Repurposing drugs to target nonalcoholic steatohepatitis
2019-05-08SilviaSookoianCarlosPirola
Silvia Sookoian, Carlos J Pirola
Abstrac t Nonalcoholic fatty liver disease (NAFLD) is a complex disorder that has evolved in recent years as the leading global cause of chronic liver damage. The main obstacle to better disease management pertains to the lack of approved pharmacological interventions for the treatment of nonalcoholic steatohepatitis(NASH) and NASH-fibrosis-the severe histological forms. Over the past decade,tremendous advances have been made in NAFLD research, resulting in the discovery of disease mechanisms and novel therapeutic targets. Hence, a large number of pharmacological agents are currently being tested for safety and efficacy. These drugs are in the initial pharmacological phases (phase 1 and 2),which involve testing tolerability, therapeutic action, and pharmacological issues.It is thus reasonable to assume that the next generation of NASH drugs will not be available for clinical use for foreseeable future. The expected delay can be mitigated by drug repurposing or repositioning, which essentially relies on identifying and developing new uses for existing drugs. Here, we propose a drug candidate selection method based on the integration of molecular pathways of disease pathogenesis into network analysis tools that use OMICs data as well as multiples sources, including text mining from the medical literature.
Key words: Drug discovery; Drug repositioning; Fibrosis; Genetics; Treatment; Systems biology
INTRODUCTION
Nonalcoholic fatty liver d isease (NAFLD) is a complex d isorder that has emerged as the leading global cause of chronic liver damage in recent years[1]. The disease course progresses through highly dynamic histological stages, ranging from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), NASHfibrosis and cirrhosis[1,2]. NASH-fibrosis and its complications, including cirrhosis and hepatocellular carcinoma, not only significantly reduce life expectancy by increasing liver-related mortality[3]but also rep resent a challenge for the healthcare system because much of the affected p op ulation is also affected by NAFLD-associated comorbid ities, includ ing obesity, typ e 2 d iabetes (T2D), and card iovascular disease[1,4-6]. Absence of reliable noninvasive biomarkers that allow identification of patients at a high risk of fibrosis and /or disease progression is one of the obstacles facing d isease management[7,8]. Similarly, w hile a large number of d rugs against NASH are currently being tested for efficacy and safety, no p harmacological interventions are presently approved for treating NASH[2,5,9,10].
Information retriev ed from p u blic d omain d ata sour ces and clinical ClinicalTrials.gov (updated December 2018), a resource provided by the U.S. National Library of Medicine, indicates that approximately 47 different drugs that target NASH and NASH-fibrosis are currently being tested in different pharmacological stages,includ ing 188 drugs in phase 1 and 162 in p hase 2 stud ies (Figure 1). A significant proportion of these drugs are small molecules or proteins that either antagonize or act as exogenous agonists of one or more targets of interest; the 47 aforementioned NASH d rugs are in fact p red icted to be linked to 151 molecular targets (Figure 1).Considering that a large majority of these drugs are in the earliest pharmacological phases that involve testing tolerability, therap eutic action, and p harmacological issues, it is reasonable to conclude that there will be a significant time lag before the next generation of NASH drugs is available for clinical use.
One potential solution to this expected delay is d rug repurposing or repositioning,w hich relies on id entifying and d evelop ing new uses for existing d rugs[11]. The advantage of drug repurposing is not limited to the fact that drugs selected for a novel ind ication hav e alread y p assed the time-consu ming p harmacok inetics,pharmacodynamics, and toxicity profiling evaluation, but are also already approved by major regulatory agencies, includ ing the United States Food and Drug Administration and/or the European Medicines Agency.
Drug repurposing can be add ressed by d ifferent approaches. Most common ones involve the selection of d rug candid ate/s based on know n targets involved in the pathogenesis of the disease of interest. More recently, system biology strategies based on a broad search into genomic resources, as w ell as large-scale gene expression libraries, have been proposed as an attractive and innovative solution, particularly for the treatment of complex diseases like NAFLD that shares d isease mechanisms with d iseases of the metabolic synd rome[12-14]. Hence, w e p rop ose a d rug cand id ate selection method based on the integration of molecular p athw ays of d isease pathogenesis into netw ork analysis tools that use OMICs d ata as w ell as multiples sources, including text mining from pertinent medical literature.
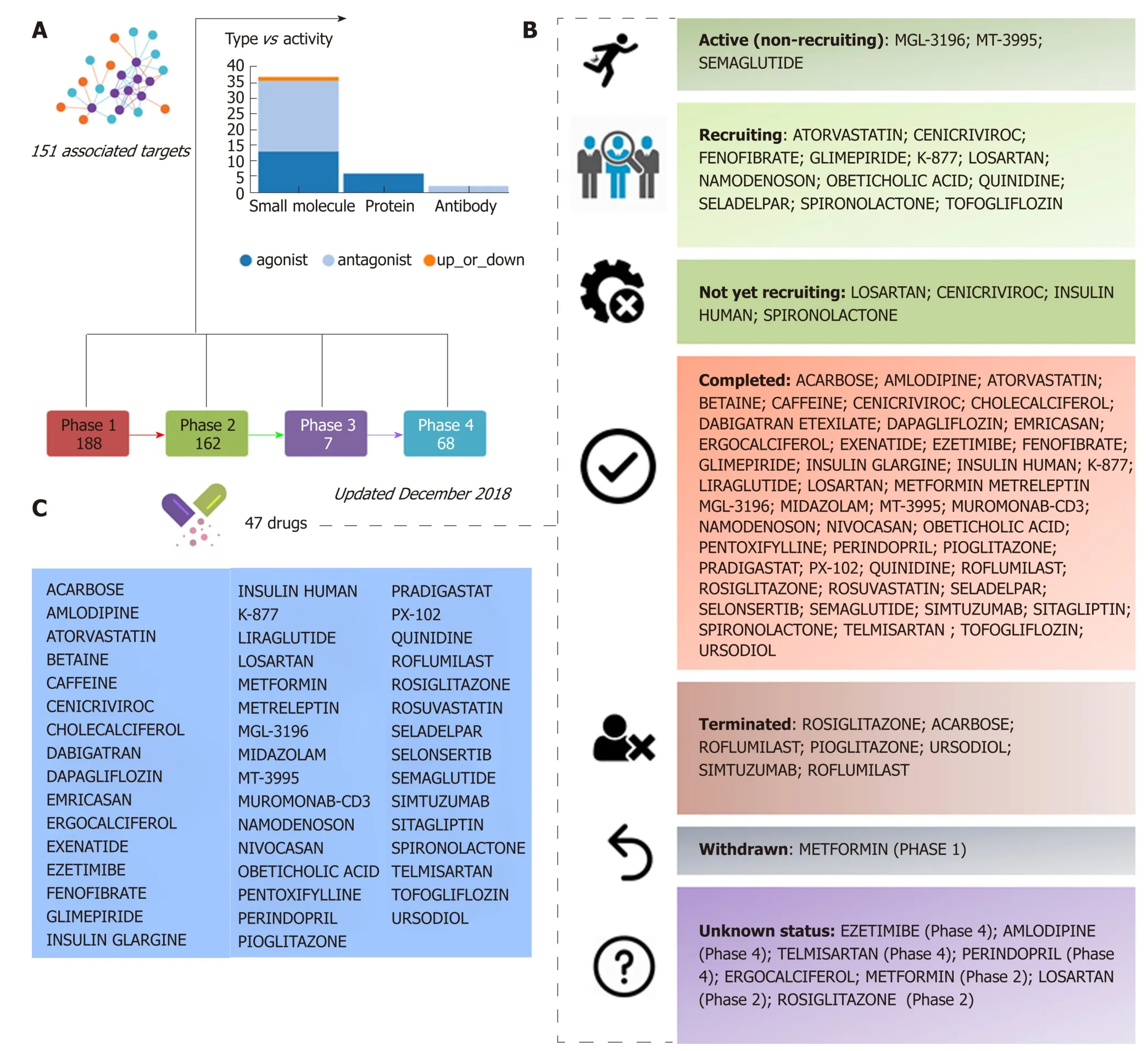
Figure 1 Clinical trials for the treatment of nonalcoholic steatohepatitis. A and B: Figure highlights 47 drugs that are currently under investigation for the treatment of nonalcoholic steatohepatitis in different pharmacological phases (from phase 1 to phase 4): Information on clinical trial status (recruitment status) as well as prediction of potential associated targets were retrieved from the Target Validation Platform available at https://www.targetvalidation.org; C: Drugs listed in the most advanced pharmacological phase updated December 2018 concerning to privately and publicly funded clinical studies. Not yet recruiting: The study has not started recruiting participants; Recruiting: The study is currently recruiting participants; Active, not recruiting: The study is ongoing, and participants are receiving an intervention or being examined, but potential participants are not currently being recruited or enrolled; Terminated: The study has stopped early and will not start again; participants are no longer being examined or treated; Completed: The study has ended normally, and participants are no longer being examined or treated (that is, the last participant's last visit has occurred); Withdrawn: The study stopped early, before enrolling its first participant; Unknown: A study on ClinicalTrials.gov whose last known status was recruiting; not yet recruiting; or active, not recruiting but that has passed its completion date, and the status has not been last verified within the past 2 years).
DRUG REPURPOSING FOR THE TREATMENT OF NASH BASED ON THE NAFLD-KEGG CONNECTIVITY MAP
As a p roof-of-concep t of the ad vantages of using multi-omics systems-based approaches for the analysis of potential NASH treatment candidates, w e selected the Kyoto Encyclop ed ia of Genes and Genomes (KEGG) p athw ay map of NAFLD(p athw ay ID: hsa04932), w hich illustrates a stage-d ep end ent p rogression of the disease (Figure 2). This pathway is composed of 149 genes/proteins involved not only in the progression of NAFL to NASH and to cirrhosis, but also genes/proteins shared w ith obesity and T2D (Table 1). Significant d isease-related pathogenic processes,including de novo fatty acid biosynthesis, lipid peroxidation, endoplasmic reticulum stress and mitochondrial dysfunction[15-17], as w ell as apoptosis and cell death related mechanisms are rep resented in the NAFLD-KEGG p athw ay (Figure 2). Thus, w e generated a p rotein-chemical interaction netw ork by map p ing the significant genes/p roteins that are rep resented in the p athw ay to chemicals/d rugs that are annotated in the Comparative Toxicogenomics Database. The 149 genes (seed s)yielded by our analysis were then mapped to the corresponding molecular interaction database; this procedure produced an extensive netw ork comprising of approximately 2000 nod es. One of the largest subnetw orks includ ed 3212 smaller nod es (that represent the number of gene/protein-chemical interactions in this subnetwork), with 13314 interactions among node members. For simplicity, we manually curated some chemical-d rug interactions focusing sp ecifically on certain genes/p roteins of potential interest, includ ing members of the casp ase family (CASP3 and CASP7),interleukins (IL1A, IL1B, and IL6), tumor necrosis factor α (TNFα), nuclear factor kappa B subunit 1 (NFKB1) and inhibitor of nuclear factor kappa B kinase subunit beta, Ju n p roto-oncogene (JUN), transcrip tion factor su bu nit, and AKT serine/threonine kinase 1 (Figure 3). Remarkably, several drugs w ere pred icted to have a significant interaction w ith the highlighted targets. For example, minocycline that is a broad spectrum long-acting d erivative of the antibiotic tetracycline w as mapped in the pathway of caspases, whereas IL1B (Figure 3) or pomalidomide that is a d eriv ativ e of thalid omid e w ith immuno-mod ulating, antiangiogenic and antineoplastic activities was mapped in the network of TNF, NFKB1, and interleukins(Figure 3).
Ad d itional targets p red icted in the minoclycline interaction netw ork are arachidonate 5-lipoxygenase (which is involved in the synthesis of leukotrienes from arachidonic acid), cytochrome C (a central component of the electron transport chain in mitochond ria), matrix metallop ep tid ase 9 (involved in the breakd ow n of extracellular matrix), v ascular end othelial grow th factor A (w hich ind uces p roliferation and migration of vascular end othelial cells, p articularly d uring pathological angiogenesis) and Poly(ADP-ribose) polymerase 1 (which is involved in the regulation of a myriad of cellular processes, such as differentiation, proliferation,and tumor transformation, as well as in the regulation of the molecular events implicit in the cell recovery from DNA d amage). Further two candidate targets pred icted in the netw ork of p omalid omid e are p rostagland in-end op eroxid e synthase 2 (also know n as cyclooxygenase, w hich is the key enzyme in prostaglandin biosynthesis)and CRBN (a calcium channel membrane p rotein, thought to play a role in brain development).
Additional examples of drugs that could be potentially tested for the treatment of NASH based on the concept of drug repositioning are illustrated in Figure 3. Drugs in the category of angiotensin II recep tor typ e 1 (AGTR1) antagonists that w ere p red icted in the netw ork of JUN, for instance irbersartan-a nonpep tid e AGTR1 antagonist w ith antihypertensive activity-might indeed be regarded as an indication expansion rather than drug repositioning because, as mentioned above, NAFLD and components of the Metabolic Synd rome, including arterial hypertension, present shared disease mechanisms (12-14). Therefore, given the pleiotropic effects of AGTR1 blockers[18]it is plausible to suggest that drugs in this pharmacological group-sartanswould synergize or potentiate the benefits of blocking the renin angiotensin system in the liver[19-22]. Remarkably, the pharmacological properties and toxicity profiles of some of the drugs presently undergoing NASH clinical trials are already known, such as atorvastatin, ezetimibe, fenofribrate, losartan, and pioglotazone, just to mention a few (Figure 1).
PLEIOTROPY: CHALLENGES AND OPPORTUNITIES FOR THE TREATMENT OF NASH
It is also important to acknow led ge the possibility that some of the novel pharmacotherapy options for the treatment of NASH might eventually present pleiotropic effect/s. This point represents the paradox of a drug covering multiple pathw ays and cell types, which could be either harmful or beneficial for patients.Remarkable examples of the advantages of pleiotropic effects of pharmacological targets for the treatment of complex traits are, as already mentioned, agents that modulate or interfere with the rennin-angiotensin system, which not only reduce cardiovascular risk but also improve systemic inflammation, oxidative stress, and even present anti-fibrogenic properties in the liver. Similar effects have also been demonstrated for statins[23,24].
When focusing on the new generation of NASH targets, obeticholic acid (OCA), a synthetically-modified bile acid (a dihydroxy-5beta-cholanic acid), is a remarkable example of the potential systemic effects of a drug targeting nuclear receptors. OCA exhibits a potent agonist effect on the farnesoid X nuclear receptor (FXR). Moreimportantly, its target-FXR (formally Nuclear hormone receptor subfamily 1 group H member 4, NR1H 4, also know n as BAR) is p red icted to be inv olved in the pathogenesis of multiple phenotypes that practically cover the full range of human diseases and traits (Figure 4). It is w ell known that OCA is currently used to treat not only NASH but other chronic liver d iseases as w ell, includ ing p rimary biliary cholangitis[25]. How ever, there are at least 65 registered clinical trials in various pharmacological phases for ~50 different diseases (Figure 4).

Table 1 Non-alcoholic fatty liver disease-Kyoto Encyclopedia of Genes and Genomes pathway(hsa04932)

JUN; Jun proto-oncogene, AP-1 transcription factor subunit IL1A; interleukin 1 alpha IL1B; interleukin 1 beta IKBKB; inhibitor of nuclear factor kappa B kinase subunit beta XBP1; X-box binding protein 1 CEBPA; CCAAT enhancer binding protein alpha CYP2E1; cytochrome P450 family 2 subfamily E member 1 FASLG; Fas ligand CXCL8; C-X-C motif chemokine ligand 8 TGFB1; transforming growth factor beta 1 EIF2AK3; eukaryotic translation initiation factor 2 alpha kinase 3 EIF2S1; eukaryotic translation initiation factor 2 subunit alpha ATF4; activating transcription factor 4 DDIT3; DNA damage inducible transcript 3 BCL2L11; BCL2 like 11 BAX; BCL2 associated X, apoptosis regulator FAS; Fas cell surface death receptor CASP8; caspase 8 BID; BH3 interacting domain death agonist CYCS; cytochrome c, somatic CASP3; caspase 3 CASP7; caspase 7 NDUFV1-3; NADH:ubiquinone oxidoreductase core subunit V1 -V3 NDUFA1-3; NADH:ubiquinone oxidoreductase subunit A1-3 NDUFA4; NDUFA4, mitochondrial complex associated NDUFA4L2; NDUFA4, mitochondrial complex associated like 2 NDUFA5-13; NADH:ubiquinone oxidoreductase subunit A5-A13 NDUFAB1; NADH:ubiquinone oxidoreductase subunit AB1 NDUFB1-11; NADH:ubiquinone oxidoreductase subunit B1-B11 NDUFS1-S8; NADH:ubiquinone oxidoreductase core subunit S1 -S8 NDUFC1; NADH:ubiquinone oxidoreductase subunit C1 NDUFC2; NADH:ubiquinone oxidoreductase subunit C2 NDUFC2-KCTD14; NDUFC2-KCTD14 readthrough SDHA; succinate dehydrogenase complex flavoprotein subunit A SDHB; succinate dehydrogenase complex iron sulfur subunit B SDHC; succinate dehydrogenase complex subunit C SDHD; succinate dehydrogenase complex subunit D UQCRFS1; ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 CYTB; cytochrome b CYC1; cytochrome c1 UQCRC1; ubiquinol-cytochrome c reductase core protein 1 UQCRC2; ubiquinol-cytochrome c reductase core protein 2 UQCRH; ubiquinol-cytochrome c reductase hinge protein UQCRHL; ubiquinol-cytochrome c reductase hinge protein like UQCRB; ubiquinol-cytochrome c reductase binding protein UQCRQ; ubiquinol-cytochrome c reductase complex III subunit VII UQCR10; ubiquinol-cytochrome c reductase, complex III subunit X UQCR11; ubiquinol-cytochrome c reductase, complex III subunit XI COX3; cytochrome c oxidase III COX1; cytochrome c oxidase subunit I COX2; cytochrome c oxidase subunit II COX4I2; cytochrome c oxidase subunit 4I2 COX4I1; cytochrome c oxidase subunit 4I1 COX5A; cytochrome c oxidase subunit 5A COX5B; cytochrome c oxidase subunit 5B COX6A1; cytochrome c oxidase subunit 6A1
https://www.genome.jp/kegg-bin/show_pathway? hsa04932.
Based on this evidence, one may presume that the pleiotropic effects, and thus the clinical consequences, of the novel NASH d rugs that are p redicted to concurrently modulate a broad range of molecular pathways could be surprisingly extensive and therefore largely beneficial for treating multip le phenotyp es. How ever, p otential pleiotropic effects of the novel anti-NASH drugs could produce undesirable effects that w e need to understand in ord er to anticipate their management. Some of these potential pleiotropic effects are indeed related to the primary biological and molecular netw ork associated w ith the drug target itself. To illustrate the importance of this issue, w e rand omly selected five molecular targets (MAP3K5 or ASK1, FXR,PPARα/δ, THRβ, and MPC1) against w hich five drugs are currently being tested in p atients w ith NASH (selon sertib[26], OCA[27], elafibranor[28], M GL-3196(http s://clinicaltrials.gov/ct2/show/NCT02912260), and MSDC-0602K[29]https://clinicaltrials.gov/ct2/show/NCT02784444). Next, w e explored the potential p leiotrop ic effect/s of mod ulating these targets in humans by searching for associations of genetic variants in the aforementioned targets w ith d ifferent phenotypes and traits, know n as PheWAS (Phenome-w id e association studies). We sp ecifically retrieved p ublically available information from the United Kind om Biobank that exp lored genetic variations in 452264 United Kind om Biobank White British individuals (http://geneatlas.roslin.ed.ac.uk/)[30].
As show n in Figure 5 and Table 2, MAP3K5/ASK1, FXR, PPARα/δ, THRβ, and MPC1 variants are involved in multiple pleiotropic effects, including modulation of blood cell count, bod y mass ind ex, and general bod y ad ip osity, along w ith comp lex systemic d isord ers, such as asthma, acute p ancreatitis, migraine, intestinal malabsortium, thyroid disease, and malignant neoplasm. Hence, understanding the pleiotropic effects of the novel NASH d rugs is the key to optimizing their use as w ell as preventing emergent-yet poorly und erstood-und esirable systemic complications that could potentially jeopardize their short- or long-term use.
CONCLUSION
We provide new strategies and approaches by which known drugs can be repurposed for the treatment of NASH. Although we explored and mapped NAFLD-chemical interaction networks, it will be necessary to perform clinical trials not only to assess therapeutic response and optimize dosage and delivery routes, but also to explore the possibility that new uses of existing (old) drugs could act on novel or unanticipated targets. The presence of potential “off target”-pleiotropic-effects raises the mandatory necessity of pharmacological optimization, including the assessment of drug interactions and adjustment according to liver function tests.
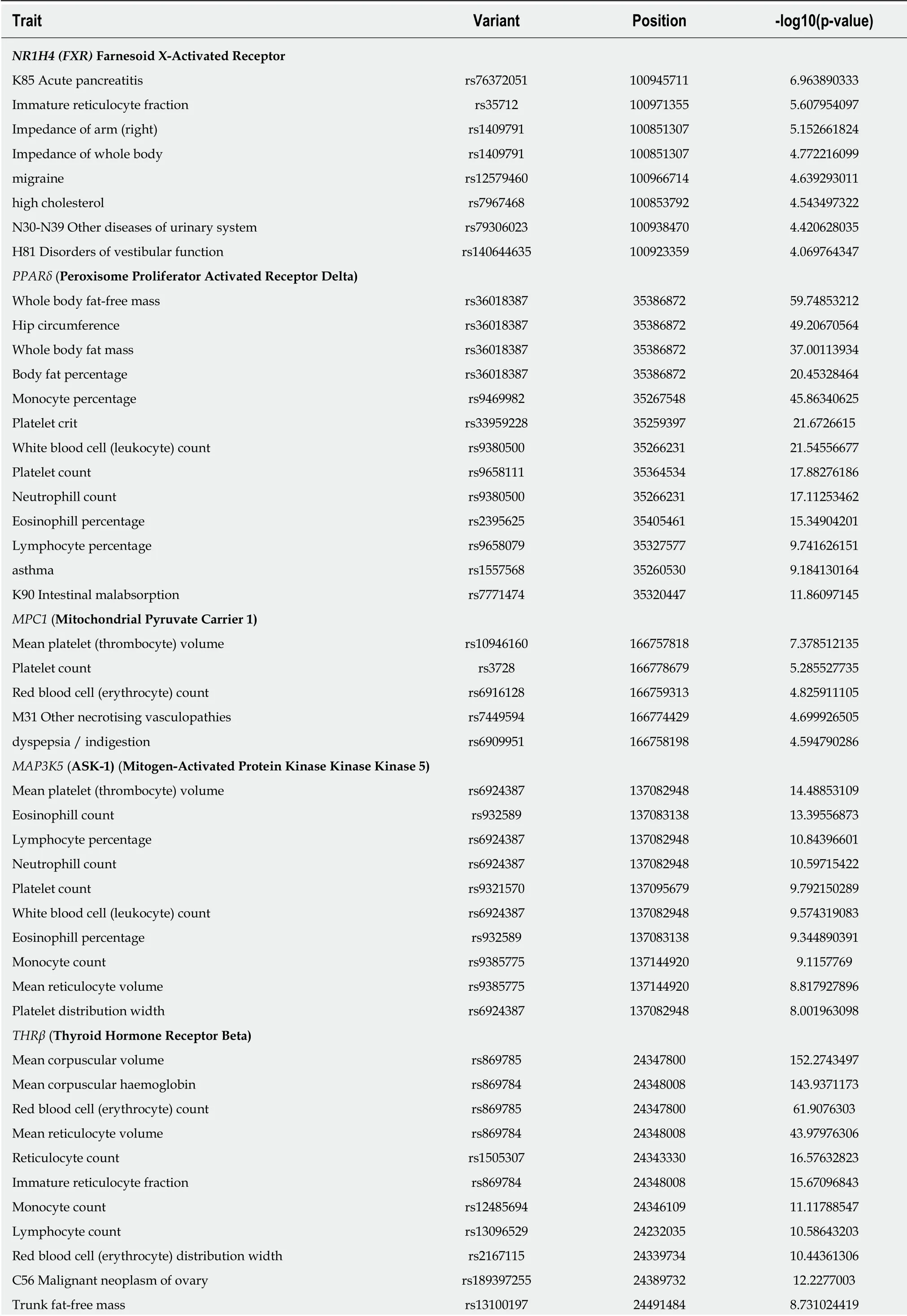
Table 2 Associations between variants in locus that are targets of novel drugs for the treatment of nonalcoholic steatohepatitis and multiple traits from individuals of the United Kindom Biobank

The associations have been computed using 452264 United Kindom Biobank White British individuals. http://geneatlas.roslin.ed.ac.uk/.
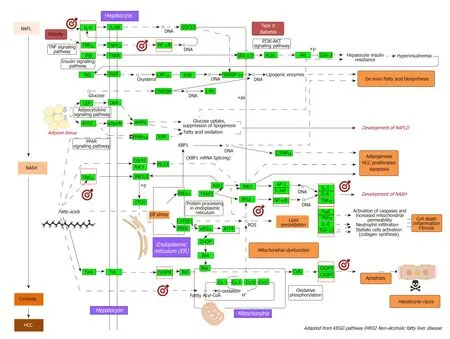
Figure 2 Nonalcoholic fatty liver disease-Kyoto Encyclopedia of Genes and Genomes pathway and mechanisms of disease pathogenesis. Pathway was retrieved from https://www.genome.jp/dbget-bin/www_bget?pathway+hsa04932; figure was modified to highlight key molecular processes. This map shows a stagedependent progression of nonalcoholic fatty liver disease (NAFLD). In the first stage of NAFLD, pathway highlights excess lipid accumulation associated with the induction of insulin resistance, which leads to a defect in insulin suppression of free fatty acids (FAAs) disposal. In addition, two transcription factors, SREBP-1c and PPARα, activate key enzymes of lipogenesis and increase the synthesis of FAAs in liver. In the second stage, pathway is presented as a consequence of the progression to nonalcoholic steatohepatitis (NASH); the production of reactive oxygen species is enhanced due to oxidation stress through mitochondrial betaoxidation of fatty acids and endoplasmic reticulum (ER) stress, leading to lipid peroxidation. The lipid peroxidation can further cause the production of cytokines [Fas ligand, tumor necrosis factor α (TNF-α), IL-8 and transforming growth factor], promoting cell death, inflammation and fibrosis. The activation of JNK, which is induced by ER stress, TNF-α and FAAs, is also associated with NAFLD progression. Increased JNK promotes cytokine production and initiation of hepatocellular carcinoma.Major organelles involved in the pathogenesis of NASH are also highlighted in the NAFLD-pathway, including mitochondria and mitochondrial dysfunction. In the figure, molecular targets that were further selected to explore protein-chemical interactions are highlighted by red squares. NAFLD: Nonalcoholic fatty liver disease;NASH: Nonalcoholic steatohepatitis; ER: Endoplasmic reticulum; HCC: Hepatocellular carcinoma; NAFL: Nonalcoholic fatty liver; FAAs: Free fatty acids; TNFα: tumor necrosis factor α.
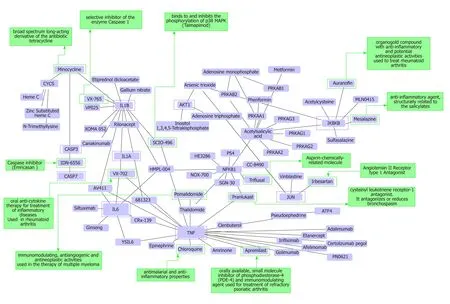
Figure 3 Protein-chemical interactions and potential repurposing drugs to target nonalcoholic steatohepatitis. We generated a protein-chemical interaction network by mapping the significant genes/proteins that are represented in the nonalcoholic fatty liver disease-Kyoto Encyclopedia of Genes and Genomes pathway to chemicals/drugs that are annotated in the Comparative Toxicogenomics Database. The 149 genes (seeds) from our analysis were mapped to the corresponding molecular interaction database; full list of seed genes is listed in Table 1. This analysis generated a huge network composed of approximately 2000 nodes. Current figure shows chemical-drug-interactions specifically focused on selected genes/proteins of potential interest, including members of the caspase family (CASP3 and CASP7), interleukins (IL1B, IL1A, and IL6), tumor necrosis factor α (TNF-α), NFKB1 (Nuclear factor kappa B subunit 1) and IKBKB (inhibitor of nuclear factor kappa B kinase subunit beta), JUN (Jun proto-oncogene, AP-1 transcription factor subunit), AKT1 (AKT serine/threonine kinase 1). In green charts we summarized information on current use and known action of selected drugs. Interaction network was predicted by the Networkanalyst resource available at https://www.networkanalyst.ca/faces/home.xhtml. The network is shown as a Cytoscape graph.
杂志排行
World Journal of Gastroenterology的其它文章
- Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development
- Considerations of elderly factors to manage the complication of liver cirrhosis in elderly patients
- Lysyl oxidase and hypoxia-inducible factor 1α: biomarkers of gastric cancer
- Predictive and prognostic implications of 4E-BP1, Beclin-1, and LC3 for cetuximab treatment combined with chemotherapy in advanced colorectal cancer with wild-type KRAS: Analysis from real-world data
- Extract of Cycas revoluta Thunb. enhances the inhibitory effect of 5-f luorouracil on gastric cancer cells through the AKT-mTOR pathway
- Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation
