surface. Through a series of preliminary experiments,we found that there was no obvious interactions among the three factors of temperature,shaking rate and pH value.In addition,the three factors have been investigated to optimize the parameters for improving MRR and Ra by Muhammad etal.[1-6]without reporting interactions as well. The optimization objective of instantaneous MRR shared the same data source with MRR,so the interactions among the three factors were avoided. However,the other factor of cell concentration was obviously affected by the temperature,shaking rate and pH value(environmental condition). Simultaneously,considering the relatively short experiment time(8 h)and limited growth rate(under a good culture state,the cell concentration of 1.36 × 107cells/ml increased to 3.32 × 107cells/ml),the interaction on the cell concentration could be neglected given the concentration difference is 1 or 2 magnitudes of order for different cell concentration levels used in the experiment. Therefore,a L9(34)orthogonal array including nine experiments without concerning of interactions was designed in this study.

Table 2 Levels for orthogonal array
1.3 Experimental procedure
The pure copper was selected as the workpiece and the metal sheets(15 mm × 15 mm × 1 mm)used in this experiment were inlaid in polystyrene resin blocks(ø25 mm × 7 mm),which ensured a constant machining surface area. The surface dimensions of metal sheet exposed to be machined are illustrated in Fig.2(a). In this work,the surfaces were polished to a surface roughness of 30—60 nm with successively decreasing grit(final grit of 1 μm). The specimens were successively rinsed with distilled water and ethanol(analytical reagent)in ultrasonic cleaner and then dried in the oven at 60 ℃prior to biomachining.
Once the workpiece was ready to machine,the next step was to prepare the A. ferrooxidans culture solution(tool for biomachining). The solution was incubated for 12 h in the same conditions as described above. After the incubation,the Fe3+concentration increased to approximately 3 g/L and the cell concentration increased to approximately 3.32 ×107cells/ml. The solution colour would be as the one presented in Fig.2(b). For biomachining tests,the specimens were independently placed on the bottom of the 250 ml flasks with 100 ml A. ferrooxidans culture solution,and the surfaces to be machined faced up(Fig.2(c))during the processing.
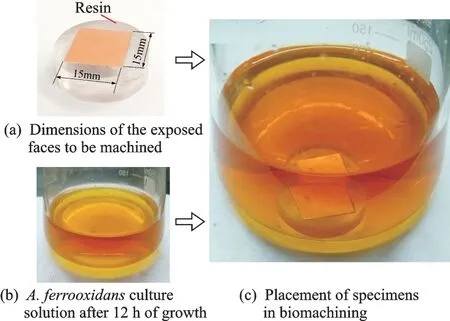
Fig.2 Experimental details
According to the optimization plan,the specimen of pure copper was machined under the specified four factors of three levels. For the pH of three levels of culture solution,they were adjusted by slowly adding 1∶1(volumetric ratio)sulfuric acid and distilled water for obtaining lower pH and 1∶1(volumetric ratio)ammonia water and distilled water for obtaining higher pH. The temperature and shaking rate could be adjusted by a thermostatic shaker. It must be noted that the cell concentration of the highest level of 3.32 × 107cells/ml was obtained by continuously culturing for 12 hours from the initial solution with cell concentration of 1.36 ×107cells/ml. In addition,the cell concentration of the other two levels can be realized by removing all the bacteria of specific volume of solution using 0.22 μm filter.
For periodical measurement,the workpieces were removed from the solution and then rinsed and dried as described above after each hour. The weights of each workpiece were measured using a precise electronic balance machine (resolution 0.000 1 g)and then immersed in the solution again.This process repeated each hour for 8 times,and each measuring process took approximately 25 min.The polystyrene resin is corrosion resistant and is not bibulous,so the weight loss of the specimen comes solely from the material removal of metal sheet. Therefore,the weight loss per square centimeter with time could be obtained. Finally,the surface roughness(Ra)of machined surfaces was examined using a white-light interferometer after 8 h of machining. Each experiment was performed in triplicate and the results were averaged from three values.
1.4 Characterization methods
The Fe3+concentration was determined by ethylene diamine tetraacetic acid (EDTA) titration.The titration procedure of Fe3+was as follows:A 1 ml sample was diluted to 20 ml in flask keeping the pH between 1.5 and 2.5. Then,a 1 ml indicator(sulfosalicylic acid aqueous solution,10% weight percent)was added to form a purplish-red complex.Finally,the Fe3+concentration of the sample was titrated with EDTA standard solution until the purplish-red complex changed to yellow. The Fe3+concentration value can be calculated with a titration formula.
In order to measure the total iron concentration,all the Fe2+was oxidized to Fe3+in a short time by saturated ammonium persulfate. Then the total iron concentration,in state of(+3),was determined by the same procedure as described above.The Fe2+concentration was calculated as the difference between the concentration of the total iron(Fe2++Fe3+)and that of Fe3+.
Living A. ferrooxidans moving in the solution was observed as moving spot directly under an optical microscope and the magnified view was synchronously transferred to a computer screen using a charge coupled device(CCD)camera. The number of living A. ferrooxidans in certain volume of solution could be counted by a blood cell counter. The living A. ferrooxidans density was obtained satistically by measuring large number of random sample statistic.
According to Refs.[8-9],the amount of material removed presented a linear behavior over the test time. Hence,a linear relationship could be fitted from the scattered points by the least square method. The slope of the fitted line is the weight loss of the samples per square centimeter per hour,which is a commonly used term defined as the MRR calculated from the equation below

The MRR comes from the slop of fitted line is the mean value in whole machining process. However,the instantaneous value,theoretically,is always changing in a certain range which is affected by many factors. Stability of instantaneous MRR,defined as linearity(R2)in this study,was used for evaluating the fluctuation quantity of instantaneous MRR. High R2value means the smaller fluctuation quantity of the instantaneous MRR. The Ra values of each specimen were averaged from nine measurements on fixed positioning with the determination scope of 0.70 mm × 0.53 mm.
The information of the main instruments used is listed in Table 3.

Table 3 Main instruments
2 Results
2.1 Effect of the four factors for different biomachining properties
Nine experiments designed by L9(34)orthogonal array for this study are listed in Table 4. The values of MRR,R2and Ra were measured and calculated from the mean of three replicates results of the nine experiments conducted based on L9(34)orthogonal array. The corresponding measured responses are shown in columns. According to the Taguchi methodology,the measured responses were converted into signal-to-noise(S/N)ratio,which measured the impact of noise factors on performance. Depending on the desired characteristics,generally,there are two types of calculation methods:the larger the better and the smaller the better,as follows:
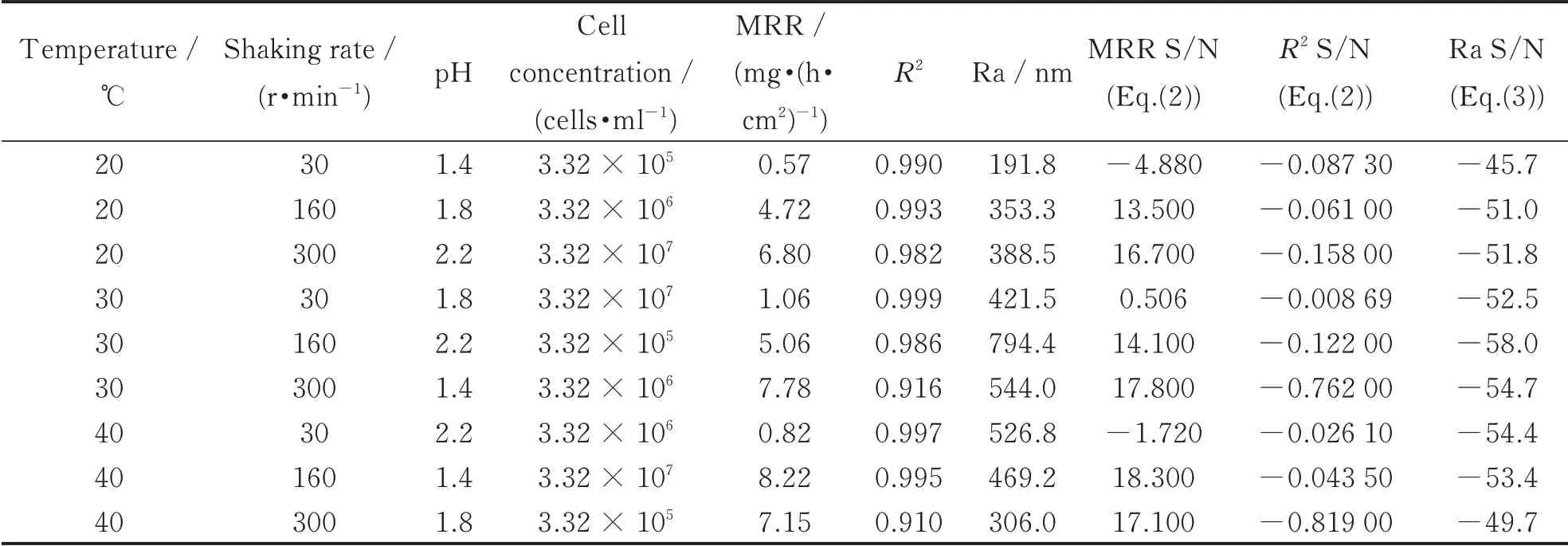
Table 4 Responses corresponding to a L9 (34)orthogonal array
The larger the better
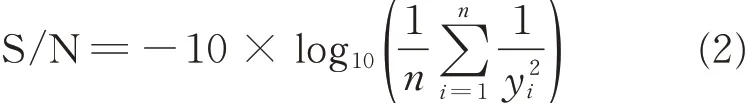
The smaller the better

where n is the total test number and y is the experiment results[10]. In this study,n is equal to one and the y values are responses of the MRR,R2and Ra.The corresponding S/N ratios are also listed in Table 4.
To obtain the impact relationships between the input process parameters and machining performances,the results were evaluated individually for each response characteristics. The influence of the input four variables on each machining performance was ranked individually using the corresponding S/N ratios based on Eqs.(2)and(3). The results are shown in Table 5 and the plots of S/N ratio vs MRR,R2and Ra are shown in Figs.3—5,respectively. With the figures,effects of all the four factors can be evaluated in visualization.
The S/N ratio for MRR is calculated using Eq.(2)(criterion of the larger the better),which is plotted in graph as shown in Fig.3. The graph evidently shows that the shaking rate is the most influential parameter for MRR and the influence is far more significant than the other three factors. It can be observed that the increase of shaking rate results in a significantly increasing of MRR. A similar result for the influence of shaking rate on MRR wasreported[6]. However,the increased magnitude of MRR,presenting obvious unsymmetrical variation,is much higher in the range of 30 r/min to 160 r/min than that in range of 160 r/min to 300 r/min. The result is consistent with that in Ref.[11]that indicated the MRR reached a constant value at an increasing shaking rate until 160 r/min.The other three factors,compared with shaking rate,show relatively smaller effects. MRR increases with the increasing of shaking rate and cell concentration increasing,while decreases with the increasing of pH value.

Table 5 The mean S/N responses for all parameters analyzed individually
By using Eq.(2)as well,Fig.4 indicates that all the four factors have important effects on R2.

Fig.3 S/N ratio (the larger the better)for MRR

Fig.4 S/N ratio (the larger the better)for R2

Fig.5 S/N ratio (the smaller the better)for Ra
The shaking rate is the extremely influential parameter for R2as well as MRR. It is noted that the influence of cell concentration presents markedly only after shaking rate. On the whole,R2decreases as the increase of temperature and shaking rate but conversely increases with pH increasing of value and cell concentration.
It can be seen in Fig.5 that the difference of effects of all four factors on Ra is not significant. Temperature and pH value affect Ra with ranks 1 and 2.According to the figure,the S/N ratio for Ra calculated using Eq.(3)(the smaller the better),the middle levels of temperature,shaking rate and cell concentration lead to the highest Ra. Besides,pH value shows inverse trend that the middle level of pH value obtain the smallest Ra.
2.2 Selection of optimum process parameters
The contributions of four factors to the three biomachining characteristic targets have already been analyzed separately in the last section. However,the three machining performances were influenced by four process parameters simultaneously.The ultimate aim of this study is to obtain the optimal process parameter combination for the best machining performance of higher MRR and stability of instantaneous MRR and lower Ra. Therefore,the overall machining property of the three machining performances responses to orthogonal array need to be evaluated synthetically. In this study,the response magnitudes of the three biomachining characteristic differed greatly due to different unit. According to Table 5,the total delta values of S/N ratios for MRR,R2and Ra were 25.8,1.2 and 14.7,respectively. In order to unify the response magnitudes of each machining performance,the synthetic evaluation weight was given 4.1%∶88.6%∶7.3%applied to MRR,R2and Ra. The corresponding S/N ratios for overall performance were calculated,as listed in Table 6.

Table 6 S/N ratio for overall score
The S/N ratio of each factor for overall score is plotted in Fig.6. According to the selection principle that the biggest is the best,the pursuit of machining performance is the biggest S/N ratio for MRR,R2and Ra. As is indicated in the figure,the optimal parameter combination for overall machining performance is as follows:Machining temperature is 20 ℃,shaking rate is 160 r/min,pH is 1.8 and cell concentration is 3.32 × 107cells/ml.

Fig.6 S/N ratio (the larger the better)for overall score
The selected parameters were applied to an experiment for validating the optimization results. The weight loss of copper per square centimeter with time was obtained,as shown in Fig.7. According to the slope of the linear curve fitted from the scattered points,the MRR of copper under the selected parametric combination was 7.69 mg/(h·cm2)and the corresponding R2was 0.998. From the view of R2,an extraordinary linearity was realized,which indicated that the fluctuation of instantaneous MRR was very small. The Ra examined after 8 h of machining was 314.8 nm and the topography of machined surface was also observed,as shown in Fig.8. The experimentally obtained S/N ratio was -2.940,as shown in Table 7,which is higher than those of groups in orthogonal array. From the results in confirmation experiment,it is clear that the overall performance is improved by using the Taguchi methodology.

Fig.7 Mass loss of specimen per square centimeter with time

Fig.8 Topography of copper surface after 8 h of machining

Table 7 Experimental validation
3 Discussion
3.1 Biomachining mechanisms
It is a general consensus that biomachining includes two main processes[2-12]:The conversion of Fe2+to Fe3+by bacteria and the removal of metal by Fe3+,in which the Fe3+is simultaneously reduced to Fe2+by the metal. Therefore,the two processes can form a circulatory system and can repeat endlessly.
The detailed process of converting Fe2+to Fe3+by bacteria is described as follows. First,A.ferrooxidans traps Fe2+ion that exists in the culture medium,and then Fe2+ion causes iron lose one electron through an internal catalytic process,thus becomes Fe3+according to Ref.[9]. During this process,the bacteria can obtain energy from the catalytic oxidation reaction for metabolic activity and reproduction.
The detailed process of removing metals by Fe3+is discussed below. Fe3+has already been widely used as an etchant in chemical machining due to its capability of removing kinds of metals. When these metals are immersed in a solution with Fe3+,they are first oxidized by Fe3+to a high valence,and then dissolved into the solution. At the same time,the Fe3+will be reduced to Fe2+by metal material.
It can be noted that Fe3+,as a product of the reaction initated by bacteria,is the executor for material removal and the Fe2+,is the energy source of living bacteria. Therefore,a circulatory system exists in biomachining. As the redox mediator in biomachining,the Fe2+and Fe3+are always converted to each other in the whole process.
The biomachining mechanism for pure copper has already been reported by many scholars[9-14],which can be easily illustrated in Fig.9.

Fig.9 The biomachining mechanism for pure copper
3.2 Analysis of four factors for biomachining performances
As discussed above,biomachining is based on two parts of chemical reactions:Fe3+reacted with metal and Fe2+reacted with bacteria through numerous biochemical reactions inside the bacteria cells.
The machining temperature,in simple terms,directly affects the two parts of reaction intensities:Inside and outside of cells. Increasing temperature surely accelerates the reactions outside of cells(Fe3+with metals). However,biochemical reactions are catalyzed by a series of enzymatic processes in bacteria cells,which actually involve different proteins with a definite optimum temperature(around 30 ℃)for reactions[15]. Increasing temperature under the optimum value enhances the reactions both inside and outside of cells. However,increasing temperature over the optimum value leads to a suppression of the reactions inside of the cells,resulting in an intensive consumption of Fe3+in the solution and rapid decline of Fe3+concentration.
The above analysis demonstrates that the S/N ratio for MRR increases sharply with the temperature increasing from 20 ℃to 30 ℃,while increases slowly with the temperature increasing from 30 ℃to 40 ℃(Fig.3). In this study,the consumption of Fe3+is faster than the regeneration of Fe3+by bacteria. Therefore,the instantaneous MRR continuously declines with time due to the decline of Fe3+concentration and the R2ultimately obtains a smaller value at a relatively higher temperature. It is the reason that S/N ratio for R2decreases with the temperature increasing from 20 ℃to 40 ℃(Fig.4). Fig.5 shows that the temperature is the most influential parameter for surface Ra,which probably attributes to exopolysaccharides formation as discussed in Ref.[3].
According to Ref.[5],the increase of shaking rate promotes the mass transfer and mixing of ionic products in the solution. Based on the theory,increasing shaking rate enhances the speed of material transition,realizing increase the reactions outside cells. In the similar way,the quick consumption of Fe3+at the early stage of machining without a timely replenishment results in a restriction of the instantaneous MRR at an increasing shaking rate until 160 r/min. Therefore,similar to the trend of increasing temperature,the instantaneous MRR continuously declines and the R2obtains a smaller value with the increase of shaking rate. It is why the changing trends of MRR and R2are opposite to the increase of shaking rate(Figs.3,4). Meanwhile,it is noted that the shaking rate is the most influential parameter for both MRR and R2. Therefore,it can be concluded that the mass transition of ionic products is the key for reaction during biomachining.
The pH value is an important parameter affecting the bacteria metabolism[12]. The root is that metabolism based on many enzymatic reactions inside of cells,which is also affected by pH value[16].Nonetheless,the effect of pH is not remarkable and mainly on surface Ra.
The cell concentration implies that the higher concentration,the higher speed of the Fe2+to Fe3+converting. Figs.3,4 show that increasing cell concentration contributes to improving both MRR and R2,especially R2. Therefore,the cell concentration is a crucial parameter for maintaining Fe3+concentration,thus the stability of Fe3+concentration is the key for improving R2(stability of instantaneous MRR).
In order to reduce experiment error,the weight loss tests were performed in triplicate and the results were averaged from three values. And the Ra values were averaged from three specimens on fixed positioning. However,the errors were inevitable,which were mainly related to the processing form itself,using metabolic activity of bacteria. Among them,the bacteria concentration was the main source of errors due to artificial statistic,which was compensated by means of large sample characteristics. Besides,the results were also affected by the problem of precision controlling of the growing state of bacteria.
After the experiment under the selected optimal parameters combination,a relatively better overall result(MRR of 7.69 mg/(h·cm2),R2of 0.998 and Ra of 314.8 nm)was obtained. The S/N ratio for overall machining property is higher than those of groups in the orthogonal array. Therefore,the optimal parameters combination for overall machining property was validated to meet expectation.
4 Conclusions
Taguchi methodology was used to investigate the effects of temperature,shaking rate,pH and bacteria concentration on MRR,R2and Ra when machining pure Cu. Shaking rate contribution of the mass transition of ionic products in solution was the most influential parameter for both MRR and R2,while temperature was the most influential parameter for Ra. The optimal combination of process parameters for overall machining property (higher MRR and R2but lower Ra)were temperature of 20 ℃,shaking rate of 160 r/min,pH of 1.8 and bacteria concentration of 3.32 × 107cells/ml. The experiment conducted under the condition of selected optimum parameter combination obtained a higher overall machining performance than other groups.
AcknowledgementsThe work was supported by the National Natural Science Foundation of China (No.51375179),the Science and Technology Projects of Fujian Province (No.2017H6014), and the Changjiang Scholars and Innovative Research Team in University (No.IRT_17R41).
The authors would like to acknowledge the East China University of Technology for supplying the culture of A. ferrooxidans. The authors are especially grateful for the suggestion from Prof. Dekui Mu at Institute of Manufacturing Engineering, Huaqiao University in China.
AuthorsProf. HUANG Hui received his Ph.D. degree at Nanjing University of Aeronautics and Astronautics,Nanjing, China, in 2002. From 2014, he has been Vice-Dean of the Institute of Manufacturing Engineering, and Vice-Dean of the College of Mechatronics and Automation in Huaqiao University. His research interests include grinding, wire sawing, machine tools and non-traditional manufactu-ring.
Mr. MA Fei is a Ph.D. candidate in the Institute of Manufacturing Engineering in Huaqiao University.
Author contributionsMr. MA Fei contributed to implementation of the experiments, analysis of the data and preparing the manuscript. Prof. HUANG Hui guided the experiments and discussed the study as well as modified the drafts.
Competing interestsThe authors declare no competing interests.
杂志排行
Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- Alterations of Cerebral Functional Connectivity in Patients with Frontal Lobe Epilepsy:A Graph Theory Study
- Thermodynamic Modeling and Simulation of Air System Control Device
- Reflected Wavefront Modulation with Phase Array by Using Acoustic Metasurface
- Multi-factor Effects on Layout of Solar Collector
- Damage Initiation and Propagation in Composites Subjected to Low-Velocity Impact:Experimental Results,3D Dynamic Damage Model,and FEM Simulations
- Dynamics Analysis of Carrier-Based Aircraft with Off-Center Catapult Launch
