Particle selectivity of filtering by C. elegans
2019-05-28YukiSuzukiKenjiKikuchiKeikoTsurutaNumayamaTakujiIshikawa
Yuki Suzuki*, Kenji Kikuchi, Keiko Tsuruta-Numayama, Takuji Ishikawa
Graduate School of Engineering, Tohoku University, Sendai, Japan
Keywords:Nematode C. elegans Pharynx Pumping Filtering Selectivity
A B S T R A C T A nematode Caenorhabditis elegans (C. elegans) is a filter feeder, which draws a suspension of bacteria and separates bacteria from the solvent by using pharyngeal pumping motions and specific mouth parts. This mechanism has not been fully understood. We investigated the mechanism of filtering of bacteria in the pharynx of C. elegans by visualization by fluorescent particles and dyed E. coli. We succeeded in quantifying the selectivity of bacteria-sized particles by C. elegans. The most accumulated particles were those of 0.5 μm in diameter. The quantity of accumulated particles of 0.2 μm or 1.0 μm in diameter was about one third of that of particles of 0.5 μm in diameter. The least accumulated particles were those of 0.05 μm in diameter. These results suggest that the pharyngeal structures of C. elegans would be suitable for eating bacteria because the size of bacteria ingested by C. elegans worms is about 0.5 μm in diameter. We also succeeded in visualizing pharyngeal structures and pumping motions and flow in the pharynx. We found that there were phase differences in the motions among procorpus, metacorpus and isthmus. This result suggests filtering would occur at the two tips of procorpus and isthmus by the phase differences. We found that bacteria-sized particles and bacteria were flowed and trapped in the channels, which existed along the central lumen from tip of procorpus to isthmus. From our results, we proposed the novel mechanism of filtering of bacteria through the channels for flowing and trapping. In future, this selective filtering mechanism of C. elegans would be applied to development of microfluidic filtration devices for medical and biological equipment.
Recently, many researches and engineers try to understand excellent structures and functions of organisms to use serviceable ideas from them and to apply them to engineering. This attempt is called biomimetics, and the examples of biomimetic studies include drag reduction swimsuits inspired by the structure of skin of sharks, shape of Shinkansen and airplanes developed from the look of birds, and painless needles imitated from the shape of mosquito needle [1]. Biomimetics has given us new technologies inspired by biological features of organisms to solve engineering problems.
Here, we picked out a food filtration function of a nematode Caenorhabditis elegans (C. elegans), which would be applied to development of microfluidic devises for medical and biological equipment. C. elegans is easy to handle and observe and the first multicellular organism to have its whole genome sequenced and widely used as a model organism for biological studies including embryology, genetics, physiology [2]. C. elegans is a filter feeder, which draws a suspension of bacteria and separates the bacteria from the liquid by using specific mouth parts [3]. C. elegans, however, has no obvious filter or bulb in the pharynx unlike many filter feeders like fishes and shellfishes [4]. The contractions and relaxations of muscular organ in the pharynx transports bacteria to the mouth and finally accumulated the bacteria in the intestine. The bacteria are filtered in the central lumen while the extra liquid is expelled from the tip of mouth simultaneously. The liquid seems to be expelled through the channels at the tips of procorpus and isthmus around the two concentration parts. Fang-Yen et al. [5] proposed the mechanism of filtering of bacteria associated with structures of the pharynx. The mechanism has not been fully understood because relationship between the structures and flow in the pharynx is not clear.
In this paper, we investigated the mechanism of filtration of bacteria in the pharynx of C. elegans by visualizing pharyngeal structures and flow in the pharynx. We found that bacteria-sized particles and bacteria were flowed and trapped in the channels,which existed from the tip of procorpus to isthmus through posterior procorpus and metacorpus. From our results, we propose the novel mechanism of filtering of bacteria. In future, this selective filtering mechanism of C. elegans would be applied to development of filtration microfluidic devices for medical and biological equipment.
C. elegans wild-type strain N2 worms (Fig. 1) were grown at 25 °C on nematode growth media (NGM) plates (0.3% sodium chloride, 1.7% agarose, 0.25% peptone, 1% cholesterol, 97.5%mili-Q water) containing E. coli strain OP50 by using standard methods [2]. We used adult worms within 2 days after they became adult.
For visualizing flow in the pharynx, we used fluorescent particles (diameter 0.5 μm, fluorescent wavelength 514 nm:Thermo Fisher Scientific Inc, Japan). For visualizing structure of the pharynx, we used Nile Red (fluorescent wavelength 637 nm:Wako Pure Chemical Industries, Japan) and OP50 painted by dyes (Alexa FlourTM488 NHS Ester, fluorescent wavelength 519 nm: Thermo Fisher Scientific Inc, Japan) [7]. For quantifying filtering of bacteria-sized particles, we used fluorescent particles of diameters 0.05, 0.2, 0.5, and 1.0 μm: Thermo Fisher Scientific Inc, Japan).
Several worms were fed on fluorescent particles with various sizes of diameters 0.05, 0.2, 0.5, and 1.0 μm on 0.13% volume of particles per plate and a small amount (5–10 μL) of OP50 suspension for 40 min at 25 °C. After feeding, the worms were soaked in 99% ethanol for several minutes and washed three times by M9 buffer (0.3% potassium dihydrogen phosphate, 0.6%dipotassium hydrogen phosphate, 0.1% magnesium sulfide (1 mol/L), 0.5% sodium chloride) [2]. The washed worms were transferred to a small amount (40–60 μL) of M9 buffer on a glass bottom dish. Fluorescence of the particles accumulated in the worms was observed with fluorescence microscope using 4x objective. The fluorescence was measured by using ImageJ image analysis software.
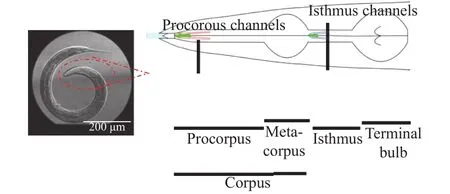
Fig. 1. Pharyngeal structures of C. elegans. The pharynx is a tubular organ divided into three parts: corpus, which is connected to the mouth and further divided into procorpus and metacorpus, isthmus,and terminal bulb, which is connected to the intestine. The muscles and luminal tissues can expand and contract. The pharynx makes pumping motions during eating bacteria [6]. Pumping is caused by expansions and contractions of corpus and anterior isthmus, so that the fed bacteria are transferred posteriorly to the intestine.
Several worms were fed on a small amount (10–20 μL) of OP50 suspension and diluted suspension of fluorescent particles of 0.5 μm in diameter or a small amount (5–10 μL) of suspension of dyed OP50 for 30 min. After feeding, the worms were dyed by Nile Red for 20 min. After dying, the worms were soaked in 99%ethanol for several minutes and washed by M9 buffer three times. The washed worms were transferred to a small amount(40–60 μL) of M9 buffer on a microscope glass and covered by a coverslip. Structures of the pharynx were observed with confocal laser scanning microscope using 60× water immersion objective. Distribution of the particles or dyed OP50 accumulated in the pharynx was investigated by the software.
Several worms, a small amount (5–20 μL) of OP50 suspension and diluted suspension of fluorescent particles of 0.5 μm in diameter were mounted on an agarose gel (M9 buffer, 2%agarose) on a microscope glass. Pharyngeal pumping motions and flow in the pharynx were observed with differential interference contrast (DIC) microscope using 60× or 100× objectives or confocal laser scanning microscope using 20× objective and recorded at from 250 to 1000 frames per second by using highspeed video camera. The time changes of inner diameter of the central parts of procorpus, metacorpus and isthmus were measured. The fluorescent particles flowing in the pharynx were traced.
We investigated how C. elegans worms eat food selectively by measuring the accumulation of fluorescent bacteria-sized particles (Fig. 2). Figure 3 shows that fluorescence of the particles with the various sizes accumulated in the worms. The highest values of fluorescence were the particles of 0.5 μm. The values of 0.2 μm and 1.0 μm were approximately one third of those of 0.5 μm. The lowest values were 0.05 μm. On the other hand, Kiyama et al. [8] reported that fluorescence of less than 0.5 μm were less than that of 0.5 μm and fluorescence of 1.0–2.0 μm was more than that of 0.5 μm when worms were fed on only fluorescent particles. The tendencies of selectivity for 0.05 μm and 0.2 μm were in good agreement with the results of study in Ref. [8]. However, the tendencies of selectivity for 0.5 μm and 1.0 μm were in conflict with the results by Kiyama et al. [8]. We found that the particles of 0.5 μm were accumulated the most in the particles with the various sizes when the particles were mixed with food E. coli. From our results, its mouth parts would be suitable for collecting the particles of 0.5 μm since the size of bacteria ingested by C. elegans worms is approximately 0.5 μm in diameter [3, 8]. Our results suggest that the pharyngeal structures of C. elegans would be suitable for eating bacteria.
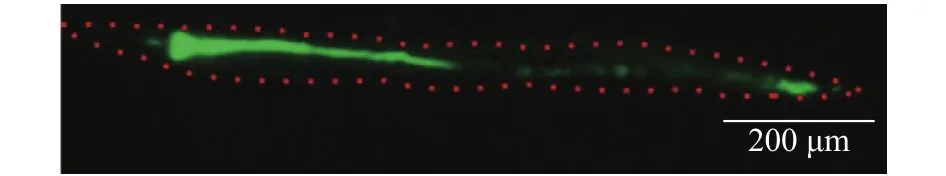
Fig. 2. An image of C. elegans worm that accumulated yellow-green particles of 0.5 μm in diameter in its body. Red dotted line shows the body of the worm. Fluorescence seen green were measured.
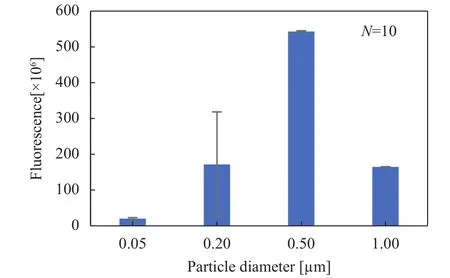
Fig. 3. Size-dependence of fluorescent particles accumulated in C.elegans worms. Data are mean ± standard deviation (SD) of 10 worms.
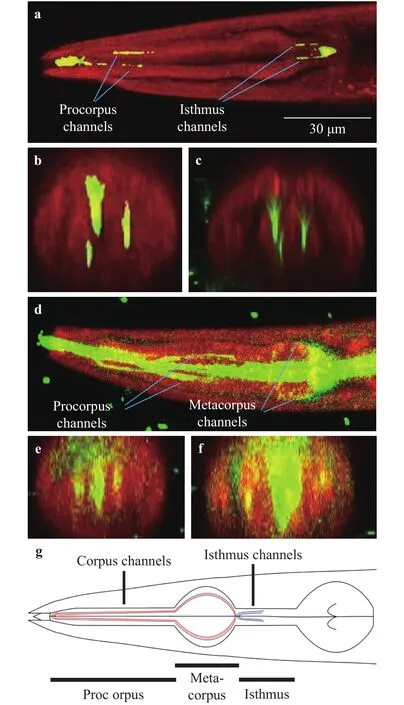
Fig. 4. Structures of the pharynx of C. elegans visualized with confocal microscopy. Red shows the pharynx of C. elegans. Yellow-green shows fluorescent particles of 0.5 μm or dyed E. coli. a The pharynx filled with the particles. The channels of anterior procorpus and isthmus are filled with the particles. b Cross-section of anterior procorpus shown in plot (a). c Cross-section of isthmus shown in plot(a). d The pharynx filled with dyed E. coli. The channels of corpus and isthmus are filled with dyed E. coli. e Cross-section of posterior procorpus shown in plot (d). f Cross-section of metacorpus shown in plot (d). g Pharyngeal structures based on our results.
We investigated pharyngeal structures, which associate with process of eating bacteria. Figure 4 shows the pharyngeal structures of C. elegans visualized by Nile Red which dyes selectively the endothelial cells with fluorescent particles of 0.5 μm (Fig.4(a)–(c)) or dyed E. coli (Fig. 4(d)–(f)). The channels of anterior procorpus and isthmus were filled with the particles, as shown in Fig. 4(a)–(c). Dyed E. coli stacked in the channels, which existed in not only anterior procorpus and isthmus, but also posterior procorpus and metacorpus, as shown in Fig. 4(d)-(f). On the other hand, Fang-Yen et al. [5] proposed that extra liquid would be expelled through the channels, which existed in the tips of anterior procorpus and isthmus based on the experiments using only fluorescent particles. We found that the channels were filled with the particles just a little when food E. coli existed, and that the channels were filled with the food far more when only the food existed. Our results suggest that characteristics of the channel vary depending on presence of food E. coli and the food would flow in the channels.
We investigated characteristics of pharyngeal pumping and how pumping would generate flow in the pharynx. Table 1 shows the maximum diameter, pumping period of procorpus,metacorpus and isthmus and dimensionless time of the peak of expansion. Figure 5 shows time change of stroke of the three parts. Procorpus and metacorpus started to expand at almost the same time and metacorpus started to contract prior to procorpus contracting. The delay of expansion between corpus and isthmus was 0.29. We observed that there were two directional flows of E. coli in the pharynx when metacorpus were contracting. One was flow from metacorpus to tip of procorpus. The other was flow from isthmus to the intestine. On the other hand,Fang-Yen et al. [5] reported that the delay between corpus and isthmus was 72.7 ± 36.4 (mean ± SD), the whole pumping periodis 305.1 ± 109.0 (mean ± SD) and the delay was 0.24. Our results of the delay between corpus and isthmus were in roughly agreement with the results of study of Fang-Yen et al. [5]. Moreover,we found that procorpus and metacorous expanded and contracted independently. In other words, there are phase differences among procorpus, metacorpus and isthmus. Our results suggest that the phase differences would generate two directional flows in the pharynx when metacorpus is contracting, so that the bacteria would be trapped at two tips of the pharynx.
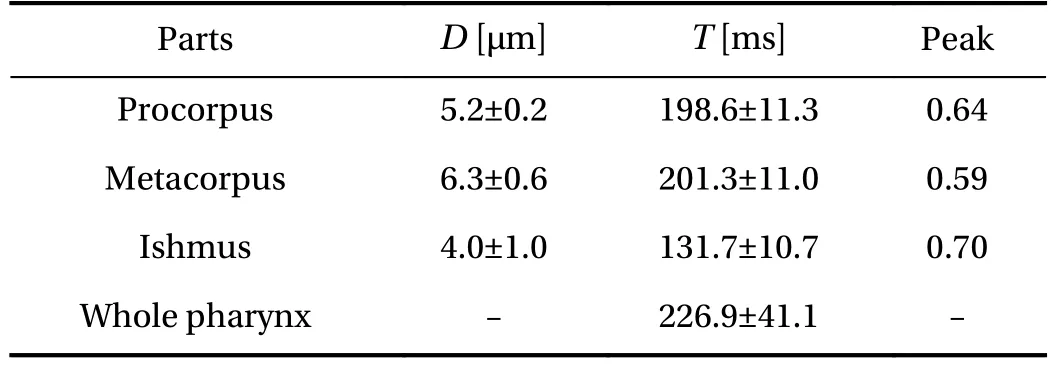
Table 1 Pharyngeal dimensions and time of one cycle. D shows the maximum diameter of procorpus, metacorpus and isthmus. T shows the pumping period. Peak shows time of the maximum diameter in pumping period. Data are means ±SD
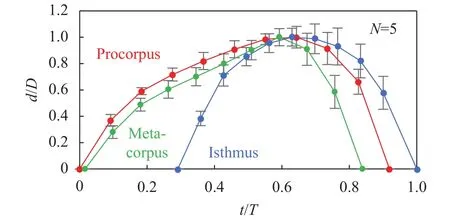
Fig. 5. One cycle of pharyngeal pumping motions. d/D means the stroke ratio divided by the max stroke. t/T means the time ratio divided by one cycle. Procorpus begins expanding at t/T = 0 and isthmus finishes contracting at t/T = 1. Error bars show SD of data of 5 worms.
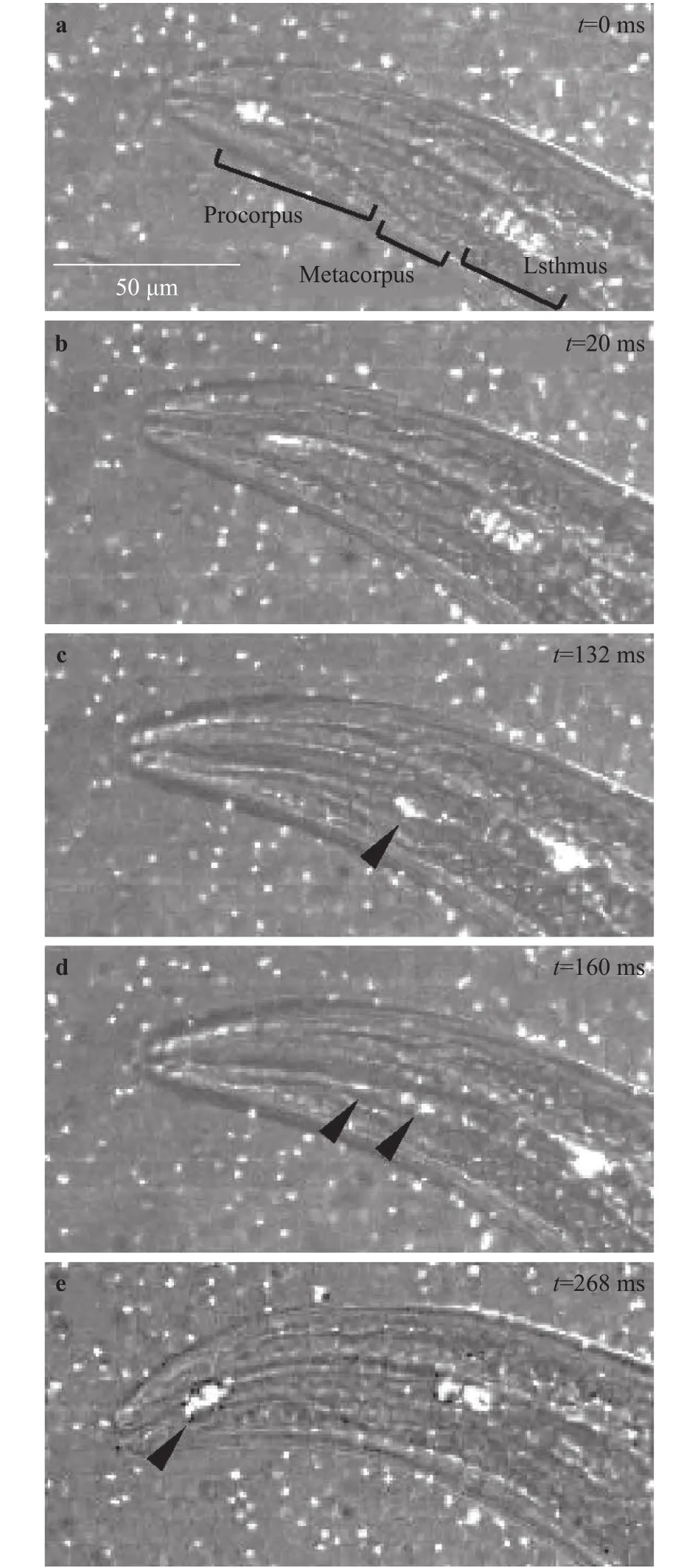
Fig. 6. Flow of bacteria-sized fluorescent particles in the pharynx visualized by a high-speed imaging. White dots show fluorescent particles. Black arrowheads show the locations of the particles observed in the sidewall of the central lumen. Time t is shown in the upper right corner of each image.
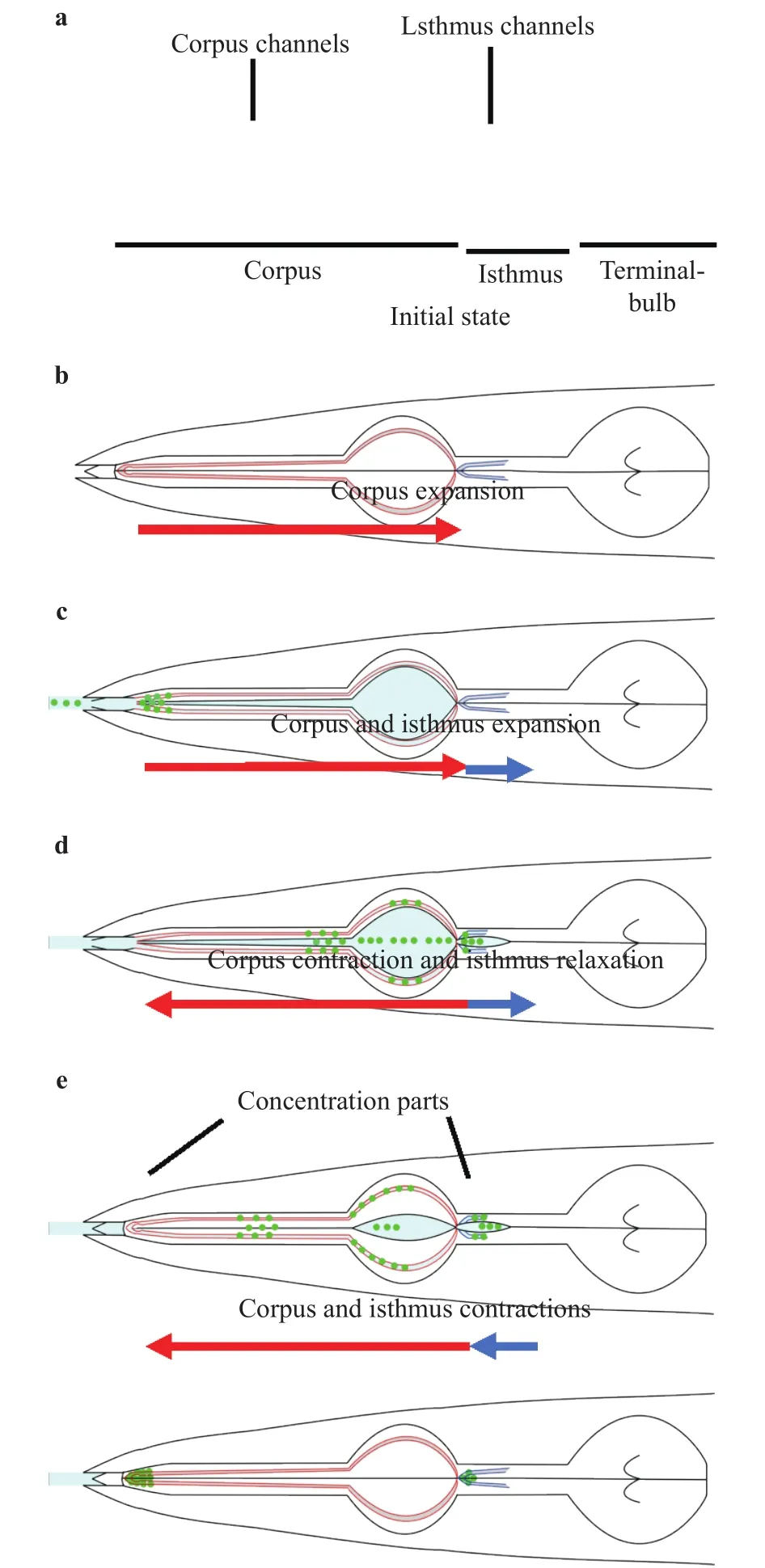
Fig. 7. Illustrations of particles flow in one pharyngeal pumping.Red and blue curves show the channels. Central black lines show the central lumen. Green dots show the particles. Red and blue arrows show the direction of flow in the pharynx.
We investigated where fluorescent particles would flow in the pharynx during pumping. Figure 6 shows movement of the particles flowing in the pharynx in case of mixing with food E.coli. At t/T = 0 (Fig. 5), the pharynx was closed and the particles were accumulated at tips of procorpus and isthmus (Fig. 6(a)).During t/T = 0–0.3, corpus expanded and the particles were flowed toward isthmus (Fig. 6(b)). During t/T = 0.3–0.6, corpus contracted and the particles in metacorpus were drifted to the sidewall of the central lumen while isthmus expanded and the particles in isthmus were flowed toward the intestine (Fig. 6(c)).During t/T = 0.6–0.7, the particles were flowed backward to the tip of procorpus along the sidewall (Fig. 6(d)). During t/T =0.7–1.0, the pharynx contracted and the particles were trapped at the tip of procorpus (Fig. 6(e)). On the other hand, Fang-Yen et al. [5] proposed that bacteria–sized particles were flowed in the central lumen and trapped at the two tips of procorpus and isthmus while the extra liquid would be expelled through the channels based on experiments using only the particles [5]. Our results are markedly different from the results of by Fang-Yen et al.[5] in terms of the particles were flowed in the channels during t/T = 0.3–0.9. We found that the particles were flowed and trapped at the channels when food E. coli existed. This result suggests that flow in the pharynx could be visualized by fluorescent particles when food exists because Fan-Yen et al. [5]demonstrated that bacteria–sized particles did not flow in the channels without bacteria.
From our results, we propose the novel mechanism of filtration of bacteria through the channels for flowing and trapping(Fig. 7). First, the pharynx is closed (Fig. 7(a)). Next, corpus expands and the particles are flowed to anterior isthmus (Fig.7(b)). Then, corpus and isthmus expand and the particles flowing in metacorpus are drifted to the sidewall of the central lumen or were flowed to posterior isthmus (Fig. 7(c)). Corpus contracts and the particle are flowed backward to the tip of procorpus through procorpus channels while isthmus expands and the particles are flowed to posterior isthmus (Fig. 7(d)). Finally, Corpus and isthmus contract and the particles are trapped at the two tips of procorpus and isthmus (Fig. 7(e)).
We succeeded in quantifying the selectivity of bacteria-sized particles by the filtration of C. elegans. The most accumulated particles were 0.5 μm in the novel mechanisms of filtration of bacteria through the channels for flowing and trapping. In future, we will apply this selective filtering mechanism of C. elegans to development of microfluidic filtration devices for medical and biological equipment.in the pharynx. We found that there were phase differences among procorpus, metacorpus and isthmus. There were two different directions of flows of bacteria in the pharynx when metacorpus was contracting. These results suggest that the phase differences would generate two directions of flows in the pharynx, so that bacteria are trapped at two tips of the pharynx. We found that bacteria-sized particles and bacteria were flowed and trapped in the channels, which existed along the central lumen from tip of procorpus to isthmus. From our results, we propose the novel mechanisms of filtration of bacteria through the channels for flowing and trapping. In future, we will apply this selective filtering mechanism of C. elegans to development of microfluidic filtration devices for medical and biological equipment.
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- The extractable hydrokinetic power from an oscillating membrane-based harvester
- Impact of spray droplets on momentum and heat transport in a turbulent marine atmospheric boundary layer
- A new numerical framework for large-eddy simulation of waves generated by objects piercing water surface
- Numerical solutions for point masses sliding over analytical surfaces: Part 1
- Numerical solutions for point masses sliding over analytical surfaces: Part 2
- Marangoni liquid film scattering over an extending cylinder
