Extract of Cycas revoluta Thunb. enhances the inhibitory effect of 5-f luorouracil on gastric cancer cells through the AKT-mTOR pathway
2019-05-08XingLiangCuiKeJiLiHaiXiaRenYongJianZhangXiaoDongLiuBaoGuoBuLeiWang
Xing-Liang Cui, Ke-Ji Li, Hai-Xia Ren, Yong-Jian Zhang, Xiao-Dong Liu, Bao-Guo Bu, Lei Wang
Abstrac t BACKGROUND Gastric cancer is one of the most common and deadly malignancies worldwide.Despite recent medical progress, the 5-year survival rate of gastric cancer is still unsatisfactory. 5-f luorouracil (5-Fu) is one of the first-line antineoplastic treatments for gastric cancer, as it can effectively induce cancer cell apoptosis.However, the effect of 5-Fu is limited due to drug resistance of the malignant tumor. Previous studies have reported that Sotetsuflavone from Cycas revoluta Thunb. can markedly suppress lung cancer cell proliferation by apoptosis,though its effect on gastric cancer remains unknown.AIM To investigate the inhibitory effect of Cycas revoluta Thunb. and to determine whether it can overcome gastric cancer cell drug resistance to 5-Fu.METHODS Cell viability was examined to determine whether the natural extract of Cycas revoluta Thunb. induced gastric cancer cell death. The half-maximal effective concentration and the half-maximal lethal concentration were calculatede.Wound-healing and transwell assays were performed to examine gastric cancer cell motility. Clonogenic assays were performed to investigate the synergistic effects of Cycas revoluta Thunb. with 5-Fu, and apoptotic bodies were detected by Hoechst staining. Western blotting was performed to examine the expression of related proteins and to investigate the molecular mechanism of Cycas revoluta Thunb.-induced cancer cell apoptosis. The expressions of proteins, including mammalian target of rapamycin (mTOR) and p-AKT, were detected in different combinations of treatments for 48 h, then analyzed by ECL detection.RESULTS Gastric cancer cells were more sensitive to the natural extract of Cycas revoluta Thunb. compared to normal gastric epithelial cells, and the extract effectively inhibited gastric cancer cell migration and invasion. The extract improved the anti-cancer effect of 5-Fu by enhancing the chemosensitization of gastric cancer cells. Extract plus 5-Fu further reduced the expression of the drug-resistancerelated proteins p-AKT and mTOR after 48 h compared to 5-Fu alone. Compared to 5-Fu treatment alone, mTOR and p-AKT expression was significantly reduced by about 50% and 75%, respectively. We also found that the natural extract of Cycas revoluta Thunb. further increased 5-Fu-induced gastric cancer cell apoptosis. Expression of apoptosis-related protein X-linked inhibitor of apoptosis protein and apoptosis inducing factor were significantly reduced and increased,respectively, in the 5-Fu-resistant gastric cancer line SGC-7901/R treated with extract plus 5-Fu, while the expression of survivin did not change.CONCLUSION The natural extract of Cycas revoluta Thunb. effectively inhibited gastric cancer cell growth and enhanced the anti-cancer effect of 5-Fu through the AKT-m TOR pathway.
Key words: Gastric cancer; 5-f luorouracil; Cycas revoluta Thunb.; Apoptosis
INTRODUCTION
Gastric cancer remains the fourth most common malignancy d iagnosed worldw ide,especially in Eastern Asia, Eastern Europe and Central and South America[1-3]. It also is the third main cause of d eath related to malignancy, just behind lung and liver cancer[4]. In 2012, there w ere about 951,600 new p atients d iagnosed w ith gastric cancer, and over 700,000 deaths related to gastric cancer have been recorded[5].
With a broad spectrum of activity against malignant cells, 5-f luorouracil (5-Fu) is commonly employed against gastric, liver and colorectal cancers[6-8]. As a prevalent chemotherapeutic d rug in clinical practice, 5-Fu can inhibit cancer cell proliferation and DNA rep lication, includ ing gastric, breast and colorectal cancer cells, by inhibiting thymidylate synthase from synthesizing thymine, which ultimately induces apoptosis[9-11].
Apoptosis is an important molecular process for stable and orderly human growth.It is strictly controlled and its dysregulation is linked to many d iseases, includ ing cancer[12,13]. This complex process is regulated by a series of key proteins, such as Xlinked inhibitor of apop tosis p rotein (XIAP), ap op tosis ind ucing factor (AIF) and survivin. XIAP is a strong apoptotic regulator[14-18]and inhibits caspase-3, -7, and -9,which are all part of the mammalian apoptotic signaling pathway. AIF is released and promotes apoptosis by intrinsic signaling cascades[19,20]w hen mitochondria respond to apoptotic stimuli, such as the translocation of BH3 interacting d omain death agonist(Bid)[21]. Survivin is a unique inhibitor of ap optosis (IAP), as it d oes not d irectly interact w ith caspases but with some adaptors or cofactors[22-26].
Although 5-Fu is w idely used as an anticancer drug, it has some serious problems,such as low effective response rate and severe side effects. One of the most critical concerns is the increasing cases of drug resistant malignant tumor. Many 5-Fu drugresistance-related proteins have been id entified. For example, P-glycoprotein (P-gp)functions as a molecular ‘pump' to expel chemotherapy drugs from the inside of the cell, and resistance to 5-Fu can be reversed w hen P-gp expression is reduced[27]. AKT is considered a key protein in the phosphiotidylinositol-3-kinase (PI3K)/Akt signaling pathw ay. It is activated at the plasma membrane by phosphorylation of Thr308 and Ser473 resid ues, and it can phosphorylate various downstream substrates related to d rug sensitivity[28]. Mammalian target of rapamycin (m TOR), a serine/threonine kinase, is a main downstream effector of the PI3K/AKT signaling pathw ay[29]. It has also been rep orted that 5-Fu d rug resistance may be mediated by the AKT-m TOR pathway[30,31].
Fortunately, d rug resistance can be reduced when used in combination with other compounds. Previous studies have reported that chemosensitization of cancer cells to 5-Fu can be achieved by using d ietary fats, particularly n-3 p olyunsaturated fatty acids (PUFAs), puerarin, iRGD, and troxerutin[32-35].
Some Chinese med icines, includ ing Cycas revoluta Thunb., have d emonstrated chemosensitization effects, w hich p rovid es some novel insights for anti-cancer treatments. According to ancient records, Cycas revoluta Thunb. is an evergreen palm w oody plant[36]w ith useful medicinal value, such as reducing fever and alleviating congestion. A component of the extract, Sotetsuflavone, was identified to have strong anti-tumor activity against lung cancer cells[37,38]. Because it can effectively induce lung cancer cell ap optosis, w e studied w hether it could inhibit grow th, migration and invasion of malignant gastric cancer cells. Furthermore, w e evaluated its potential for chemosensitization in combination with 5-Fu and investigated its potential molecular mechanism.
MATERIALS AND METHODS
Materials, reagents and antibodies
The leaf of Cycas revoluta Thunb. was acquired from AnGuo herbal medicine market in HeBei Province of China. DMEM (12800017) and tryp sin (25300054) w ere purchased from Life Technologies (Carlsbad, CA, United States). MTT (M 2128) and 5-Fu (F6627) w ere purchased from Sigma (Saint Louis, MO, United States). Antibod ies against p-gp, XIAP, p-Akt, AIF, mTOR, survivin, and GAPDH were purchased from Abcam (Shanghai, China).
Extraction of Cycas revoluta Thunb.
The powder of the Cycas revoluta Thunb. leaf was extracted by reflux extraction with 80% ethanol. The extracts were collected and concentrated under reduced pressure until there was no irritating odor. The product was dissolved in water and filtered.The filtrate was then extracted with dichloromethane, concentrated under reduced pressure and dried.
Cell culture
The MGC-803, SGC-790, and HGC-27 cell lines were obtained from ATCC (Manassas,VA, United States). SNU-5 cells w ere obtained from the Cell Resource Center of Shanghai Institute of Life Sciences, Chinese Academy of Sciences. GES-1 cells were obtained from the Genetics department of Beijing Cancer Research Institute. The SGC-7901/R line was obtained from Shanghai Institute of Medicine, Chinese Academy of Sciences. Cell lines were cultured at 5% CO2and 37˚C in DMEM medium containing fetal bovine serum (10%), penicillin (100 U/mL), and streptomycin (100 U/mL). Cells were used and analyzed at logarithmic growth phase.
Cell viability and clonogenic assay
Cells were grow n in 96-well plates for cell viability tests. Gastric cell lines were treated with the extraction of Cycas revoluta Thunb. or 5-Fu after 24 h. The viability rate was measured by ATPlite assay (Perkin Elmer, Waltham, MA, United States)[39].One thousand cells w ith d ifferent treatments were seeded into culture d ish in clonogenic assays. The number of colonies was measured after 9 d.
Wound-healing migration assay
MGC803 and HGC27 cells were cultured in six-well tissue culture plates and tested w hen the confluence reached 80%. Wounds were created by sterile pipette tips (10-μL), and loosely attached cells were w ashed out with phosphate-buffered saline. Light microscope was employed to photograph the progression of cell migration at different times, and the number of migrated cells was calculated in the scratched region.
Transwell invasion assay
Twenty-four-well Boyden chambers w ith 8-mm pore size filters (BD Falcon, Corning-Costar, New York, NY, United States) w ere used for this assay. Samp les w ere suspend ed and seed ed in the insert chamber w ith DMEM/F12 med ia and w ere incubated at 37˚C in 5% CO2for 24 h to allow cells to migrate into the bottom, w hich contained DMEM/F12 med ia and 10% FBS. The number of cells that migrated w as counted after staining with DAPI.
Western blotting analysis
Total protein w as extracted w ith NP40 lysis buffer. Sod ium dod ecyl sulfate p olyacrylamid e gel electrop horesis (commonly know n as SDS-PAGE) and polyvinylidene difluoride membrane w ere used for separating and transferring samples. Membranes were blocked in tris buffered saline tween-20 (TBST) solution containing 5% nonfat dry milk for 1 h. Primary antibodies were added overnight at 4˚C and then rinsed three times for 10 min in TBST. Membranes were incubated in secondary antibodies for 1.5 h before being washed. The results were analyzed by ECL detection system.
Cell apoptosis and Hoechst 33258 staining
After treatment with extract, samples w ere collected and w ashed with precooled phosphate-buffered saline (PBS). They were resuspended in 300 μL of binding buffer diluted in PBS. After incubation for 10 min, 5 μL Annexin V-FITC w as add ed,followed by 5 μL PI for 5 min. Samples were then rinsed three times with precooled PBS and fixed in 4% paraformaldehyde for 30 min. After washing with PBS for three times, Hoechst was added to the plate dropwise and incubated at room temperature for 15 min. The results w ere observed und er a fluorescence microscope and photographed after a final PBS wash.
Statistical analysis
The statistical methods used in this study were reviewed by Zhimin Shi from the College of Hebei University of Engineering. Experiments were repeated at least three times, and the data were processed by SPSS 20.0 statistical software. The standard deviation and Least Significant Difference were calculated by Student-Newman-Keuls test or Dunnett T3 test, in whichaP < 0.05 andbP < 0.01.
RESULTS
Gastric cancer cell growth was inhibited by Cycas revoluta Thunb. extract
To investigate tumor inhibition effects of d ifferent doses (0 μg/m L-350 μg/m L) of Cycas revoluta Thunb. extract, w e performed cell viability assays. The results show ed that Cycas revoluta Thunb. extract significantly inhibited gastric cancer cell viability after 24 h, especially at the low and medium doses (0 μg/m L-250 μg/mL) (Figure 1A).For treatments under 250 μg/m L extract, gastric cancer cell viability (MGC-803, SGC-790, HGC-27 and SUN-5) d ramatically decreased w ith increasing concentrations of extract, w hile that of normal human gastric epithelial cells (GES-1a0 remained stable,which suggested that gastric cancer cells were more sensitive to Cycas revoluta Thunb.natural extract than normal gastric cells. We then analyzed the half-maximal effective concentration (EC50) and the half-maximal lethal concentration (LC50) of all cell lines(Figure 1B). The EC50 values of gastric cancer cells ranged from 176.44 μg/m L to 194.88 μg/m L and the LC50 values ranged from 135.23 μg/m L to 152.20 μg/m L.Compared to the EC50 (291.32 μg/m L) and LC50 (280.27 μg/m L) values for GES-1 cells, the Cycas revoluta Thunb. Extract w as obviously more effective against gastric cancer cells. Additionally, high concentrations of extract (250 μg/mL-350 μg/mL), the viability rate of gastric cancer cells increased, which may be due to a screening effect for resistant cells or other adaptive mechanism.
Cycas revoluta Thunb. extract reduced gastric cancer cell migration and invasion
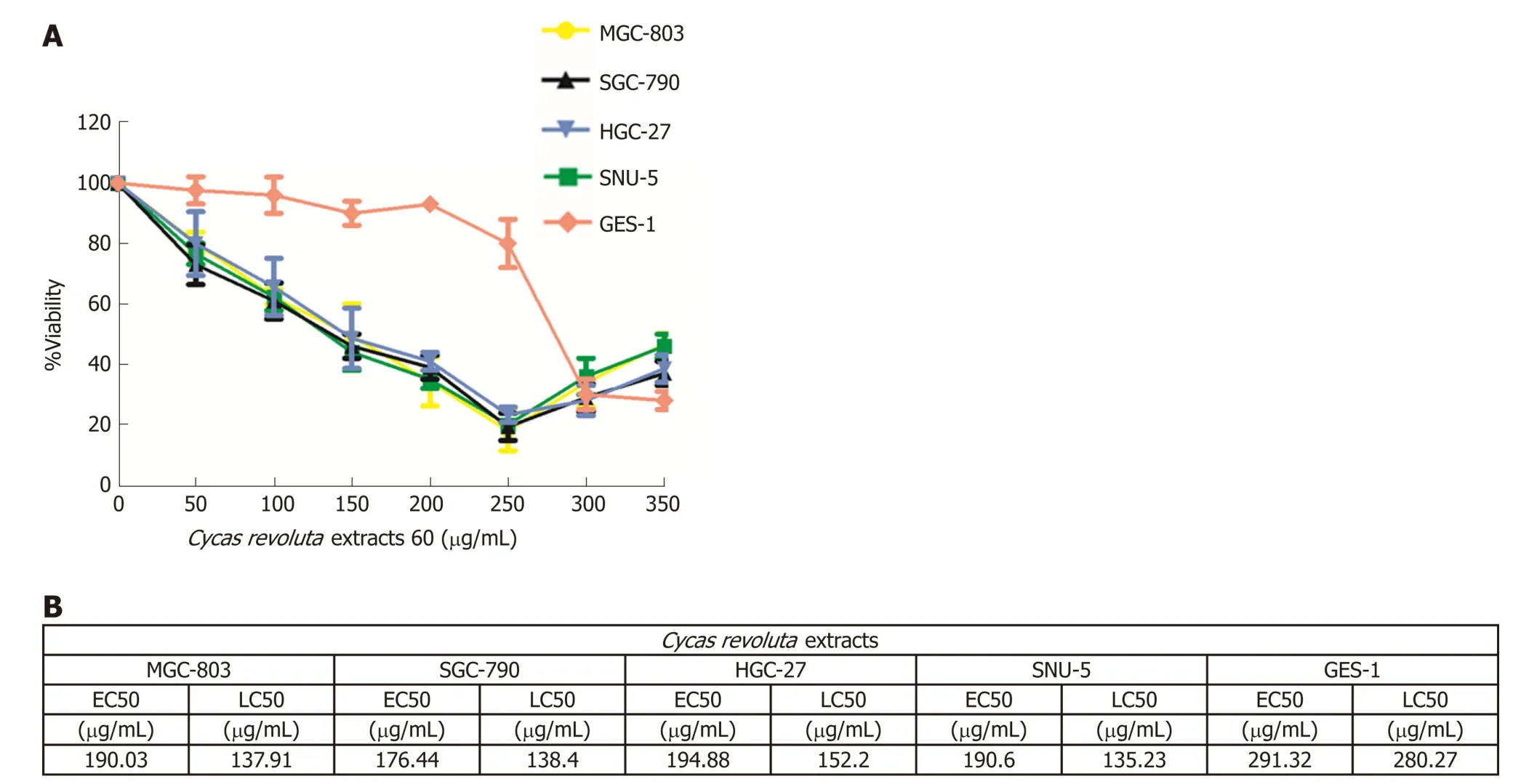
Figure 1 Cycas revoluta Thunb. extract effectively inhibits gastric cancer cell growth. A: Dose-response assays of the extract. Gastric cancer cells were analyzed after treatment with increasing concentrations of the extract for 24 h. The percentage of cell viability was normalized to the control, and three independent experiments were performed for calculating ± STDEV; B: Half-maximal effective concentration and the half-maximal lethal concentration for each cell line. EC50: Half-maximal effective concentration; LC50: Half-maximal lethal concentration.
To d etermine the effect of Cycas revoluta Thunb. natural extract on gastric cancer cell migration, we performed wound-healing assays. MGC-803 and HGC-27 gastric cancer cell lines w ere selected for the test, and cells were treated w ith a low dose of extract(60 μg/mL), w hich reduced cell viability by about 20%. Our results showed that Cycas revoluta Thunb. extract significantly reduced gastric cancer cell migration after 24 h of extract treatment, especially for MGC-803 cells, whose wound width was over twice that of control cells (Figure 2A). To further investigate its effect on gastric cancer cell invasion, we performed transw ell invasion assays. Cycas revoluta Thunb. natural extract markedly reduced the invasion ability of both MGC-803 and HGC-27 cell lines(Figure 2B). Taken together, these results demonstrate that the Cycas revoluta Thunb.natural extract effectively inhibited malignant gastric cancer cell migration and invasion.
Cycas revoluta Thunb. extract enhanced the anti-cancer effect of 5-Fu by chemosensitization
To determine whether Cycas revoluta Thunb. natural extract can be used in combination with other anti-cancer drugs, we chose 5-Fu, one of the most widely used chemotherapy drugs, for clonogenic assays.
By assessing colony formation ability, we found that although the inhibitory effect of 5-Fu was stronger than that of the extract, combining the two drugs enhanced the inhibitory effect of 5-Fu (Figure 3A).
To investigate w hether the increased inhibitory effect w as d ue to chemosensitization, we performed cell viability assays with a low dose of extract (60 μg/mL)and increasing doses of 5-Fu for 24 h. We found that the Cycas revoluta Thunb. natural extract significantly sensitized gastric cancer cells to 5-Fu (Figure 3B). The EC50 and LC50 values dramatically decreased in MGC-803 (1.6 times and 2.8 times) and HGC-27 cells (1.8 times and 3.5 times), suggesting that Cycas revoluta Thunb. natural extract had additive and synergistic effects with 5-Fu in inhibiting gastric cancer cells. To further confirm this result, we examined the expression of three key drug-resistancerelated proteins including p-gp, p-AKT and mTOR by western blot. We found that all three proteins significantly decreased when 5-Fu was used in combination with Cycas revoluta Thunb. natural extract (Figure 3C), which suggested reduced drug-resistance of gastric cancer cells.
Cycas revoluta Thunb. extract mediates 5-Fu chemosensitization through apoptosis
Because the Sotetsuflavone in Cycas revoluta Thunb. ind uced lung cancer cell apoptosis, we performed Hoechst 33258 staining in the 5-Fu-resistant gastric cancer line SGC-7901/R after treatment with the extract, 5-Fu or both. Both the extract and 5-Fu induced SGC-7901/R cell apoptosis, demonstrating that Cycas revoluta Thunb.extract can similarly induce gastric cancer cell apoptosis (Figure 4A). Moreover, the combination of 5-Fu and extract dramatically increased the extent of apoptosis (Figure 4A), suggesting that the Cycas revoluta Thunb. extract-induced chemosensitization of 5-Fu may be mediated via apoptosis . To confirm this hypothesis, we examined the expressional level of three important proteins involved in the apoptosis pathway.XIAP and AIF expression significantly decreased and increased, respectively, while the survivin expression remained stable (Figure 4B). This result demonstrated that the chemosensitive enhancement of 5-Fu and Cycas revoluta Thunb. extract may be due to further activation of apoptosis.
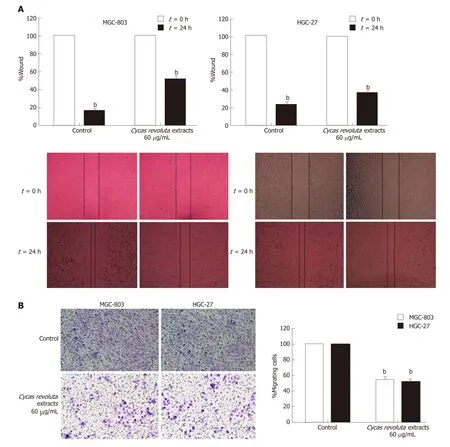
Figure 2 The effect of Cycas revoluta Thunb. natural extract on cell migration and invasion. A: Wound healing assays; and B: transwell invasion assays were performed with a low dose of extract (60 μg/mL). The wound width or migrated cells normalized to the control is shown in histograms, and the error bars represent ±STDEV from n = 3 independent experiments, b P < 0.01.
DISCUSSION
As one of the most common cancers, gastric cancer has been frequently diagnosed and has led to thousands of deaths worldwide. Around 500,000 people in China died from it just in 2015[3]. Although 5-Fu is often employed as chemotherapy against gastric cancer, its effect varies, likely due to the drug resistance of gastric tumors[10,11,27]. To overcome the problem of increasing drug resistance, 5-Fu is usually used with other compound s to enhance cancer cell sensitivity. Accord ing to p revious stud ies, the traditional Chinese medicine Cycas revoluta Thunb. exhibited this potential synergistic effect[37,38]. Therefore, w e investigated its inhibitory effect and the effect of chemosensitive enhancement with 5-Fu in gastric cancer.
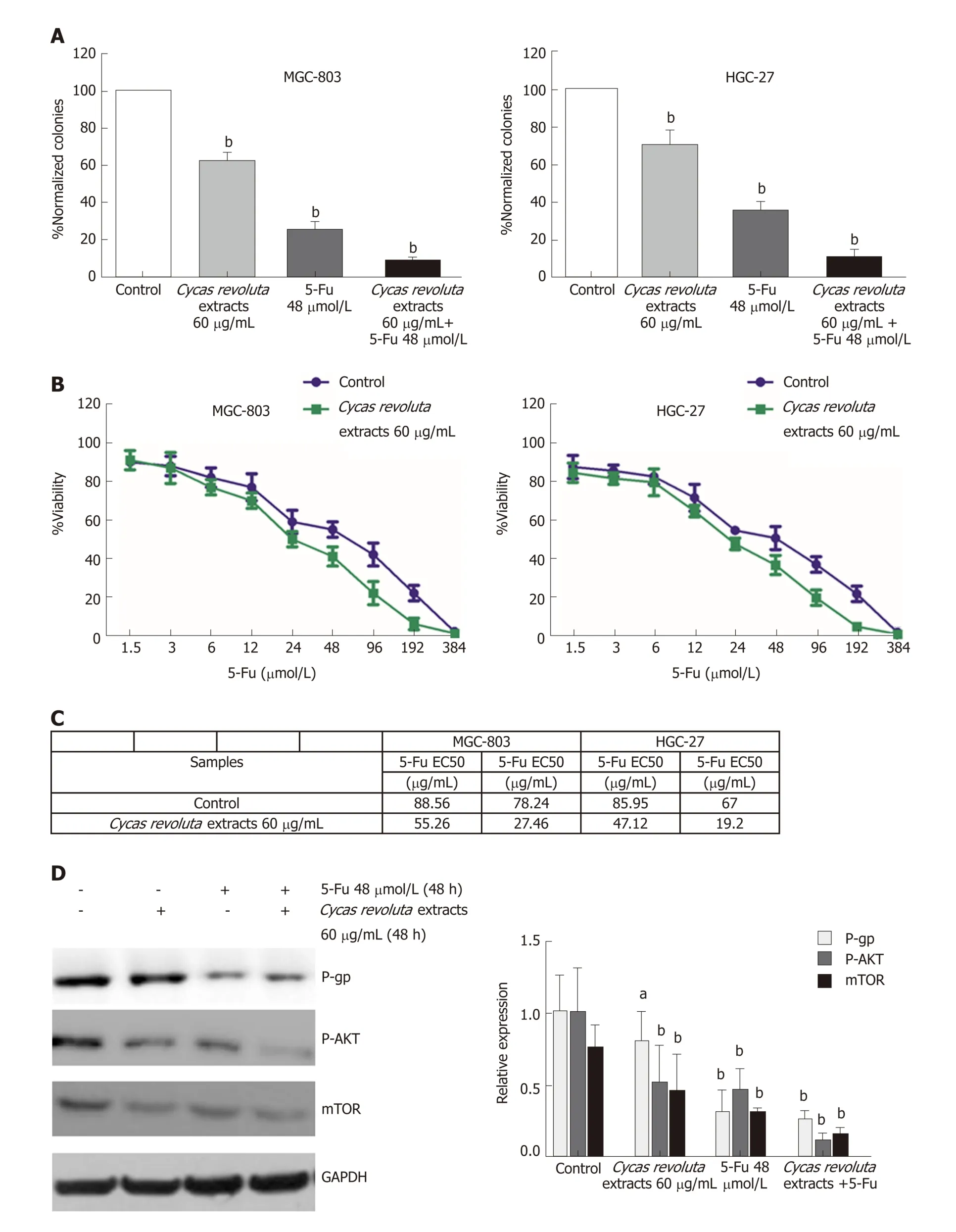
Figure 3 The chemosensitization of Cycas revoluta Thunb. natural extract with 5-fluorouracil. A: Clonogenic assays. The error bars represent ± STDEV from n = 3 independent experiments, b P < 0.01; B: Dose-response assays of 5-fluorouracil (5-Fu) with the extract; C: The half-maximal effective concentration and the halfmaximal lethal concentration of 5-Fu in combination with the extract. The error bars represent ± STDEV from n = 3 independent experiments; D: Western blot analysis of drug-resistance-related proteins. GAPDH was used as a control. Relative expressions are shown in the left histogram, and the error bars represent ± STDEV from n= 3 independent experiments a P < 0.05, b P < 0.01. 5-Fu: 5-fluorouracil; EC50: Half-maximal effective concentration; LC50: Half-maximal lethal concentration; mTOR:Mammalian target of rapamycin; P-gp: P-glycoprotein.
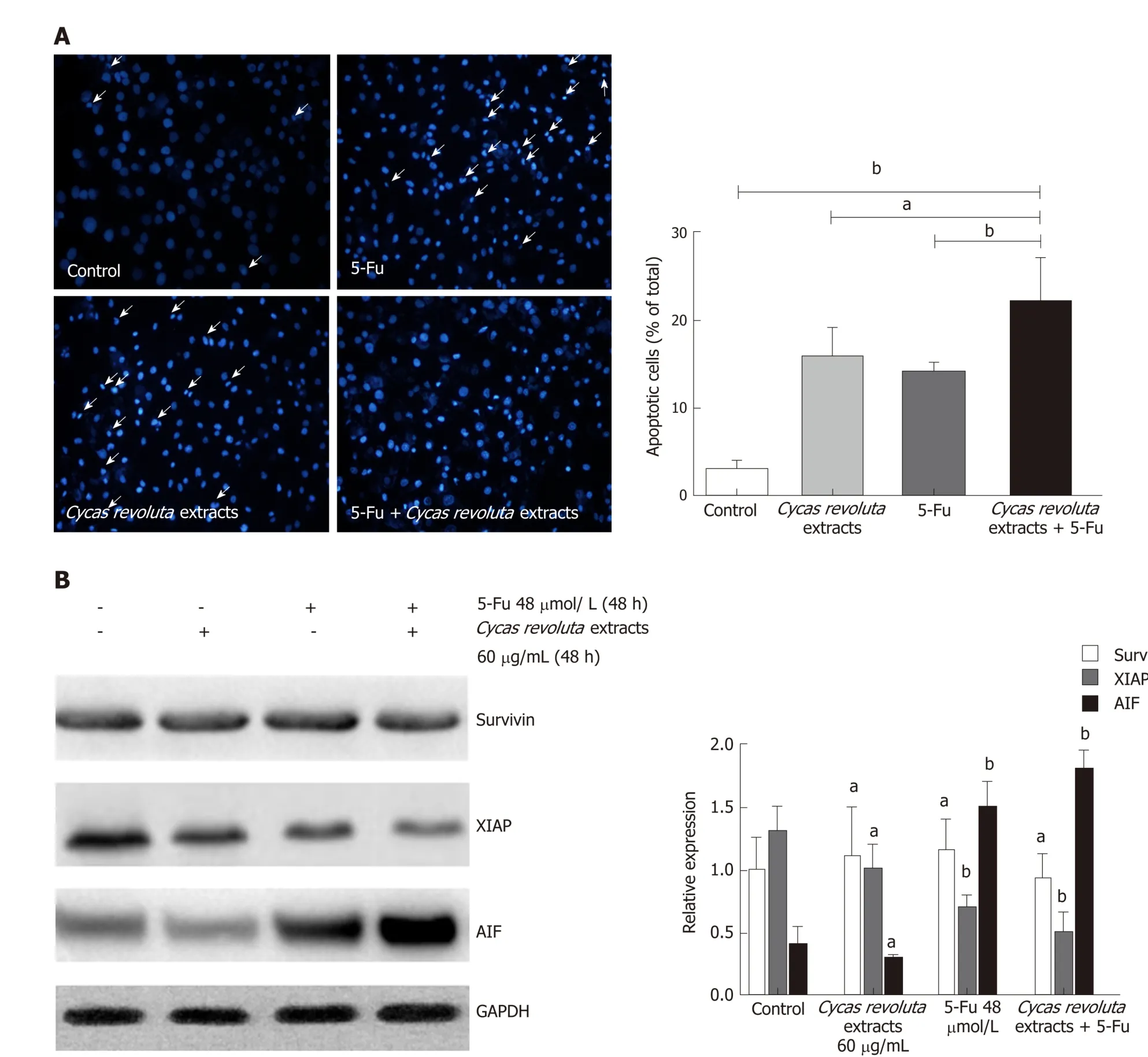
Figure 4 The mechanism of chemosensitization mediated by Cycas revoluta Thunb. extract. A: Hoechst 33258 staining to confirm apoptosis induced by 5-fluorouracil and the extract. The error bars represent ± STDEV from n = 3 independent experiments a P < 0.05, b P < 0.01; B: Western blot analysis of apoptosis-related proteins. Relative expressions are shown in the left histogram, and the error bars represent ± STDEV from n = 3 independent experiments a P < 0.05, b P < 0.01. 5-Fu:5-fluorouracil; XIAP: X-linked inhibitor of apoptosis protein; AIF: Apoptosis inducing factor.
In this stud y, w e found that Cycas revoluta Thunb. extract effectively inhibited gastric cancer cell growth with little effect on normal gastric cells at low and medium doses (0 μg/m L-250 μg/m L). Combined with the significant decrease in EC50 and LC50 values for gastric cancer cells, w e conclude that gastric cancer cells are more sensitive to Cycas revoluta Thunb. extract than normal gastric cells. How ever, the inhibitory effect on normal cells dramatically increased when the concentration of the extract w as over 250 μg/m L. In contrast, the viability rate of cancer cells increased.This finding may be due to the strong screening effect of high concentrations of Cycas revoluta Thunb. natural extract, in w hich d rug-resistant cancer cells rap id ly proliferate, or employ other adaptive mechanisms. This result also suggests that the d ose of Cycas revoluta Thunb. natural extract should be strictly controlled d uring p ractical app lication. Ad d itionally, w e d emonstrated that Cycas revoluta Thunb.natural extract significantly d ecreased the migration and invasion ability of gastric cancer cells, further confirming its inhibitory effect on gastric cancer cells.
To d etermine w hether Cycas revoluta Thunb. natural extract could be used w ith 5-Fu, w e carried out clonogenic assays. The results show ed that 5-Fu exhibited a stronger inhibitory effect than the extract, but combining the tw o d rugs further inhibited cancer cell colony formation. By analyzing EC50 and LC50 values, we can conclude that cancer cell sensitivity to 5-Fu increased in the presence of Cycas revoluta Thunb. natural extract. This result w as further confirmed by detecting the expression of the d rug-resistance-related p roteins p-gp, p-AKT and m TOR. p-AKT and mTOR exp ression w ere even further d ecreased in 5-Fu treatments w hen combined w ith Cycas revoluta Thunb. natural extract, suggesting that the changes in expression are highly related to Cycas revoluta Thunb-mediated enhancement of sensitivity of gastric cancer cells. mTOR is involved in AKT phosphorylation, which activates this enzyme.The activation of the AKT-m TOR pathw ay has been w id ely observed in various cancers, such as bladder cancer, breast cancer and non-small cell lung cancer[40-45]. This pathway plays an important role in regulating proliferation, survival, metastasis, and d rug resistance of tumors, such as p aclitaxel or end ocrine therap y[44,45]. Some preclinical and clinical evidence has also suggested that NEAT1, BAG-1 and XPC are involved in the enhanced drug resistance of cancer cells med iated by the AKT-mTOR p athw ay[41,42,44,45], w hich may p rovid e some clues for us to further exp lore the mechanism that the extract sensitizes gastric cancer cells to 5-Fu through the AKTmTOR pathway.
Hoechst 33258 staining proved that using the tw o compounds together obviously increased the rate of cancer cell apoptosis, suggesting that Cycas revoluta Thunb.natural extract further induces apop tosis in 5-Fu treatments. This hypothesis w as further confirmed by examining the expression of the ap op tosis-related p roteins XIAP, AIF and survivin. We observed that the activator AIF increased and inhibitor XIAP d ecreased, further explaining the increased ap optosis of gastric cancer cells.However, survivin expression remained stable, which suggests that this enhancement of apoptosis may not be mediated by survivin.
In conclusion, this stud y suggests that Cycas revoluta Thunb. natural extract can inhibit gastric cancer cell growth, migration and invasion. Moreover, it can be used to enhance the effect of 5-Fu through the AKT-m TOR pathw ay, w hich p rovid es a promising strategy in chemotherapy against gastric cancer.
ARTICLE HIGHLIGHTS
Research background
As one of the most frequent cancers, gastric cancer caused more than 700,000 deaths in just 2012 w orldw ide. Although 5-f luorouracil (5-Fu) is often employed as treatment against gastric cancer,its effect is severely affected by drug resistance of gastric cancer cells. Cycas revoluta Thunb.Extract has show n promise as a cancer treatment, though its effect on gastric cancer remains unknow n.
Research motivation
To find new w ays for chemical sensitization of cancer cells and improve the effect of 5-Fu during chemotherapy against malignancies.
Research objectives
To explore the anti-cancer effect of Cycas revoluta Thunb. in gastric cancer and investigate its chemical sensitization effect against gastric cancer cells during 5-Fu treatment.
Research methods
The half-maximal effective concentration and the half-maximal lethal concentration of drugs w ere determined by cell viability test. The effect of Cycas revoluta Thunb. on gastric cancer cell migration w as investigated by w ound-healing and transw ell assay. The synergistic effect between Cycas revoluta Thunb. and 5-Fu w as confirmed by clonogenic assay and apoptosis detection. The expression of crucial proteins was measured by western blotting.
Research results
We found that the natural extract of Cycas revoluta Thunb. Preferentially killed gastric cancer cells compared to normal gastric cells. In addition, the extract significantly inhibited gastric cancer cell grow th, migration and invasion. Cycas revoluta Thunb. can also improve the inhibitory effects of 5-Fu and effectively induce cell apoptosis. Western blotting analysis show ed that Pglycoprotein, p-AKT and mammalian target of rap amycin (m TOR) expression markedly d ecreased, suggesting that AKT-m TOR p athw ay p lays an important role in chemical sensitization induced by Cycas revoluta Thunb.
Research conclusions
Our study demonstrated that the natural extract of Cycas revoluta Thunb. can significantly inhibit gastric cancer cell grow th, migration and invasion. Furthermore, it can also improve the effect of 5-Fu and promote apoptosis during chemotherapy. Therefore, our study provides a new drug for improving the clinical effect of chemotherapy in gastric cancer. Our study also show ed that Cycas revoluta Thunb. Enhanced the effects of 5-Fu through the AKT-m TOR pathw ay, offering a novel mechanism for the chemical sensitization effect of Cycas revoluta Thunb.
Research perspectives
In the future, research may reveal the main component of Cycas revoluta Thunb. that enhances the sensitivity of cancer cells and further develop for its application in anti-cancer treatments.The id entification of the molecular pathw ay related to AKT-m TOR may further explain the underlying mechanism.
杂志排行
World Journal of Gastroenterology的其它文章
- Repurposing drugs to target nonalcoholic steatohepatitis
- Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development
- Considerations of elderly factors to manage the complication of liver cirrhosis in elderly patients
- Lysyl oxidase and hypoxia-inducible factor 1α: biomarkers of gastric cancer
- Predictive and prognostic implications of 4E-BP1, Beclin-1, and LC3 for cetuximab treatment combined with chemotherapy in advanced colorectal cancer with wild-type KRAS: Analysis from real-world data
- Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation
