Pd1-M (M=Al,Cu)Single-atom Catalysts for CO Oxidative Coupling to DMO:a DFT Study*
2019-07-31LINHaoHANBingyingLINGLixiaLIUPingZHANGRiguangWANGBaojun
LIN Hao HAN Bingying LING Lixia, LIU Ping ZHANG Riguang WANG Baojun
ABSTRACT In order to reduce the amount of noble Pd catalyst in CO oxidation coupling to dimethyl oxalate(DMO).Two kinds of single-atom catalysts(SACs)were designed by density functional theory(DFT).The influence of Pd1-M (M=Al,Cu)single-atom catalysts on the reaction were studied in detail.Firstly,it is found that Pd1-Al SAC has a great surface deformation,which cannot exist stably,therefore it is not suitable for this reaction as a catalyst.Secondly,Pd1-Cu SAC has shown an outstanding activity for the synthesis of DMO,and it achieves the goal of reducing Pd consumption at the same time.But the selectivity of Pd1-Cu SAC to DMO is unsatisfactory and needs to be further improved in the future studies.
KEYWORDS CO oxidative coupling to DMO,single-atom catalysts,Pd catalyst,density functional theory
0 Introduction
Single-atom catalysts (SACs)are new catalysts,which can minimize the size of the metal clusters and evenly distribute the active centers to the limit[1-2].Past studies have shown that SACs have different properties and characteristics compared to traditional catalysts,such as significant size effect[3]and the low coordination environment of active metal center[4].With the continuous development of catalyst preparation technology,SACs have been successfully prepared in experiment[5].Furthermore,SACs also exhibit excellent catalytic performance in a large number of chemical reactions[6-13].Because of the unique properties of SACs,studying the catalytic mechanism of SACs has a great significance for understanding the nature of catalytic reactions.
CO oxidative coupling to dimethyl oxalate(DMO)is an important part of C1chemistry[14-15].In addition,the reaction is also the key technology to relieve the problem of the energy crisis in our country[16-17].The core catalyst for this reaction has been proved to be the palladium-based catalyst[18-19].But the price of Pd is so expensive that the development of this reaction in industry has been greatly restricted[20]; and reducing the amount of Pd in this reaction has become a hot topic in academia[21-22].This problem can be well solved by making metal Pd into single-atom catalysts.Besides,Pd SACs also show excellent performance in a large number of catalytic reac-tions[23-24].In the study of PEI et al[25],Pd SACs were applied in selective hydrogenation of acetylene,the results show that Pd SACs have an excellent activity and selectivity for the reaction,and the utilization ratio of precious metal Pd has been greatly improved by Pd SACs.
The interactions between two kinds of metals make bimetallic catalysts show a unique electronic structure and chemical properties[26-27].The key step in the preparation of alloy catalysts is the selection of second metal.In our past research studies,Pd-Al and Pd-Cu bimetallic catalysts had been widely used in a large number of chemical reactions due to their excellent properties[28].For example,Pd-Al bimetallic catalysts show a good catalytic performance for the generation of DMO in our previous work[29-30].And PARK et al[31]synthesized CeO2-supported Pd-Cu alloy catalyst with one-pot method for the oxidation of formic acid,the experimental results show that the catalytic performance of this catalyst is much better than those of other catalysts.
As mentioned above,Pd-Al and Pd-Cu bimetallic catalysts all have played important roles in the field of catalytic,however,the effect of Pd1-M(M=Al,Cu)single-atom catalysts on the reaction mechanism of CO oxidative coupling to DMO is not clear.In our previous studies,we have found that the surface structure of Pd(100)had a high activity in CO oxidative coupling to DMO,but the selectivity of Pd(100)surface to generate DMO needed to be improved.So in this work,the models of Pd1-M (M=Al,Cu)bimetallic single-atom catalysts with(100)surface properties have been constructed.And based on theoretical calculation,the reaction process of CO oxidative coupling to DMO on these catalysts has been clarified.It is expected that this work can provide a new idea for preparing catalysts for synthesis of DMO with superior performance and low Pd consumption in industry.
1 Method and models
1.1 Computational methods and parameters
All data in this work were calculated by using Vienna Ab-initio Simulation Package(VASP)software[32-34].The core-valence interaction was described by the projector-augmented wave (PAW)method.And the generalized gradient approximation (GGA)with the Perdew-Burke-Ernzerhof(PBE)[35]exchange-correlation functional was used in this work.The plane wave basis set with cutoff energy of 400eV was used.The k-point was selected as 3×2×1for Pd1-Al and Pd1-Cu SACs.The atomic structures were relaxed until the electronic energy was less than 1×10-5eV and force was less than 0.3eV/nm for unconstrained atoms.The Climbing-image Nudged Elastic Band method(CI-NEB)[36-37]combining with the dimer method[38-39]was used for locating transition state(TS)for every elementary step,in which the force was converged to 0.5eV/nm.Vibrational frequency calculation was done and only one virtual frequency was obtained to confirm the TS structure.
The activation energy(Ea)can be obtained by the following Equation:
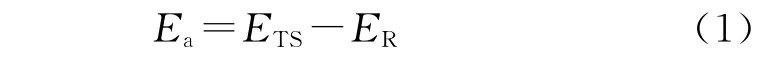
And the adsorption energy(Eads)is defined as the following formula:
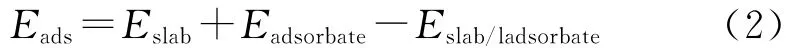
Where Eslaband Eadsorbaterepresent the energies of different surfaces and the isolated adsorbate in the gas phase,respectively.Eslab/adsorbateis the total energy of an adsorption system.
1.2 Construction of models
First of all,the pure Al(100)and Cu(100)surfaces are modeled using ap(3×4)unit cell with three layers.The optimized lattice parameters of Al and Cu are 0.399 2nm and 0.362 3nm,respectively.Which is in line with the experimental dates(Al,0.404 9nm;Cu,0.361 5nm)[40].Next,an atom in the top layer of pure Al(100)and Cu(100)surfaces will be replaced by a Pd atom.Finally,through structural optimization we will get Pd1-Al and Pd1-Cu SACs.
For Pd1-Al and Pd1-Cu SACs,the third layer was frozen in the bulk position,other layers and adsorbed species were allowed to relax.All species on these surfaces were allowed to relax.The structures and adsorption sites on Pd1-Al and Pd1-Cu SACs are shown in Fig.1.Considering the location of the active center,there are only three possible active sites on Pd1-Al and Pd1-Cu SACs(bridge,top-Pd and hollow)being studied for DMO formation.
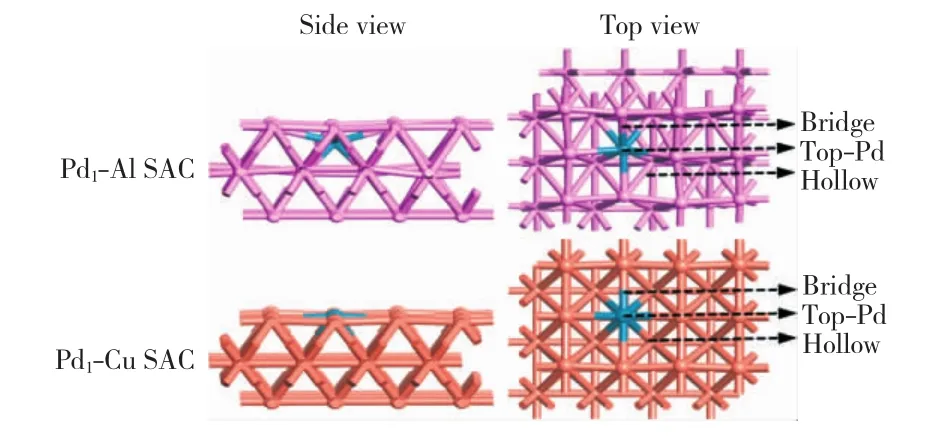
Fig.1 Structures of Pd1-Al and Pd1-Cu SACs and corresponding adsorption sites
2 Results and discussion
2.1 Pathway to generate DMO
For the reaction process of CO oxidative coupling to DMO,some conclusions can be obtained by referring to our early work and previous studies:the dissociation of CH3ONO is favorable[41-42],and the product OCH3will directly initiate the oxidative coupling reaction;there are mainly two ways to generate DMO[43],as shown in Fig.2,including COOCH3coupling with COOCH3(Path 1)and COOCH3coupling with CO (Path 2).The byproduct dimethyl-carbonate (DMC)comes from Path 1.Through studying the formation of DMC,the selectivity of catalysts to the target product will be clarified.
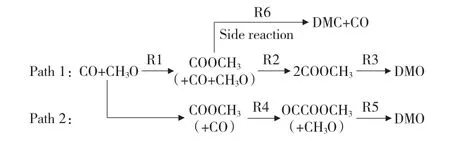
Fig.2 Pathways of CO oxidative coupling to DMO
2.2 Adsorption properties of reactants,possible intermediates and products
First of all,the optimized structure of Pd1-Al SAC is very unstable as shown in Fig.1.This is mainly due to the relatively softer physical structural property of Al.We have further studied the adsorbability of Pd1-Al SAC to reactive species,the results show that after adsorbing reaction species the surface structure of Pd1-Al SAC is greatly deformed,and cannot continue to participate in the reaction.So we can conclude that Al is not a suitable metal to act as the substrate of a single-Pd-atom catalyst.In this study,a disadvantage of SACs is that the structural deformation during the reaction process is obviously comparable with that of other types of bimetallic catalysts[29-30].Thus it can be seen that there are still some challenges in the comprehensive application of SACs in the field of catalysis.
The adsorption configurations and corresponding adsorption energies of related species on Pd1-Cu SAC are shown in Fig.3.
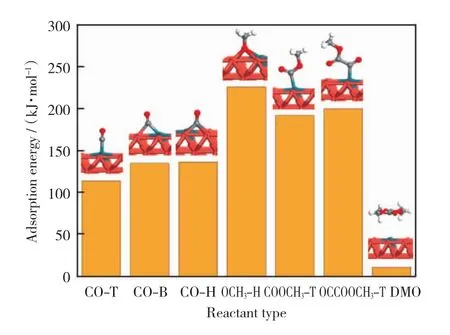
Fig.3 Adsorption configurations of reactive species on Pd1-Cu SAC and corresponding adsorption energies
CO has three adsorption modes on Pd1-Cu SAC,they are adsorbed at the top,bridge and hollow sites of the catalyst surface by C atom.And the corresponding adsorption energies of CO on Pd1-Cu SAC are 113.9kJ/mol,134.9kJ/mol,and 136.4kJ/mol,respectively.Unlike CO,OCH3can only be adsorbed stably on the hollow site with the adsorption energy of 226.1kJ/mol.The adsorption mode of COOCH3on Pd1-Cu SAC is the top adsorption on the Pd atom.The adsorption energy of COOCH3Pd1-Cu SAC is 192.1kJ/mol.And the adsorption mode of OCCOOCH3on Pd1-Cu SAC binds with Pd atom on surface through C atom with the adsorption energy of 200.1kJ/mol.
DMO exists on Pd1-Cu SAC in the form of physical adsorption which is in good agreement with our previous studies[15,29-30].And the adsorption energy of DMO on Pd1-Cu SAC is only 10.6 kJ/mol.It is worth mentioning that the OCCO which acts as an important intermediate on pure Pd(111)surface[29]cannot exist stably on Pd1-Cu SAC.
2.3 CO oxidative coupling to DMO on Pd1-Cu SAC
The generation of DMO on Pd1-Cu SAC begins with the formation of co-adsorption configuration of CO and OCH3.At this time,CO and OCH3are adsorbed at the bridge site formed by Pd and Cu atoms.Then CO reacts with OCH3to form COOCH3.This process requires a transition state(TS1)and overcomes an energy barrier of 55.3 kJ/mol with an endotherm of 20.1kJ/mol.In this step,the distance between C of CO and O of OCH3has been shortened from 0.365 2nm to 0.133 7nm in COOCH3.After the generation of COOCH3,the next reaction will be divided into two paths down(Path 1and Path 2).
In Path 1,the COOCH3will be formed via a transition state(TS2),as shown in Fig.4.Activation energy of 25.6kJ/mol is needed to complete this process with an exothermic heat of 27.8kJ/mol.The distance between C of CO and O of OCH3has been shortened from 0.340 1nm to 0.135 1nm.The generation of DMO is accomplished by the coupling of two COOCH3.This process has to overcome an energy barrier of 97.9kJ/mol and releases exothermic heat of 12.7kJ/mol,meanwhile the distance between two C atoms changes from 0.296 3nm to 0.198 1nm in TS3and eventually becomes 0.154 7nm in DMO.
In Path 2,the formed COOCH3will be coupled with a CO to produce OCCOOCH3at the beginning.This process needs to go through a transition state(TS4)by overcoming an energy barrier of 152.0kJ/mol and endothermic heat of 43.0 kJ/mol.The distance between those two carbon atoms is reduced from 0.307 6nm to 0.155 6nm.In the final phase of Path 2,the OCCOOCH3reacts with another OCH3to form DMO.The energy barrier of this elementary reaction is 90.6kJ/mol and exothermic heat is 25.7kJ/mol.
It is not difficult to find that Path 1is the favorable route for the formation of DMO on Pd1-Cu SAC.The rate-determining step of each path is the coupling reaction of two C atoms in both paths of generating DMO.That is,the coupling reaction of two COOCH3and Path 1and in Path 2is the coupling reaction of COOCH3and CO.This is in good agreement with our previous research[29].
When COOCH3,CO and OCH3are adsorbed on the Pd1-Cu SAC at the same time,OCH3does not react with CO to form a second COOCH3,but react with the formed COOCH3to produce the DMC,as shown in Fig.4.Similarly,this process also needs to undergo a transition state (TS6).The energy barrier of this reaction is 82.8kJ/mol,simultaneous exothermic heat is 115.3kJ/mol.The distance between O of OCH3and C of COOCH3is reduced from 0.289 9nm to 0.134 3nm.
2.4 Micro-kinetic modeling
The micro-kinetic modeling is an effective way to analyze the performance of catalysts.Its reliability has been confirmed by numerous studies in the past[44-48].In our previous work,the formation rate of DMO on Pd(111),Pd-Cu(111)and Pd-Al(111)surfaces has been well investigated by microkinetic modeling.In order to further understand the effects of Pd1-Cu SAC toward CO oxidative coupling to DMO,we have also carried out the calculation of corresponding kinetic and Turnover Frequency(TOF).At the same time,the selectivity of catalyst to DMO will be further clarified by kinetic model.First of all,the elementary reactions involved in the whole reaction process are listed in Table 1.
The rate constants of all elementary reactions as shown in Table 1are calculated by the harmonic transition state theory (TST),and the formula(3)for calculation is as follows[49-50]:
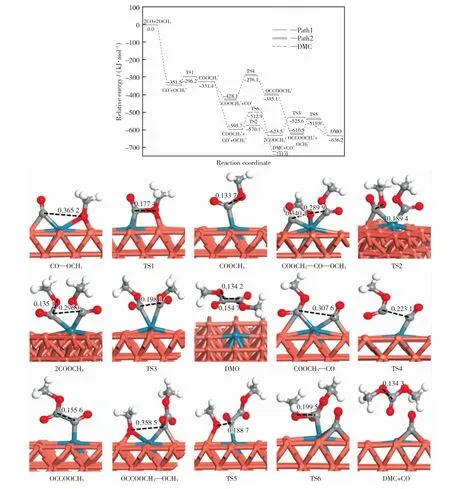
Fig.4 Relative energy diagram of DMO formation and the configuration of the species involved in the reaction on the Pd1-CuSAC(energy unit:nm)

Table 1 Related reactions and the corresponding rate constants

And the surface coverage of CO,OCH3and intermediates(θCO,θOCH3,θCOOCH3,andθOCCOOCH3)can be obtained from the steady-state approximation theory[51].At the same time,the equilibrium relationship between reactive species and free site(θ*)can also be obtained.The formulas used in the calculation are as follows(4)-(8):
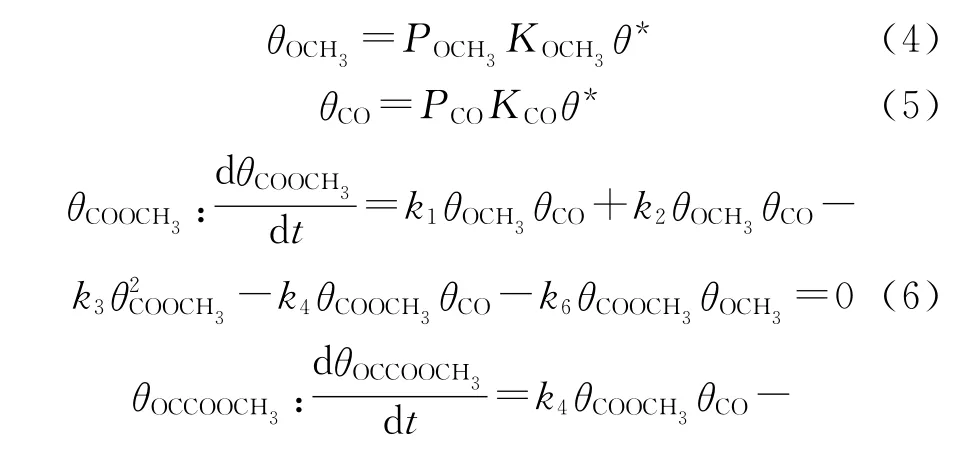

Previous experimental studies have shown that the suitable temperature range for the reaction is 375K-415K,and the partial pressures of CO and OCH3are 280kPa and 200kPa[52],respectively.The relevant computational data are listed in Table 2.
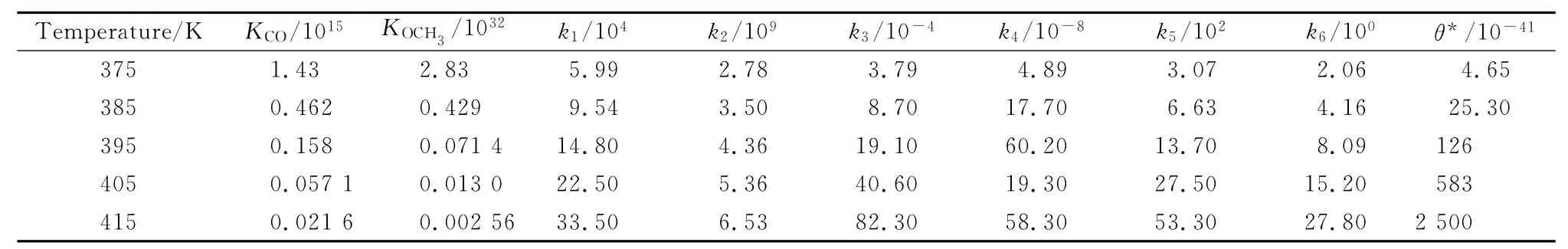
Table 2 Reaction equilibrium constants,rate constants and the free site(θ* )in the reaction on the Pd1-Cu SAC (375K≤T≤415K)
The meaning of turnover frequency(TOF)is a conversion amount of products at a single active site in unit time[53-55].And this concept focuses on the description of the active sites of catalysts.Because of the special structure of SACs,the formation rate of products is equal to the TOF value of products.It is generally known that the formation rate of DMO and DMC is directly proportional to the concentration of reactants and the rate constants[56].And the formation rate of DMO and DMC can be obtained via the following formula(9)-(10):
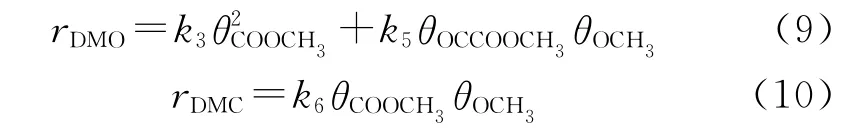
The TOF values of DMO and DMC on Pd1-Cu SACs are listed in Table 3.Comparing this study with our previous work,it can be found that the formation rate of DMO on Pd1-Cu SAC is much higher than that on pure Pd surface[29].And the formation rate of DMO increases with the increase of temperature,so it can be concluded that increasing temperature is beneficial to the formation of DMO.
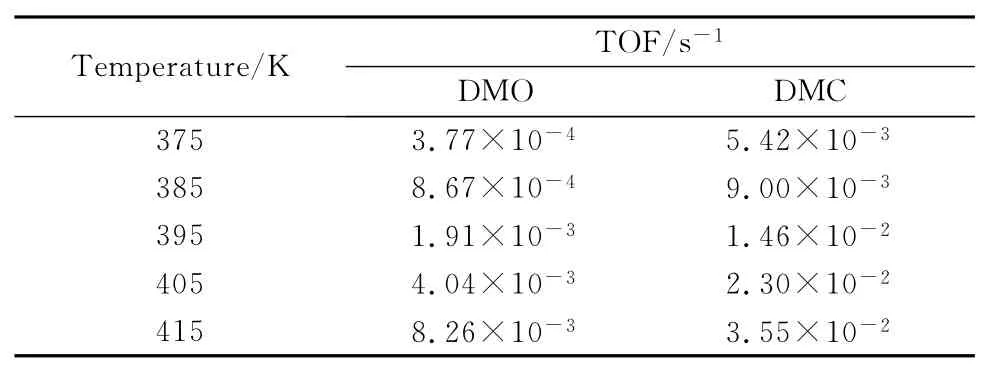
Table 3 TOF(s-1)of DMO and DMC on the Pd1-Cu SAC(375K≤T≤415K)
In order to further describe the catalytic performance of catalyst to CO oxidative coupling to DMO,we plot a temperature-dependent curve of the selectivity of catalyst to DMO.As shown in Fig.5,it is not difficult to find that the selectivity of Pd1-Cu SAC to DMO formation is not ideal.At 375K,the selectivity of Pd1-Cu SAC to DMO formation is only 6.5%.Even when the temperature rises to 415K,the selectivity is still relatively low(only 18.8%).And the selectivity of Pd1-Cu SAC to DMO also increases with the increase of temperature;therefore,we can promote the selectivity of catalyst to the formation of DMO by increasing temperature.
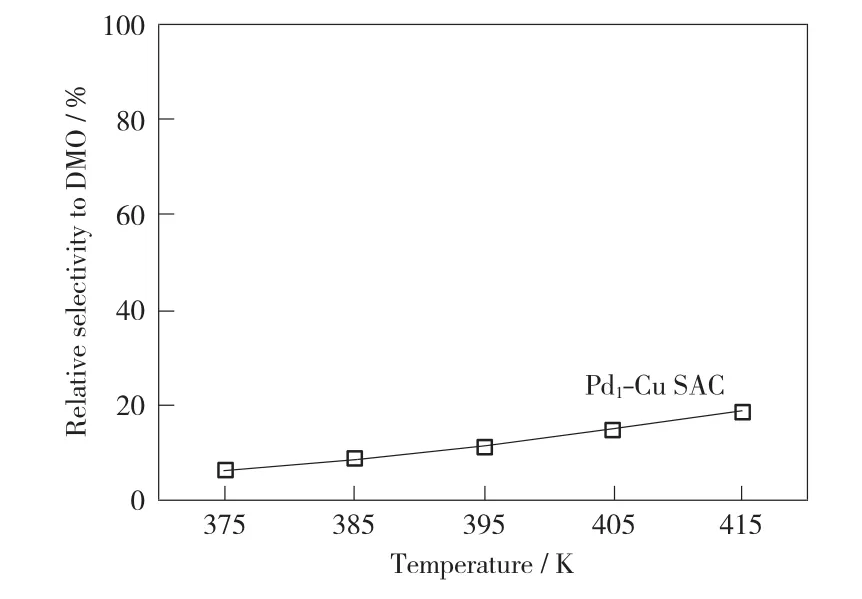
Fig.5 Relative selectivity of DMO formation on the Pd1-Cu SAC
3 Conclusions
In this study,we have constructed Pd1-M(M=Al,Cu)single-atom catalysts(SACs).And the reaction mechanism of CO oxidative coupling to DMO over these catalysts has been simulated by DFT calculation.Through the analysis of the calculation results,we can get the following conclusions.Firstly,the structural of Pd1-Al SAC is instable,so it cannot further catalyze the oxidative coupling reaction of CO to achieve the purpose of preparing DMO.Secondly,Pd1-Cu SAC has a high activity for the generation of DMO,and DMO mostly comes from the coupling of two COOCH3.Last but not least,the application of Pd1-Cu SAC has greatly reduced the amount of precious metal Pd.However,Pd1-Cu SAC is more conducive to the generation of DMC,and the selectivity of Pd1-Cu SAC to DMO is not ideal.In the future,we will conduct further in-depth studies on this issue in order to obtain better catalysts.
