Predictive and prognostic implications of 4E-BP1, Beclin-1, and LC3 for cetuximab treatment combined with chemotherapy in advanced colorectal cancer with wild-type KRAS: Analysis from real-world data
2019-05-08GuiFangGuoYiXingWangYiJunZhangXiuXingChenJiaBinLuHaoHuaWangChangJiangHuiQuanQiuLiangPingXia
Gui-Fang Guo, Yi-Xing Wang, Yi-Jun Zhang, Xiu-Xing Chen, Jia-Bin Lu, Hao-Hua Wang, Chang Jiang,Hui-Quan Qiu, Liang-Ping Xia
Abstrac t BACKGROUND Colorectal cancer (CRC) is one of the main causes of cancer-related deaths in China and around the world. Advanced CRC (ACRC) patients suffer from a low cure rate though treated with targeted therapies. The response rate is about 50%to chemotherapy and cetuximab, a monoclonal antibody targeting epidermal growth factor receptor (EGFR) and used for ACRC with wild-type KRAS. It is important to identify more predictors of cetuximab efficacy to further improve precise treatment. Autophagy, showing a key role in the cancer progression, is influenced by the EGFR pathway. Whether autophagy can predict cetuximab efficacy in ACRC is an interesting topic.AIM To investigate the effect of autophagy on the efficacy of cetuximab in colon cancer cells and ACRC patients with wild-type KRAS.METHODS ACRC patients treated with cetuximab plus chemotherapy, with detailed data and tumor tissue, at Sun Yat-sen University Cancer Center from January 1, 2005,to October 1, 2015, were studied. Expression of autophagy-related proteins[Beclin1, microtubule-associated protein 1A/B-light chain 3 (LC3), and 4Ebinding protein 1 (4E-BP1)] was examined by Western blot in CRC cells and by immunohistochemistry in cancerous and normal tissues. The effect of autophagy on cetuximab-treated cancer cells was confirmed by MTT assay. The associations between Beclin1, LC3, and 4E-BP1 expression in tumor tissue and the efficacy of cetuximab-based therapy were analyzed.RESULTS In CACO-2 cells exposed to cetuximab, LC3 and 4E-BP1 were upregulated, and P62 was downregulated. Autophagosome formation was observed, and autophagy increased the efficacy of cetuximab. In 68 ACRC patients,immunohistochemistry showed that Beclin1 levels were significantly correlated with those of LC3 (0.657, P < 0.001) and 4E-BP1 (0.211, P = 0.042) in ACRC tissues.LC3 was significantly overexpressed in tumor tissues compared to normal tissues(P < 0.001). In 45 patients with wild-type KRAS, the expression levels of these three proteins were not related to progression-free survival; however, the expression levels of Beclin1 (P = 0.010) and 4E-BP1 (P = 0.005), pathological grade(P = 0.002), and T stage (P = 0.004) were independent prognostic factors for overall survival (OS).CONCLUSION The effect of cetuximab on colon cancer cells might be improved by autophagy.LC3 is overexpressed in tumor tissues, and Beclin1 and 4E-BP1 could be significant predictors of OS in ACRC patients treated with cetuximab.
Key words: 4E-binding protein 1; Beclin-1; Microtubule-associated protein 1A/B-light chain 3; Advanced colorectal cancer; Cetuximab efficacy; Prognosis
INTRODUCTION
Colorectal cancer (CRC) is one of the main causes of cancer-related deaths both in China and worldwide. In past years, the median overall survival of advanced CRC(ACRC) p atients w as 20 mo d ue to the d evelop ment of new combinations of chemotherapy drugs, including oxaliplatin-based[1]and irinotecan-based regimens[2].However, drug resistance and/or off-target toxicity limit the efficiency of current chemotherapy drugs. The introduction of new targeted therapies, such as monoclonal antibodies targeting epidermal grow th factor receptor (EGFR) (cetuximab and panitumumab) or against vascular endothelial grow th factor (bevacizumab, zivaflibercept, and ramucirumab), has significantly increased the overall survival of ACRC patients to 30 mo[3]. Currently, the only antibody targeting EGFR that has been approved for use in CRC by the Chinese Food and Drug Administration is cetuximab.Stud ies have d emonstrated that only ACRC p atients w ith w ild-type KRAS benefit from cetuximab[4]and thus have better overall survival; this beneficial effect is observed especially in patients with left-sided ACRC[5]. How ever, the response rate(RR) is only 40%-60% for patients w ith wild-type KRAS[6]. It is important to id entify novel p red ictors to exclud e patients w ith w ild-type KRAS w ho w ill not respond to cetuximab, which will further improve precise treatment.
Autophagy, an important intracellular homeostatic pathw ay for the degradation of d ysfunctional organelles and p roteins, can p rovid e energy for survival und er cond itions of d iverse cellular stresses[7]. Desp ite accumulating evid ence that autop hagy plays a critical role in cancer metastasis, autophagy can also supp ress pathological p rocesses, including cancer metastasis[8]. Multip le autophagy-related genes and proteins are involved in metastatic CRC[9]. Mammalian target of rapamycin(mTOR), a member of the phosphatidylinositol-3-kinase family, plays a major role in inhibiting the autophagic process and is associated w ith the p roliferation, survival,invasion, and metastasis of CRC cells[10]. Several m TOR signaling comp onents,including mTOR, p70-S6 Kinase 1 (S6K), and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), are highly exp ressed and activated in gland ular elements of CRC[11]. The current paradigm suggests that mTOR phosphorylates 4E-BP1 at multiple sites, w hich promotes the dissociation of eIF4E from 4E-BP1, reducing the inhibitory effect of 4E-BP1 on eIF4E-d ep end ent translation initiation and d ecreasing the translational activity[12]. At the molecular level, the complex autophagy machinery is orchestrated in several stages. One w ell-characterized regulatory event is the interaction betw een Beclin1 and Beclin2, w hich d isrupts the interaction of Beclin1 with the class III phosphoinositide 3-kinase (PI3K), a core regulator of autophagy[13].Autophagy-related genes (Atgs) control autophagosome formation through Atg12-Atg5 and microtubule-associated protein 1A/B-light chain 3 (LC3) complexes, and the modified form of LC3 is attached to the autophagosome membrane[14].
It has been reported that autophagy was induced by EGFR siRNA in cancer cells[15].Few reports d escribe autop hagy and the anti-cancer role of cetuximab, antibod y targeting EGFR, and most of these stud ies have been performed on cancer cells. In this study, we examined the critical autophagic proteins, Beclin-1, 4E-BP1, and LC3, in CRC cells and investigated w hether autop hagy d etermines cell sensitivity to cetuximab in vitro. We also investigated the relationships betw een clinicopathological characteristics and the exp ression of Beclin-1, LC3, and 4E-BP1 in ACRC tissue specimens, and evaluated the influence of these autophagy-related proteins on ACRC p rognosis. We p reviously stud ied the association betw een Beclin-1 and LC3 expression levels and the effect of cetuximab on ACRC[16], but cetuximab w as not given as a first-line therapy in all patients, and the KRAS status of those patients was unknown because KRAS was not used as a predictor of the efficacy of cetuximab at that time. The present study focused on patients w ith ACRC w ith w ild-type KRAS who were administered with cetuximab plus chemotherapy as the first-line therapy to explore the predictive value of the expression levels of Beclin-1, LC3, and 4E-BP1. The differences in their expression levels between cancerous and normal tissues as well as the correlation of their expression w ith clinical and pathological grade, progressionfree survival (PFS), and overall survival (OS) w ere also examined in a group of patients w ild-typ e KRAS w ho w ere initially or later treated w ith cetuximab and chemotherapy.
MATERIALS AND METHODS
Cell line and culture
The CRC cell line CACO-2, w hich has the wild-type KRAS gene, w as obtained from the Cell Bank of the Chinese Acad emy of Sciences (Shanghai, China). The cells were maintained at 37 °C in an atmosphere containing 5% CO2.
MTT assay
MTT assays were performed to determine the anti-proliferative effect of cetuximab on CACO-2 cells. After treatment with cetuximab for 24, 48, and 72 h, 20 μg (5 mg/mL)of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma, United States) was added to 96-well plates and incubated at 37 °C for 5 h; then, 150 μL of dimethyl sulfoxide (DMSO) was added to each well and incubated for 10 min at room temperature to dissolve the formazan crystals. The absorbance of each w ell was measured with an ELISA reader (BIO-TEK, United States) at a wavelength of 562 nm.The experiment w as performed in trip licate, and the d ata w ere analyzed by comparison to DMSO-treated control cells.
Western blot analysis
The tissue samp les w ere homogenized in sod ium d od ecyl sulphate (SDS) buffer containing the protease inhibitor PMSF. The homogenates w ere incubated on ice for 20 min and then centrifuged at 12000 rpm for 30 min at 4 °C. The supernatant w as collected and equal volume of 2 × SDS buffer w as added. The mixture was boiled for 10 min and preserved at -20 °C. The protein extracts (50 μg) were separated by SDSp oly acrylamid e gel electrop horesis (SDS-PAGE) and then transferred onto polyvinylid ene d ifluorid e membranes (Millipore, United States). The membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 at room temperature for 90 min and then incubated w ith primary antibodies against Beclin-1 (3 μg/m L; ab55878, Abcam, Cambrid ge, United Kingdom), LC3 (2 μg/m L;ab48394, Abcam), 4E-BP1 (2 μg/m L; ab2606, Abcam), and actin (0.5 μg/mL, ab3280,Abcam). The protein bands w ere d etected w ith secondary antibodies conjugated to horserad ish peroxid ase (1:5000, Abcam, United Kingd om) and visualized w ith enhanced chemiluminescence reagents. Each band w as quantified through densitometry, and the results are presented as the relative expression of each protein from different samples.
Fluorescence microscopy
For fluorescence microscopy, 500 μL of cultured cells were removed from the flask at the desired time points, centrifuged for 3 min at 5000 × g, and resuspended in an appropriate volume of w ater. A total of 4 μL of each sample w as spotted on a microscope slide and viewed using an Olympus 1 × 51 inverted fluorescence microscope.
Patients and variables
In this retrospective study, ACRC patients with definitive pathological diagnoses,paraffin-embed d ed p athological sp ecimens, and comp lete clinicop athologic information who received cetuximab combined with first-line or later chemotherapy at our institution (Sun Yat-sen University Cancer Center) between January 1, 2005 and October 1, 2015 were enrolled.
Our primary study endpoints were OS, which was defined as the time from the date of the first cycle of front-line therapy to the date of death from any cause, and first-line PFS, w hich w as defined as the time from the initial therapy to tumor progression, death from any cause, or the last follow-up before the initiation of second-line therapy. In addition, the objective RR (ORR) and disease RR (DCR) to cetuximab combined with chemotherapy as a first-line treatment were also studied.The study was approved by the ethics committee of our institution.
Tissue samples
CRC tissues were surgical or biopsy specimens taken from primary colorectal tumors,and the negative margins of the surgical specimens w ere considered as normal tissues. All tissue samples were acquired from the sample bank of the pathology department at our cancer center, and all formalin-fixed paraffin-embedded samples in this stud y w ere p reserved intact. All pathological d iagnoses of tissues w ere adenocarcinoma.
Immunohistochemical staining
The expression of Beclin-1, LC3, and 4E-BP1 in primary tumors was detected through immunohistochemical staining (IHC) and then compared w ith the expression in the normal tissue samples. The formalin-fixed paraffin-embedded tissue was cut into 4-μm thick sections, d ewaxed, rehydrated, and blocked with hyd rogen peroxide. After incubation with primary antibod ies against 4E-BP1 (1:200), Beclin-1 (1:100), and LC3(1:400) (all from Cell Signaling Technology, United States) d iluted in 1% PBS, the sections w ere incubated with an anti-rabbit secondary antibod y (Invitrogen, United States) for 30 min. After an additional two washes in PBS, the sections were incubated with diaminobenzid ine (DAB, Invitrogen, United States) for 10 min to detect signals.Finally, the sections w ere counterstained w ith hematoxylin (Invitrogen, United States). Negative controls were obtained by substituting nonimmune rabbit serum for the primary antibodies.
Immunohistochemical score determination
Protein expression was evaluated via an Olympus CX31 microscope (Olympus, Center Valley, PA, United States) by tw o ind ivid uals w ho w ere blind ed to the p atient outcomes and other clinical findings. The immunoreactivity was evaluated according to the intensity of staining and the percentage of stained cells. Immunostaining intensity was rated as follows: 0, pale yellow or no staining; 1, yellow; 2, deep yellow;and 3, brow n. The p ercentage of stained cells p ositive for Beclin-1 and LC3 w as graded as follow s: grad e 0 (0%-10%); 1 (10%-25%); 2 (25%-50%); and 3 (50%-100%).The percentage of stained cells positive for 4E-BP1 was graded as grad e 0, 0%; 1 (1%-20%); 2 (20%-40%); 3 (40%-60%); 4 (60%-80%); and 5 (80%-100%). The staining intensity and percentage of stained tumor cells w ere scored for at least 10 high-power fields (400×). The mean score w as calculated as [(intensityreader1× percentagereader1) +(intensityreader2× percentagereader2)] / 2. Then, the total scores for Beclin1, LC3, and 4EBP1 w ere categorized as low expression (=0) or high expression (>0).
Statistical analysis
All statistical analyses w ere performed w ith SPSS for Window s, version 24.0. The associations betw een Beclin1, LC3, and 4E-BP1 expression and clinicop athological characteristics w ere assessed using the chi-square test or Fisher's exact test, as appropriate. The chi-square test w ith continuity correction w as used to analyze data derived from small cohorts. The relationships among the expression of Beclin1, LC3,and 4E-BP1 w ere assessed by Sp earman's correction analysis. The Kaplan-Meier method w as used to estimate tumor progression in mCRC, and the log-rank test w as used to determine the statistical significance. The groups w ere compared w ith respect to survival using Cox regression analysis. Hazard ratios w ere d etermined using univariate and multivariate Cox regression. P < 0.05 indicated a significant difference.
RESULTS
Autophagy induced by cetuximab in CRC cells
Autophagy was induced by cetuximab treatment in CACO-2 cells with wild-type KRAS, w hich were sensitive to cetuximab (Figure 1A). Abundant characteristic autophagosomes in CACO-2 cells were present 72 h after cetuximab treatment; in contrast, autophagosomes w ere scarce in untreated cells (Figure 1B). We also observed the conversion of LC3/Atg8 from the cytoplasmic form, LC3-1 (18 k Da), to the autophagosomic form, LC3-2 (16 k Da), in the cells exposed to cetuximab (Figure 1C) and decreased expression of P62, which indicated autophagosome formation.Moreover, we observed increased expression of 4E-BP1, indicating that the mTOR pathway was inactive (Figure 1C). MTT assay showed that autophagy increased the inhibitory effect of cetuximab on cell proliferation (Figure 1D).
Basic characteristics of patients
Initially, 235 ACRC patients treated with cetuximab plus chemotherapy were found in the clinical database of our center, but only 68 patients with detailed data and wellpreserved tumor specimens were finally enrolled in this study. The age of the patients ranged from 23 to 77 years, with a median age of 54.5 years. The Beclin1, LC3, and 4EBP1 expression levels were evaluated in all patients treated with cetuximab combined with chemotherapy; the basic characteristics of those patients and the correlations of those characteristics with the expression of Beclin1, LC3, and 4E-BP1 for all patients are reported in Table 1. Forty-five patients were confirmed to have the wild-type KRAS gene, 25 w ere given cetuximab as first-line therapy, and 20 w ere given cetuximab after having already received a different first-line therapy. The number of patients with pathological grades 3, 2, and 1 was 18, 41, and 3, respectively.
For the 45 patients with wild-type KRAS, the ORR and DCR to first-line treatment with cetuximab combined with chemotherapy were 45.5% and 72.7%, respectively,and the median OS was 31 mo (2.2-137).
Expression of Beclin1, LC3, and 4E-BP1 expression in CRC and normal tissues
There were significant correlations between the levels of Beclin1 and those of LC3 and 4E-BP1 in CRC tissues and that LC3 was significantly overexpressed in tumor tissues compared to normal tissues (Figure 2). According to the quantitative scoring method described above, the expression levels of Beclin1, LC3, and 4E-BP1 were classified as either high or low. The expression levels of Beclin1, LC3, and 4E-BP1 in cancerous and normal tissues are shown in Figure 3. LC3 was significantly upregulated in tumor specimens compared to normal specimens (64.87% vs 2.00%, P < 0.001); the sensitivity was 71.4%, with a specificity of 91.3%. The expression levels of Beclin1 (64.87% vs 81.81%, P = 0.248) and 4E-BP1 (81.09% vs 87.10%, P = 0.793) w ere not different between CRC and normal tissues.
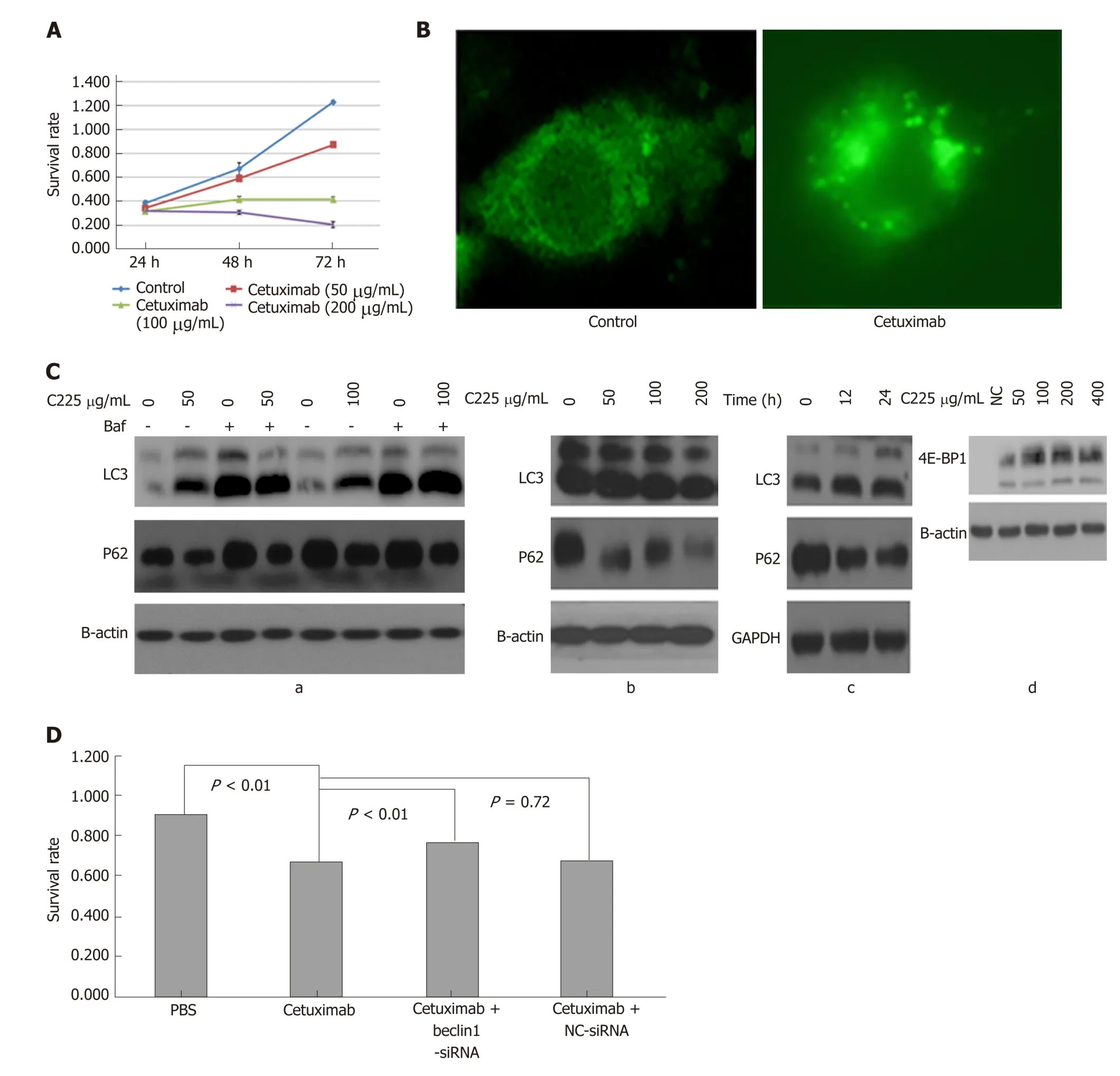
Figure 1 Cetuximab induces autophagy and upregulates 4E-BP1 expression and autophagy enhances anti-cancer effect of cetuximab in colon cancer. A:CACO-2 cell viability measured using MTT assay. Cell viability was significantly inhibited in a time- and dose-dependent manner by cetuximab. B: Cetuximab induced formation of autophagosomes. Autophagosomes in CACO-2 cells treated by cetuximab or not were examined by fluorescence microscopy. More autophagosomes were found in CACO-2 cells after treatment with cetuximab. C: Western blot analysis showing that cetuximab induced autophagy. (a) CACO-2 cells were either untreated or treated with cetuximab and Baf, and Baf inhibited autophagy-related protein degradation. Microtubule-associated protein 1A/B-light chain 3 (LC3)-II/LC3-I was increased, and P62 was decreased when treated with cetuximab, and both LC3 and P62 were increased when treated with cetuximab and Baf compared to treatment with cetuximab alone. (b and c) Cetuximab induced autophagy in a concentration and time dependent manner. With time and increasing concentration, the expression of LC3 increased, and P62 declined. (d) CACO-2 cells were treated with cetuximab for 48 h, and the expression of 4E-binding protein 1 was increased as the concentration of cetuximab increased. D: Effect of autophagy on cetuximab inhibition of cell proliferation. We added PBS, cetuximab, cetuximab + Beclin1-siRNA,and cetuximab + NC-siRNA separately, and the concentration of cetuximab was 78 µg/mL; then, cells were treated for 72 h. The results confirmed that autophagy enhanced cetuximab inhibition of cell proliferation in CACO-2 cells. LC3: Microtubule-associated protein 1A/B-light chain 3; 4E-BP1: 4E-binding protein 1.0
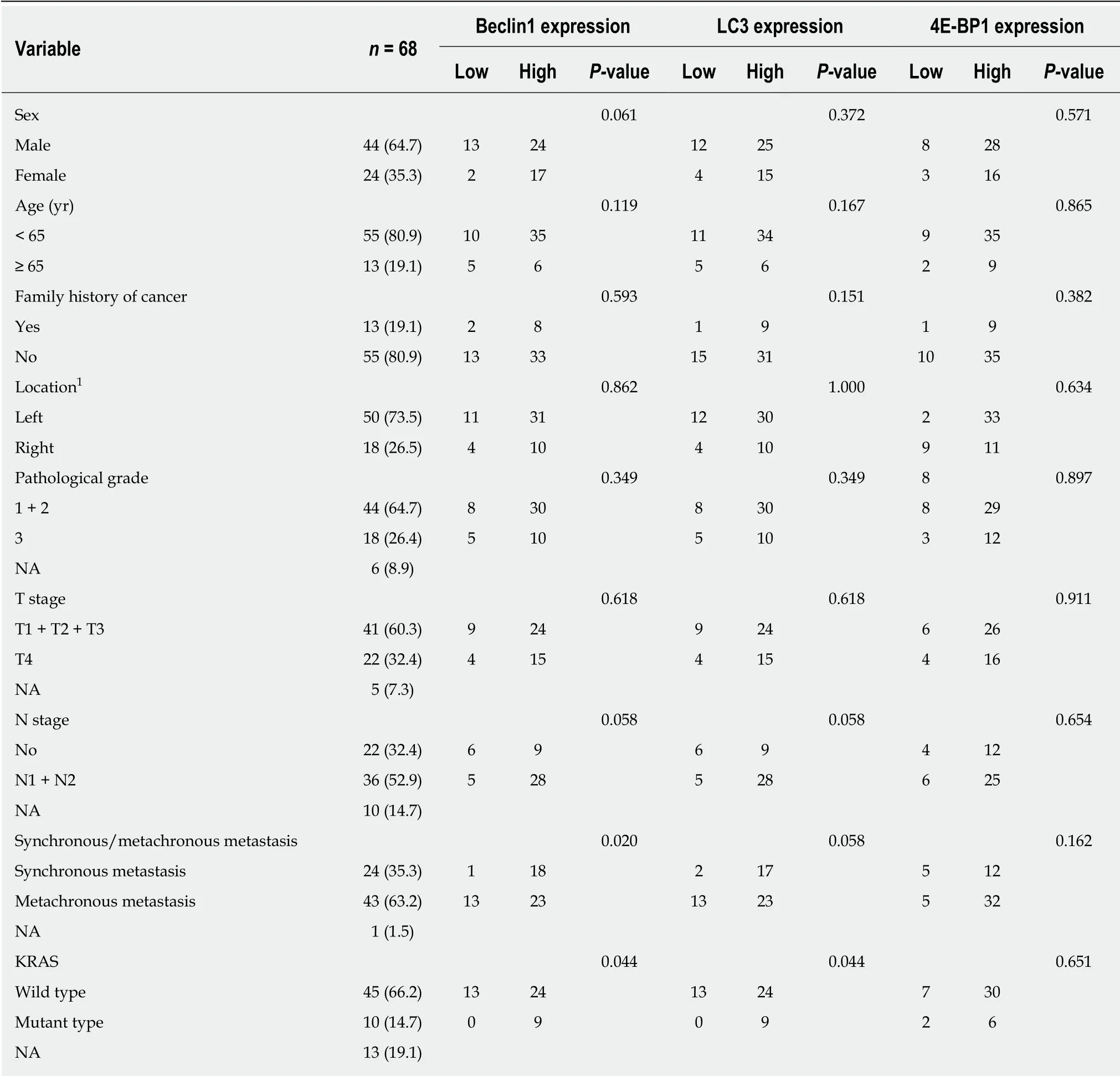
Table 1 Correlations of Beclin1, LC3, and 4E-BP1 expression with clinicopathological characteristics in 68 advanced colorectal cancer patients n (%)
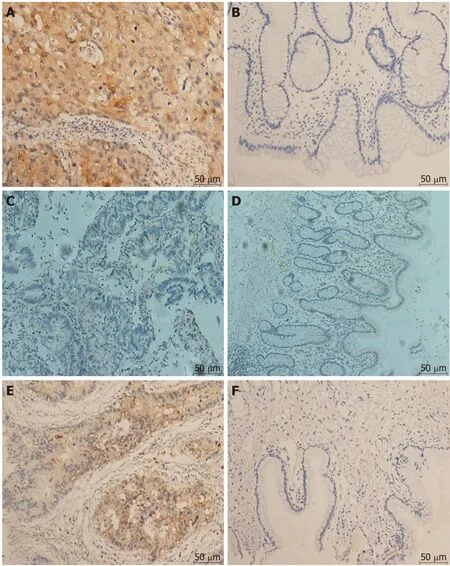
Figure 2 Immunohistochemical staining (200×). A: LC3 displayed strong cytoplasm staining in colorectal cancer tissue; B: LC3 displayed weak cytoplasm staining in normal tissue; C: Beclin1 displayed strong cytoplasm staining in colorectal cancer tissue; D: Beclin1 displayed weak cytoplasm staining in normal tissue; E: 4E-BP1 displayed strong cytoplasm staining in colorectal cancer tissue; F: 4E-BP1 displayed weak cytoplasm staining in normal tissue.
Correlations among the expression levels of Beclin1, LC3, and 4E-BP1; KRAS status; and clinicopathologic factorsexpression was not correlated with that of 4E-BP1 (P = 0.251). Compared to patients with the wild-type KRAS gene, patients with the mutated KRAS gene expressed higher levels of Beclin1 (100% vs 64.9%, P = 0.044) and LC3 (100% vs 64.9%, P = 0.044).The associations of the expression levels of Beclin1, LC,3 and 4E-BP1 w ith the clinicopathologic factors in all 68 patients are show n in Table 1. Patients w ith metachronous metastasis had higher expression levels of Beclin1 than those with synchronous metastasis (94.7% vs 63.9%, P = 0.020).
Predictive value of Beclin1, LC3, and 4E-BP1 expression for cetuximab-containing chemotherapy
For the 25 patients with the wild-type KRAS gene who received cetuximab-containing chemotherapy as the first-line treatment, the ORRs and DCRs for patients w ith low and high Beclin1, LC3, and 4E-BP1 expression levels are shown in Table 2. There were no associations betw een the expression levels of Beclin1 (P = 1.000, P = 0.325), LC3 (P= 0.362, P = 0.325), or 4E-BP1 (P = 0.303, P = 0.560) and the ORR or DCR. In the whole group, the median PFS was 8.81 mo (0.72-32.43). The expression levels of Beclin1, LC3,and 4E-BP1 did not have any relationship w ith PFS [8.80 mo vs 4.7 mo (P = 0.843) for Beclin1 high expression and low expression, respectively; 4.70 mo vs 9.96 mo (P =0.167) for LC3 high expression and low expression, respectively; and 5.32 mo vs 7.36 mo (P = 0.410) for 4E-BP1 high expression and low expression, respectively (Table 2)].
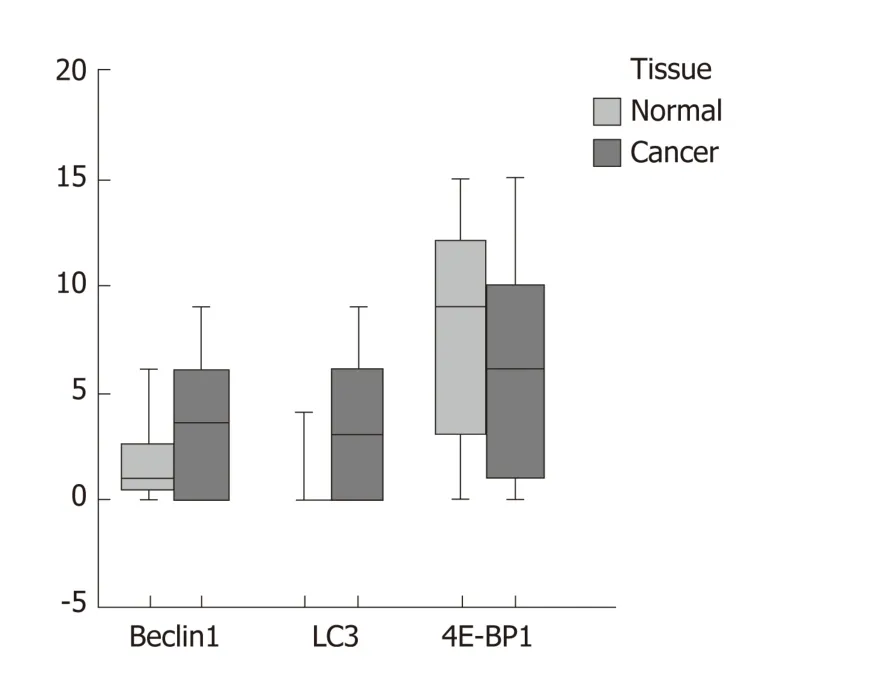
Figure 3 Differential expression of Beclin-1, LC3, and 4E-BP1 tested by immunohistochemical staining in colorectal cancer samples compared to normal tissues. LC3 (P < 0.001) showed higher expression in cancer than in normal tissue, while Beclin-1 (P = 0.248) and 4E-binding protein 1 (P = 0.793) did not. LC3: Microtubuleassociated protein 1A/B-light chain 3; 4E-BP1: 4E-binding protein 1.
Prognostic value of Beclin1, LC3, and 4E-BP1 expression
The median OS for the 45 patients w ith the wild-type KRAS gene w ho received cetuximab as either a first-line treatment or a subsequent treatment was 31 mo (2.2-137). The most common factors potentially affecting OS, such as gender, age, family history of cancer, tumor location, p athological grad e, T stage, N stage,synchronous/metachronous metastasis, and the expression of Beclin1, LC3, and 4EBP1, were analyzed, as shown in Table 3. According to univariate Cox regression analysis, pathological grade (43.00 mo vs 10.32 mo, P < 0.001), the expression level of Beclin1 (19.65 mo vs 37.82 mo, P = 0.037) (Figure 4A), and the expression level of 4EBP1 (64.82 mo vs 23.66 mo, P = 0.024) (Figure 4B) were risk factors affecting OS.Multivariate Cox regression analysis revealed that pathological grade (P = 0.002), T stage (P = 0.004), the expression level of Beclin1 (P = 0.010), and the expression level of 4E-BP1 (P = 0.005) were independent prognostic factors for OS.
DISCUSSION
Most studies on the roles of autop hagy in cetuximab-mediated cancer therap y are limited to cancer cells, but results based on clinical patients are few. The present study investigated the influence of Beclin1, LC3, and 4E-BP1 expression in tumors, which are three key factors involved in the autophagic process, on the ORR, DCR, PFS, and OS of patients treated w ith cetuximab combined w ith chemotherap y. The efficacy found in this study population was the same as that in the previous stage III clinical trials[3], w ith an ORR of 45.5%, a DCR of 72.7%, and a med ian OS of 31 mo. First, we found that LC3 and 4E-BP1 were upregulated and P62 was downregulated in CACO-2 cells exp osed to cetuximab, and autop hagosome formation w as observed by fluorescence microscopy, indicating that cetuximab induces autophagy in CRC cells.In ad dition, the effect of autophagy on the killing of cancer cells by cetuximab w as confirmed by MTT assay. We further studied the influence of these autophagic factors on cetuximab efficacy in ACRC. Using IHC, w e observed that there were significant correlations betw een the levels of Beclin1 and the levels of LC3 and 4E-BP1 in CRC tissue and that LC3 w as significantly overexpressed in tumor tissues compared to normal tissues. The expression levels of Beclin1 and 4E-BP1 as w ell as pathological grade and T stage were independent prognostic factors for OS.
Our study found that cetuximab, which targets EGFR, induced autophagy in colon cancer cells. The mechanism of the d ow nstream signaling effect of EGFR on autophagy and the role of autophagy in EGFR-driven cancer cell proliferation remain unclear. One stud y of EGFR and autop hagy in cancer cells show ed that the mechanisms und erlying the enhancement of autophagy involve anti-EGFR agentstimulated dow nregulation of HIF1 as w ell as Beclin2, w hich in turn releases Beclin1 from Beclin2 suppression; this d emonstrated that autophagy is a protective cellular resp onse to cetuximab treatment[17]. Another stud y found that autop hagy p layed different roles in the cetuximab-mediated treatment of different cancer cells, including vulvar squamous carcinoma cells, colorectal adenocarcinoma cells, and head and neck cancer cells. Autop hagy protected cancer cells from d eath w hen ap op tosis w as strongly ind uced by cetuximab and enhanced cetuximab-ind uced apop tosis w henonly a minimal level of apoptosis was induced after cetuximab treatment[18]. We also found that autop hagy w as activated by cetuximab in colon cancer cells, and cetuximab increased cell death through autophagy. Autophagy is involved in the process by which cetuximab inhibits cell proliferation[8], but the negative or positive effects of cetuximab treatment on autophagy are controversial, and further studies are needed.
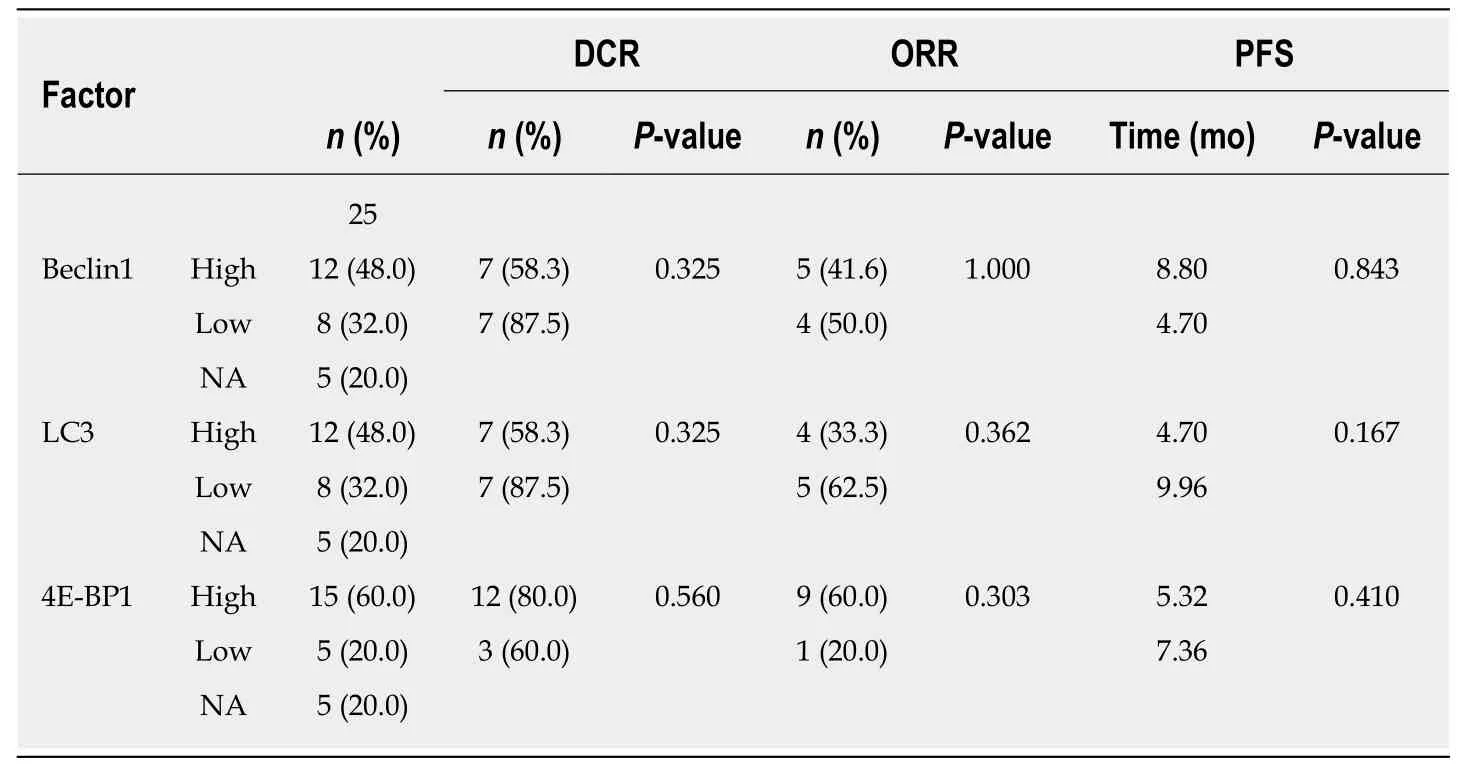
Table 2 Relationships of Beclin1, LC3, and 4E-BP1 with short-term efficacy of first-line cetuximab-containing therapy in 25 patients with wild-type KRAS
In our study, patients with metachronous metastasis showed significantly higher expression levels of Beclin1 than those w ith synchronous metastasis. Beclin1 overexpression might indicate late metastasis of CRC, which is consistent with the previous results observed in cells. Koneri et al[19]transfected the Beclin1 gene into HT29 colon cancer cells and found that ectopic Beclin1 overexpression resulted in slow cell grow th and G1 arrest w ith d ow nregulated expression levels of cyclin E and phosphorylated Rb. This finding suggested that Beclin1 overexpression may reverse aggressive phenotypes and act as a tumor suppressor in CRC. Additionally, we found that patients with mutant KRAS had higher expression levels of the autophagic markers Beclin1 and LC3. Several studies have shown that autophagy is upregulated in cancers with KRAS mutations. Alves et al[20]explained that KRAS effector pathways are involved in the regulation of autop hagy through the MEK/ERK and PI3K/AKT/mTOR signaling pathways. Guo et al[21]observed that cells with mutated KRAS require autophagy to maintain oxidative metabolism and tumorigenesis when nutrients are limiting.
The present study demonstrated that CRC tissues overexpressed LC3 compared with normal colorectal tissues, with a sensitivity and specificity of 71.4% and 91.3%,respectively, indicating that LC3 might be a means of distinguishing between normal and cancer tissues. This finding was supported by the results of a study involving 163 gastrointestinal cancer patients in which LC3 was differentially expressed in the cytoplasm of cancer cells and normal epithelial cells[22]. The overexpression of LC3 could be interpreted in several ways. During the development and progression of cancers, cancer cells are often exposed to metabolic stress, such as insufficient nutrients or oxygen, because of their high proliferation rate and insufficient vascularization[23]. It is thought that normal cells exhibit a basal level of autophagy to maintain cellular homeostasis. Moreover, increased autophagy in CRC cells may also play a crucial role in tumor metastasis and survival[24]. We observed that Beclin1 and LC3 (0.657, P < 0.001) were significantly correlated in CRC tissue, and Beclin1 and 4EBP1 (0.211, P = 0.042) were also significantly correlated. These results are the same as the conclusions drawn in a previous study[25]and our experimental results in vitro.
As a type of programmed cell death, autophagy is closely related to chemotherapy resistance[26]. Stud ies have show n that autop hagy occurs abnormally in the chemotherapy courses for liver cancer, leukemia, lung cancer, CRC, and other tumors[27-29]. In the present study, we attempted to identify the proteins involved in autophagy that could predict the efficacy of cetuximab. We observed that Beclin1,LC3, and 4E-BP1 expression levels w ere not related to the ORR, DCR, or PFS.Interestingly, the prognosis of patients w ith ACRC who received cetuximab pluschemotherapy was not only correlated with clinical and pathological factors, such as the degree of pathological grade and T stage but also with Beclin1 and 4E-BP1 expression levels. A high expression level of Beclin1 and low expression level of 4EBP1 independently predicted longer OS in ACRC patients, which might indicate a positive relationship between autophagy and cetuximab efficacy. This finding is consistent with our results in colon cancer cells, which showed that autophagy was enhanced in cells treated with cetuximab, and autophagy increased the effect of cetuximab on inhibiting cell proliferation. It is necessary to design a more rigorous clinical trial to confirm our primary results. We believe that both Beclin1 and 4E-BP1 could be used as independent predictors of cetuximab efficacy.
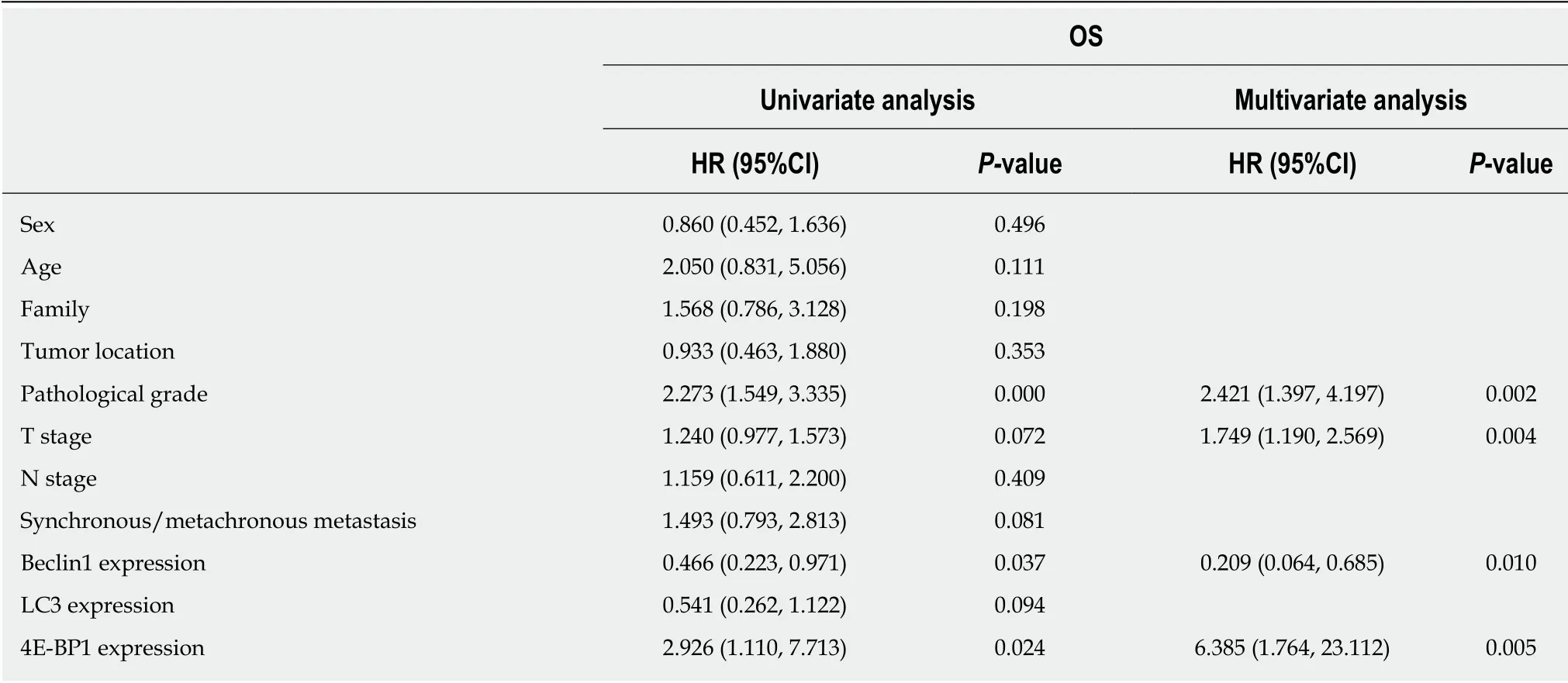
Table 3 Univariate and multivariate analyses of the influence of Beclin1, LC3, 4E-BP1, and clinicopathological factors on survival in 45 patients with wild-type KRAS who were treated with cetuximab
Our study had several shortcomings. First, it was retrospective, and the number of cases was small. Second, our study focused on the associations between the levels of expression of Beclin-1, LC3, and 4E-BP1 and the efficacy of cetuximab in patients with ACRC with wild-type KRAS; the statuses of NRAS and HRAS were not available.However, we identified two new independent predictors of the efficacy of cetuximab treatment in ACRC patients with wild-type KRAS, Beclin1 and 4E-BP1, and, to the best of our knowledge, our report of the association between 4E-BP1 and cetuximab is novel. Furthermore, our data were obtained from the clinical setting; therefore, the findings are important despite the small sample size.
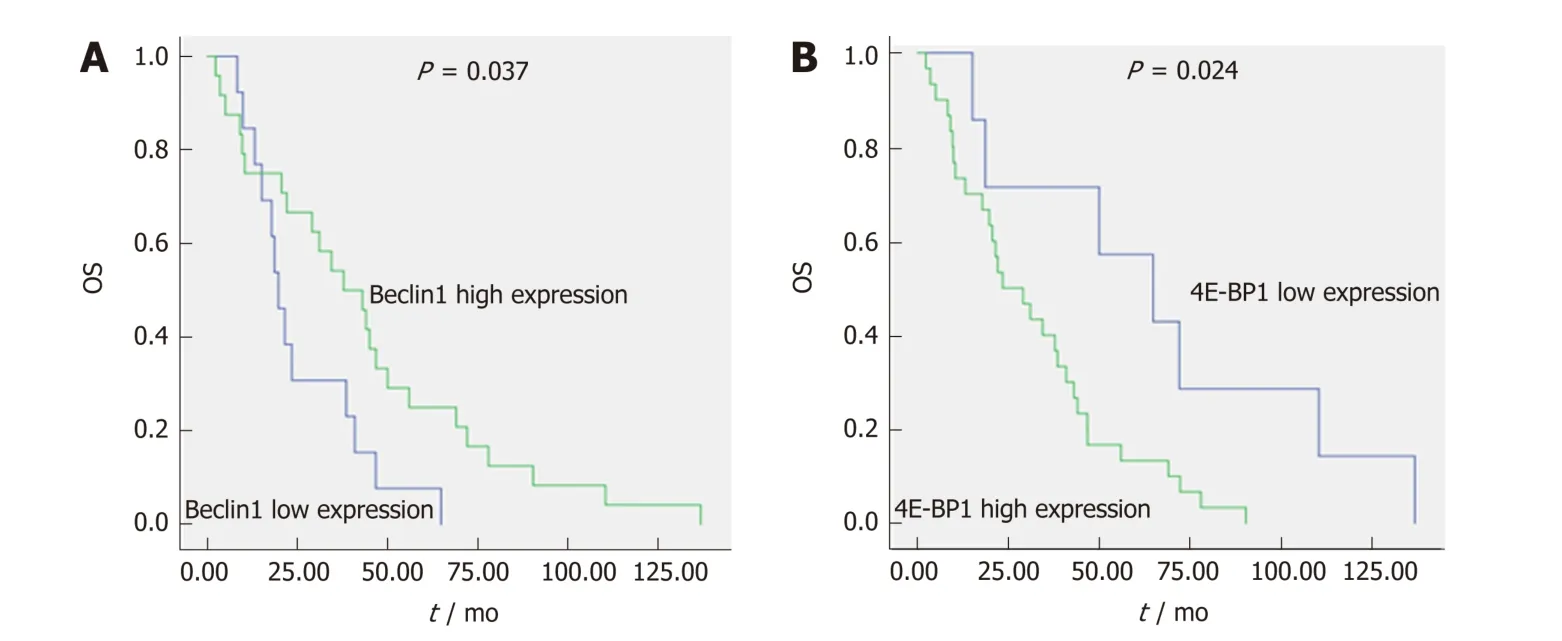
Figure 4 The expression levels of Beclin1 and 4E-binding protein 1 are independent prognostic factors for overall survival of advanced colorectal cancer patients initially treated with cetuximab-containing chemotherapy. A: The median overall survival (OS) of patients with low and high Beclin1 expression (low expression was defined as an immunohistochemistry score ≤ 0, and high expression was defined as a score > 0) was 19.65 mo vs 37.82 mo, respectively. B: The median OS of patients with low and high 4E-BP1 expression (low expression was defined as an immunohistochemistry score ≤ 0, and high expression was defined as a score > 0) was 64.82 mo vs 23.66 mo, respectively. OS: Overall survival; 4E-BP1: 4E-binding protein 1.
ARTICLE HIGHLIGHTS
Research background
Great effects have been made in exploring the treatment of colorectal cancer (CRC), but the effectiveness is still limited due to the limited drug selection. Cetuximab, a monoclonal antibody targeting epidermal growth factor receptor (EGFR), significantly increased OS (overall survival)of advanced CRC patients (ACRC). But the response rate (RR) is only 40%-60% for patients with wild-type KRAS. Autophagy, showing a key role in the cancer progression and induced by EGFR siRNA, may allow to develop effective strategies for improving cetuximab effect in CRC,while there have been few studies regarding the predictive role of autophagy in ACRC patients with wild-type KRAS.
Research motivation
Autophagy-related factors were investigated in ACRC with wild-type KRAS to find new biomarkers for cetuximab efficacy, which may offer the potential for developing novel therapeutic strategies for these patients.
Research objectives
The role of Beclin1, microtubule-associated protein 1A/B-light chain 3 (LC3), and 4E-binding protein 1 (4E-BP1), the key factors in autophagy, in predicting the efficacy of cetuximab in ACRC with wild-type KRAS was explored. It was found that Beclin1 and 4E-BP1 were independent prognostic factors for overall survival (OS). In the future research, elucidating the molecular mechanism of association of these factors with cetuximab may help us find more new biomarkers and make cetuximab treatment more accurate.
Research methods
We detected the expression of autophagy-related proteins 4E-BP1, Beclin-1, and LC3 in CRC samples and adjacent non-tumor tissues by immunohistochemistry. And the three proteins were examined by Western blot in CRC cells. The effect of autophagy on cetuximab-treated cancer cells was confirmed by MTT assay. The associations of Beclin1, LC3, and 4E-BP1 expression in tumor tissue with the efficacy of cetuximab-based therapy were analyzed.
Research results
Autophagosome formation was observed in colon cancer cells, with LC3 and 4E-BP1 upregulated, and autophagy increased the efficacy of cetuximab. Beclin1 expression was significantly correlated with LC3 and 4E-BP1 expression in ACRC tissues. LC3 was significantly overexpressed in tumor tissues compared to normal tissues. In patients with wild-type KRAS,the expression levels of Beclin1 and 4E-BP1 were independent prognostic factors for OS. The molecular mechanism of association of these factors with cetuximab effect and their weight coefficients in prognostic models need further study.
Research conclusions
LC3 is overexpressed in tumor tissues, and Beclin1 and 4E-BP1 are significant predictors of OS in wild-type KRAS ACRC treated with cetuximab. Autophagy has a role in improving the effects of cetuximab on colon cancer cell.
Research perspectives
Autophagy might play a new role to predict the effects of cetuximab in ACRC with wild-type KRAS in the future, and inducing or blocking autophagy may be a new way to improve cetuximab treating ACRC.
杂志排行
World Journal of Gastroenterology的其它文章
- Repurposing drugs to target nonalcoholic steatohepatitis
- Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development
- Considerations of elderly factors to manage the complication of liver cirrhosis in elderly patients
- Lysyl oxidase and hypoxia-inducible factor 1α: biomarkers of gastric cancer
- Extract of Cycas revoluta Thunb. enhances the inhibitory effect of 5-f luorouracil on gastric cancer cells through the AKT-mTOR pathway
- Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation
