赤霉素GA4是水稻矮化特征的重要调节因子
2019-03-29黄升财王冰谢国强刘中来张美娟张树清程宪国
黄升财,王冰,谢国强,刘中来,张美娟,张树清,程宪国
赤霉素GA4是水稻矮化特征的重要调节因子
黄升财1,王冰1,谢国强2,刘中来2,张美娟1,张树清1,程宪国1
(1中国农业科学院农业资源与农业区划研究所,北京 100081;2九江农业科学院,江西九江 332101)
【目的】以水稻幼胚组培过程中获得的一株半矮化水稻突变体为研究对象,解析水稻半矮化突变体株高变矮及分蘖增多等表型异常的原因,为克服水稻过度矮化发育障碍因子及培育抗倒伏高产水稻品种提供科学理论依据。【方法】首先统计分析半矮化水稻突变体与野生型的表型差异,利用体式显微镜和光学显微镜观察突变体花的结构及其细胞特征;通过转录组测序及qRT-PCR分析差异基因的表达特征,并通过外源喷施赤霉素GA3处理检测突变体对外源赤霉素的敏感性;最后利用高效液相色谱和质谱联仪检测突变体内赤霉素的含量与富集特征。【结果】表型观测与统计结果表明,突变体水稻株高比野生型减少56.59%,有效分蘖数高出47.44%,差异均达到极显著水平。突变体的表皮毛消失且花发育迟缓,雄蕊变小。尽管突变体分蘖数较高但结实率明显降低,仅为野生型的12.62%,且种子长度和宽度均减小,差异极显著。通过显微镜观察茎的纵切切片,发现突变体细胞长度减少23%,差异极显著。外源喷施赤霉素后突变体的株高、有效分蘖、结实率、种子大小、表皮毛和茎秆细胞长度均有不同程度的恢复,说明植物体内赤霉素合成不足可能是引起水稻矮化的主要原因。转录组测序结果显示突变体中显著上调,qRT-PCR验证结果与转录组测序结果一致。由于OsGA13ox控制GA12转化为GA53,而GA12和GA53分别转化为GA4和GA1,GA4的活性高于GA1,因此,突变体中GA4减少可能是导致半矮化的主要原因。赤霉素检测结果表明突变体中GA4含量减少94.9%,与预测结果一致。此外,D14作为SL(独脚金内酯)的特异性受体,参与调控植物SL信号转导,抑制枝条分枝或者分蘖。qRT-PCR结果显示,与野生型相比,突变体中显著下调,而经过GA3处理的野生型和突变体中均显著上调。上调可能导致有效分蘖数减少,而其下调可能致使有效分蘖数增加。统计结果表明突变体中有效分蘖显著增多,而经过GA处理之后,野生型和突变体有效分蘖数均显著低于未经GA3处理前,表明在水稻中的表达可能受到GA的调控从而影响水稻分蘖。【结论】异常表达导致活性更高的GA4在水稻中的富集减少,形成水稻半矮化突变体;赤霉素可能通过影响的表达间接调控水稻的分蘖。
水稻;半矮化突变体;;赤霉素GA4;;有效分蘖
0 引言
【研究意义】水稻是人类生存至关重要的粮食作物之一,水稻产量通常由分蘖数、穗粒数、单粒重及株高等农艺性状决定。随着人口压力逐渐增加,耕地面积逐渐减小,气候和环境恶化等问题的出现,人类对粮食产量和质量的要求愈来愈高。探明水稻矮化和分蘖增多的分子机制,为培育抗倒伏及高有效分蘖的水稻品种提供科学的理论支持,对保证人类粮食安全具有重要的意义。【前人研究进展】植物生长高度在很大程度上受内源激素的影响,包括赤霉素[1-2]、油菜素类固醇[3-4]以及独脚金内酯[5-6]等。其中赤霉素(GAs)是一类二萜类化合物,可促进植物生命周期各个阶段的生长:种子萌发、茎伸长、叶片膨大、开花和花发育[7]。赤霉素的合成受古巴焦磷酸合成酶(copalyl pyrophosphate synthase,CPS)、内根-贝壳杉烯合成酶(ent-kaurene synthase,KS)、内根-贝壳杉烯19-氧化酶(ent-kaurene oxidase,KO)、GA-20-氧化酶(gibberellin- 20-oxidase,GA20ox)及GA-3-氧化酶(gibberellin-3- oxidase,GA3ox)等多个酶统一调控[8]。在拟南芥中,(CPS突变体)[9]、(KS突变体)[10]、(KO突变体)[11]均出现矮化现象。水稻赤霉素合成基因[12]、()[13]及()[1]突变也会使水稻出现不同程度的矮化。一般情况下,赤霉素合成突变体除了株高会发生变化之外,另外一个特征是对外源GA敏感。而水稻[14]和[15]突变体均对外源GA不敏感,表明GID1(赤霉素特异性受体)和SLR1(DELLA蛋白)参与植物体内赤霉素信号的转导。相关研究表明,当细胞外GA浓度较低时,GID1不与GA结合,其N端结构域(N-Ex)呈疏松状态,使DELLA蛋白与GA早期应答基因结合,并通过抑制应答基因的翻译过程进而使其失活。当细胞外GA浓度较高时,GA诱导GID1构象发生变化,并与其结合形成GA-GID1复合物。该复合物具有疏水性的表面以便与DELLA蛋白形成三聚体,促进DELLA与泛素E3连接酶复合体结合,诱导DELLA蛋白被26s蛋白酶降解,进而GA信号得以释放[16]。矮化突变体除了株高变矮之外,通常还伴随着花发育异常和分蘖增多等现象。尹昌喜[17]研究表明下调表达及上调表达导致穗茎节间GA1含量减少是水稻出现半矮化和包穗的原因。而张玲[18]和WANG等[19]分别利用图位克隆和BSA鉴定了新的基因和,这两个基因的突变均能导致水稻半矮化且花发育异常。独脚金内酯(strigolactone,SL)是一组类胡萝卜素衍生的内酯,拟南芥多丛枝突变体[20]及水稻多分蘖突变体[21]均证实了SL在抑制腋芽生长方面具有保守作用。【本研究切入点】笔者在转基因水稻中获得稳定遗传的半矮化突变体,与已被鉴定的其他水稻矮化植株的表型有较大差异。【拟解决的关键问题】本研究利用转录组学及生物信息学分析确定造成突变体表型异常的主要基因,并利用高效液相色谱-质谱联仪对突变体内的激素进行检测,与转录组测序结果相互验证。矮化成因的解析对克服过度矮化障碍因子及培育抗倒伏高产水稻品种具有理论与应用价值。
1 材料与方法
1.1 突变体材料
在水稻日本品种Kitaake()幼胚组培过程中,获得一株半矮化突变体,通过网室自然条件下土壤培养3代之后,未出现性状分离,表型稳定,突变体用(13)表示。
1.2 突变体表型分析
挑选发育饱满的野生型和突变体种子于玻璃培养皿中,自来水浸泡,置于30℃恒温培养箱中暗发芽1 d。第2天用湿润的滤纸代替自来水继续培养,第4天转移到光照培养箱中,光照条件为白天16 h 28℃/黑夜8 h 22℃。第5天转移到土壤中,水稻土和蛭石比例为2﹕1,培养白盆的尺寸54 cm×35 cm×12 cm(长×宽×高),土壤重量为4 kg,每天上午和下午各浇一次水。培养地点是中国农业科学院资源区划所东区网室。
在水稻生长至抽穗期时,用直尺量取地上部高度(5次生物学重复)。选取5株水稻进行有效分蘖数统计。利用立体式显微镜(LECIA M165 FC,德国)观察水稻小穗及花内部构造的差异。小穗采自穗的同一位置,然后用镊子将外稃和内稃分开露出雄蕊和雌蕊。选取穗茎节间的茎,用刀片刮至茎透明为止,刮的时候需滴两滴蒸馏水以确保茎保持湿润,然后利用光学显微镜(LEICA DM6 B,德国)观察细胞大小,并统计30个细胞的长度。在水稻成熟之后,选取10株统计其结实率。然后用游标卡尺测量种子长和宽,重复6次。
1.3 转录组测序
对苗期生长3周的水稻进行外源GA处理,喷施20 ml浓度为1.5×10-4mol·L-1的GA3,3 d喷施1次,共3次,于喷施后第8天选取水稻第2片叶进行转录组测序,所有处理均按3次生物学重复进行。首先用Easy Pure Plant RNA(北京全式金)试剂盒提取RNA,然后用带有Oligo(dT)的磁珠富集mRNA,加入fragmentation buffer将mRNA打断成短片段,随后,用六碱基随机引物(random hexamers)以mRNA为模板进行反转录合成一链cDNA,再加入缓冲液、dNTPs和DNA聚合酶Ⅰ合成二链cDNA。其次利用AMPure XP beads纯化双链cDNA,对纯化后的双链cDNA进行末端修复、加A、加接头。通过AMPure XP beads对双链cDNA进行片段大小选择,最后进行PCR扩增以构建cDNA文库。使用Agilent 2100对文库的插入片段大小进行检测,文库质检合格后,利用Illumina高通量测序平台进行测序。
使用DEseq和DEseq2检测转基因与野生型株系之间的差异表达基因,差异基因筛选的条件为:差异倍数≥2和Q值(或FDR)≤0.01。然后使用Gene Ontology(GO富集)[22]和KEGG富集[23]对差异基因进行分析。
1.4 突变体外源GA敏感性检测
土培水稻外源喷施GA处理与转录组测序的水稻一致,在转录组测序取样之前,观察并记录表型差异。水培水稻外源GA处理是待水稻在恒温培养箱中发芽3 d后,进行水培,营养液(Hoagland,pH5.8)3 d更换1次,并在营养液中分别添加浓度为1.5×10-4、3×10-5和6×10-6mol·L-1的GA3,10 d后,观察突变体的表型。
1.5 qRT-PCR分析
待水稻生长至孕穗期,进行外源GA处理,与转录组测序取样之前的处理方式相同,之后选取花、穗茎节间的茎及第2片叶提取RNA。首选用液氮进行研磨,之后用Easy Pure Plant RNA试剂盒(北京全式金)进行RNA提取。将提取后的RNA用浓度测定仪(Nanodrop 2000,美国)测定其浓度。利用反转录试剂盒Transscript One-step gDNA Removal and cDNA Synthesis SuperMix(北京全式金)将1.5 μg RNA反转录为cDNA。使用ChamQTM Universal SYBR qPCR Master Mix(南京诺唯赞)进行荧光定量PCR,总反应溶液体积为20 μL,包括10 μL 2×ChamQTM Universal SYBR qPCR Master Mix、0.4 μL正反引物(表1)、2 μL cDNA模板和7.2 μL无RNA酶水,重复3次。使用ABI 7500(美国)荧光定量PCR仪进行定量PCR扩增,PCR扩增条件为94℃ 30 s;95℃ 10 s,60℃ 30 s,40个循环。以作为内参,扩增结束后使用2-ΔΔCt公式计算相对表达量。
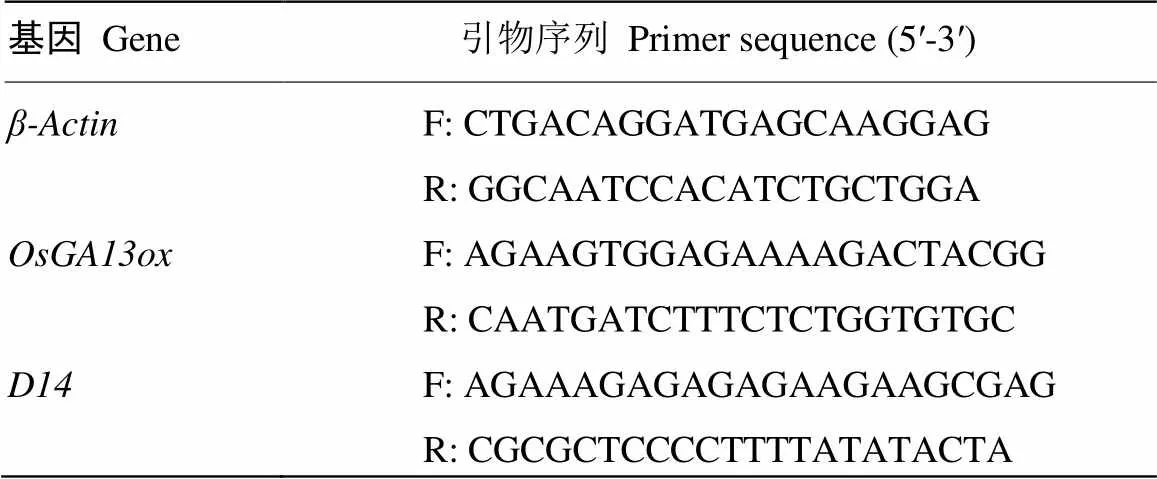
表1 荧光定量PCR引物
1.6 赤霉素含量检测
1.6.1 赤霉素的提取 称取新鲜水稻叶片约1 g于液氮中研磨粉碎,加入10 ml异丙醇/盐酸提取缓冲液和8 μl 1 μg·mL-1内标溶液,4℃震荡30 min;随后加入20 ml二氯甲烷,4℃震荡30 min;之后13 000 r/min(4℃)离心5 min,取下层有机相,避光用氮吹仪(杭州美欧)吹干有机相,用400 μl甲醇(0.1%甲酸)溶解,0.22 μm滤膜过滤,最后用高效液相色谱(Aglient1290,美国Aglient)-质谱(SCIEX-6500Qtrap,美国AB)联仪检测。
1.6.2 标准曲线绘制 以甲醇(0.1%甲酸)为溶剂配制梯度为0.1、0.2、0.5、2.0、5.0、20.0、50.0和200.0 ng·mL-1的GA1和GA4标准溶液,并加入终浓度为20.0 ng·mL-1的内标溶液。每个浓度2个重复。
1.6.3 液相和质谱条件 液相条件:色谱柱采用poroshell 120 SB-C18反相色谱柱(2.1 mm×150 mm,2.7 μm);柱温:30℃;流动相:A﹕B=(甲醇/0.1%甲酸)﹕(水/0.1%甲酸);洗脱梯度:0—1 min,A=20%;1—9 min,A递增至80%;9—10 min,A=80%;10—10.1 min,A递减至20%;10.1—15min,A=20%;进样体积:2 μl。
质谱条件:气帘气为15 psi;喷雾电压:4 500 v;雾化气压力:65 psi;辅助气压力:70 psi;雾化温度:400℃。
1.7 统计分析
利用Excel 2010和SPSS 12.0软件对实验数据进行统计分析,用Duncan法进行差异显著性检验。
2 结果
2.1 半矮化突变体sd13的表型差异
水稻半矮化突变体的表型发育异常。与野生型相比,突变体株高变矮且分蘖数增多(图1-A)并出现包穗现象,穗不能完全伸出剑叶鞘(图1-B和图1-C);突变体种子变小(图1-D)且小穗发育异常,外稃与内稃上的表皮毛消失(图2-A和图2-C);花发育延迟,雄蕊变小(图2-E和图2-G);茎节间细胞长度减小(图3)。统计分析显示突变体株高减少56.29%,且各个节间长度均减小(图4-A),差异极显著;突变体有效分蘖数比WT高出47.44%(图4-B),方差分析差异极显著;突变体结实率低,仅为WT的12.62%(图4-C);种子长度和宽度均减小,经方差分析差异显著(<0.05)(图4-D和图4-E)。利用显微镜对突变体的茎纵切片统计分析,发现突变体的细胞长度减少约23%,差异极显著(<0.01)(图4-F)。
2.2 突变体对外源GA敏感性检测
为了深入探究突变体遗传突变的分子应答与赤霉素合成基因之间是否存在依存关系,对水稻进行外源喷施GA3处理,结果表明,经赤霉素处理的突变体的株高、分蘖、包穗、结实率、种子大小、表皮毛和茎细胞长度均得到一定程度的恢复(图2-C和图2-D,图4-B—图4-F,图5-A)。与喷施GA3前相比,野生型和突变体在喷施GA3之后,有效分蘖数均减小,差异均达显著水平。喷施GA3后,与野生型相比,虽然突变体结实率差异仍然极显著,但是与喷施GA3之前相比显著增高(<0.01)。种子的长与宽在喷施GA3后均得到一定程度的恢复,但是与喷施GA前差异不明显。在喷施GA3后,突变体表皮毛出现了一定的恢复,但是没有测试具体数量指标。突变体在喷施GA3后,其茎细胞长度显著变长(<0.01)。水培的突变体经过GA3处理也出现相似的现象(图5-B),结果显示,在营养液中添加不同浓度GA3之后,突变体株高均增加,其中以低浓度处理(6×10-6mol·L-1)的突变体恢复最佳,说明突变体极有可能是GA敏感型突变体。

A:孕穗期野生型和突变体形态;B:野生型小穗(比例尺,2 cm);C:突变体小穗(比例尺,2 cm);D:野生型和突变体成熟种子大小

A—D:GA喷施前后野生型和突变体水稻小穗形态(比例尺,0.4 mm);E—H:GA喷施前后野生型和突变体水稻花的特征(比例尺,0.4 mm)
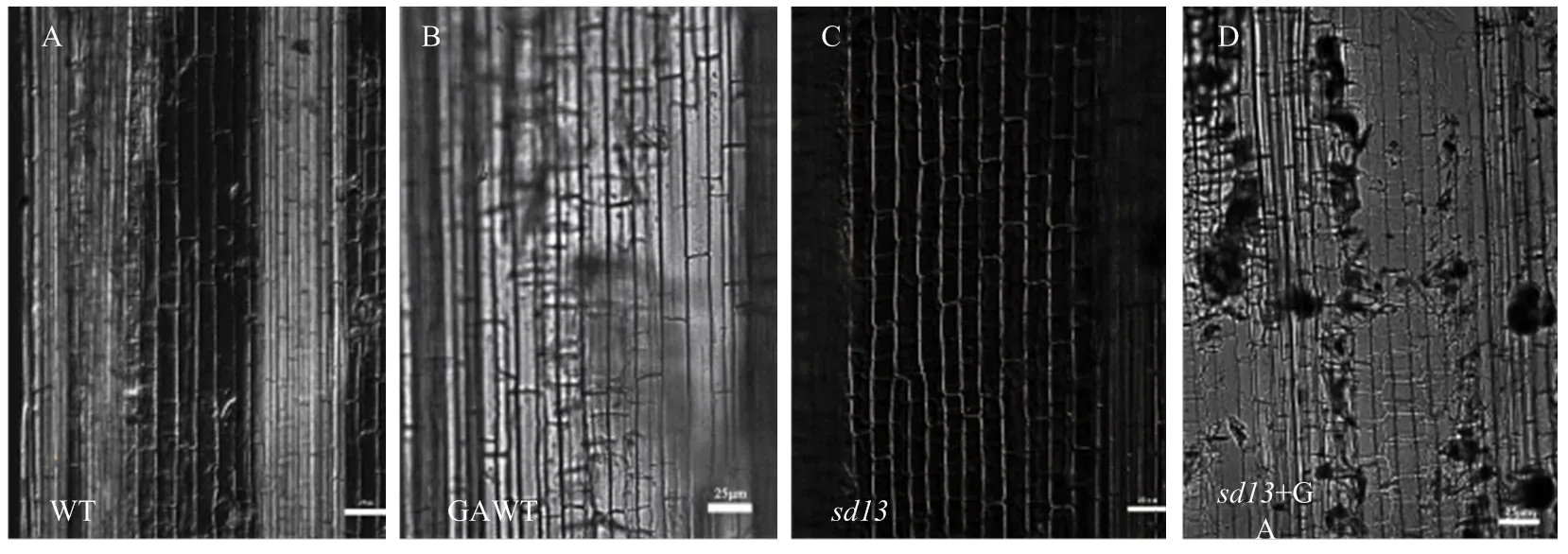
图3 喷施GA3前后野生型和突变体水稻穗茎节间茎细胞大小(比例尺,25 μm)

**代表差异极显著,P<0.01;*代表差异显著,P<0.05。下同
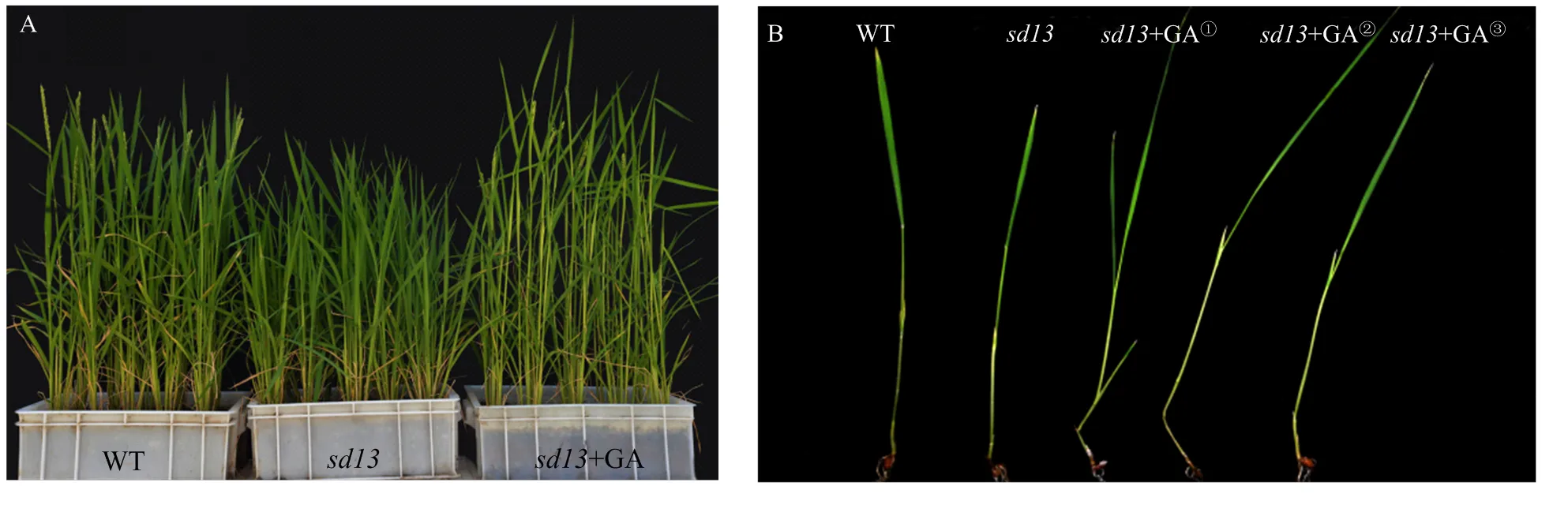
A:土培突变体喷施GA后的表型变化;B:营养液添加不同浓度GA后突变体表型变化,GA①、GA②和GA③分别代表GA3浓度为1.5×10-4、3.0×10-5和6.0×10-6 mol·L-1
2.3 基于转录组学分析的差异基因表达特征
为了深入探究遗传突变的分子机制,将培养3周的水稻进行转录组测序。测序结果显示,与WT相比,突变体共有631个差异基因,其中326个上调,305个下调(图6-B)。GO(Gene Ontology)富集显示,差异基因主要富集在电子转移过程,涉及电子转移的基因有50个(图6-C)。KEGG(Kyoto Encyclopedia of genes and genomes)富集显示差异基因主要参与糖酵解和磷酸戊糖等代谢途径(图6-D)。说明半矮化突变体的某一个或者多个基因突变导致了下游参与电子转移基因的异常表达,异常表达的基因极有可能是一些与糖代谢有关的活性酶。

A:差异基因聚类图。每一行表示一个基因,每一列表示一个样,颜色从红到蓝,表示表达水平从大到小,颜色相近聚类区内的基因表达模式相近,说明这些基因可能具有相似的功能或参与调控同一条代谢通路;B:差异基因火山图。差异表达显著的基因用红点(上调)和绿点(下调)表示,差异表达不显著的基因用蓝点表示;C:差异基因GO富集图。绿色代表生物过程,橙色代表分子功能;D:差异基因KEGG富集散点图。点的大小表示此pathway中差异表达基因个数多少,而点的颜色对应于不同的Qvalue值,值越小说明富集越显著
A: Differential gene clustering. Each row represents a gene and each column represents a sample. The color is from red to blue, indicating that the expression level is from large to small, and the expression patterns of genes in similar clusters are similar, indicating that these genes may have similar functions or participate in regulation of the same metabolic pathway; B: Differential gene volcano map. Genes with significant differential expression are indicated by red dot (up-regulation) and green dot (down-regulation), and genes with insignificant differential expression are represented by blue dots; C: Differential gene GO enrichment map. Green represents biological processes and orange represents molecular function; D: Differential gene KEGG enrichment scatter plot. The size of the dot indicates the number of differentially expressed genes in the pathway, and the color of the dot corresponds to a different Qvalue. The smaller the value, the more significant the enrichment
图6 转录组测序结果
Fig. 6 Transcriptome sequencing results
为进一步分析挖掘差异表达基因的累积分布特征,对突变体中喷施GA前后得到的8个表达不一致的差异基因进行了分析(表2),结果表明,这8个差异基因对外源GA较为敏感。利用生物信息学分析,发现有一个与赤霉素合成相关的,在突变体中表达明显上调,而喷施GA后表达出现下调。在植物体内负责将GA12转化为GA53,而GA12和GA53又分别转化为GA4和GA1,GA4的活性强于GA1,暗示半矮化突变体极有可能是表达异常引起的。对突变体的花、茎和叶提取RNA进行qRT-PCR验证,结果(图7)显示,在突变体各器官中的表达均高于野生型,尤其在茎中的表达量较高且与WT相比差异极显著;在突变体叶中的表达量也较高,与WT相比差异极显著;但在花中的差异不明显。对突变体进行GA处理之后,的表达量出现不同程度的下降,其中在茎和叶中与喷施GA之前相比差异极显著。说明突变体是由表达异常引起的,且对外源GA敏感。
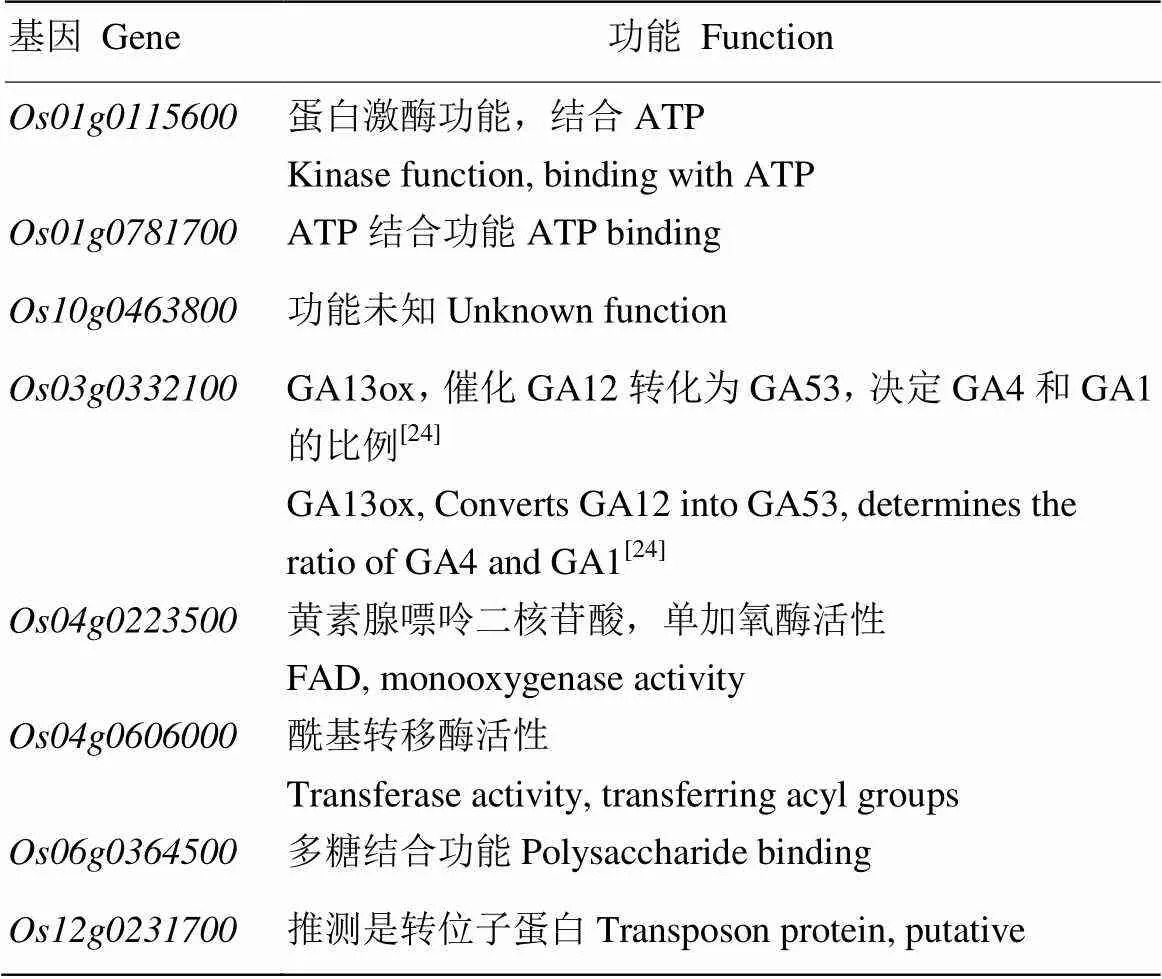
表2 突变体喷施GA前后表达相反的基因
所有基因功能均查阅自Uniprot蛋白数据库及相关文献,下同
All gene functions are reviewed from the UniProt database and related paper, the same below
2.4 水稻突变体GA的富集特征
为明确突变体中表达上调是否引起水稻中GA4含量变化,用高效液相和质谱联仪对突变体中的GA1和GA4进行检测分析。结果表明,与WT相比,突变体中的GA1含量无显著变化,但活性更强的GA4减少了94.9%(图8),差异极显著(<0.01),与预测结果一致,说明半矮化突变体是由GA4减少引起的。
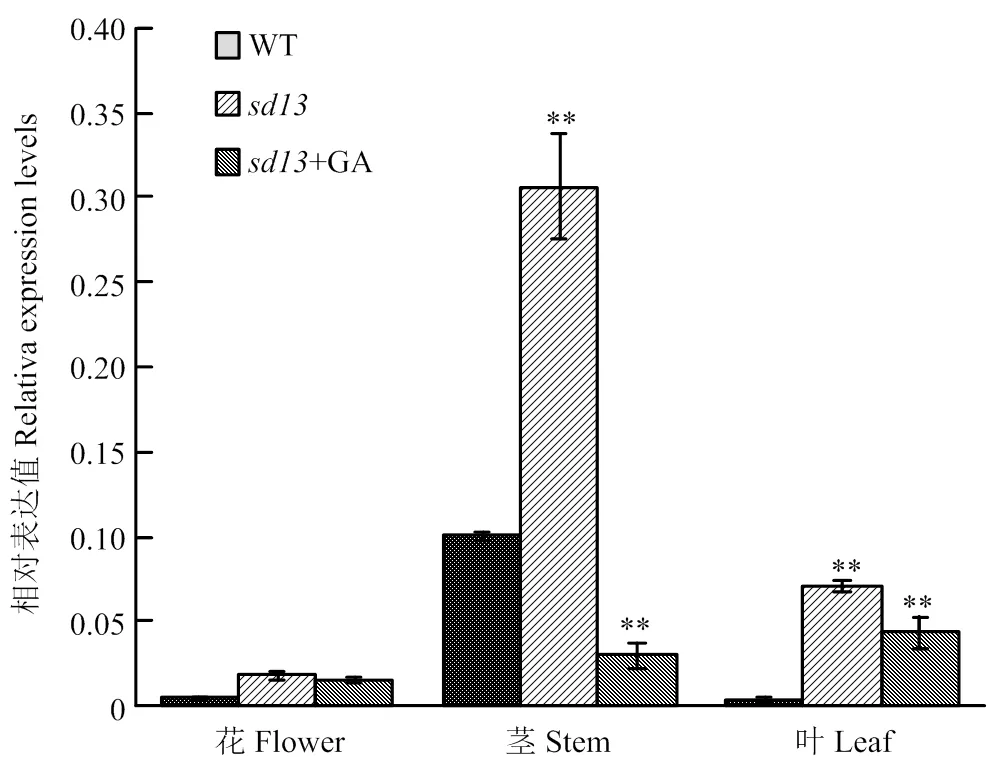
图7 OsGA13ox在突变体各器官中的相对表达量
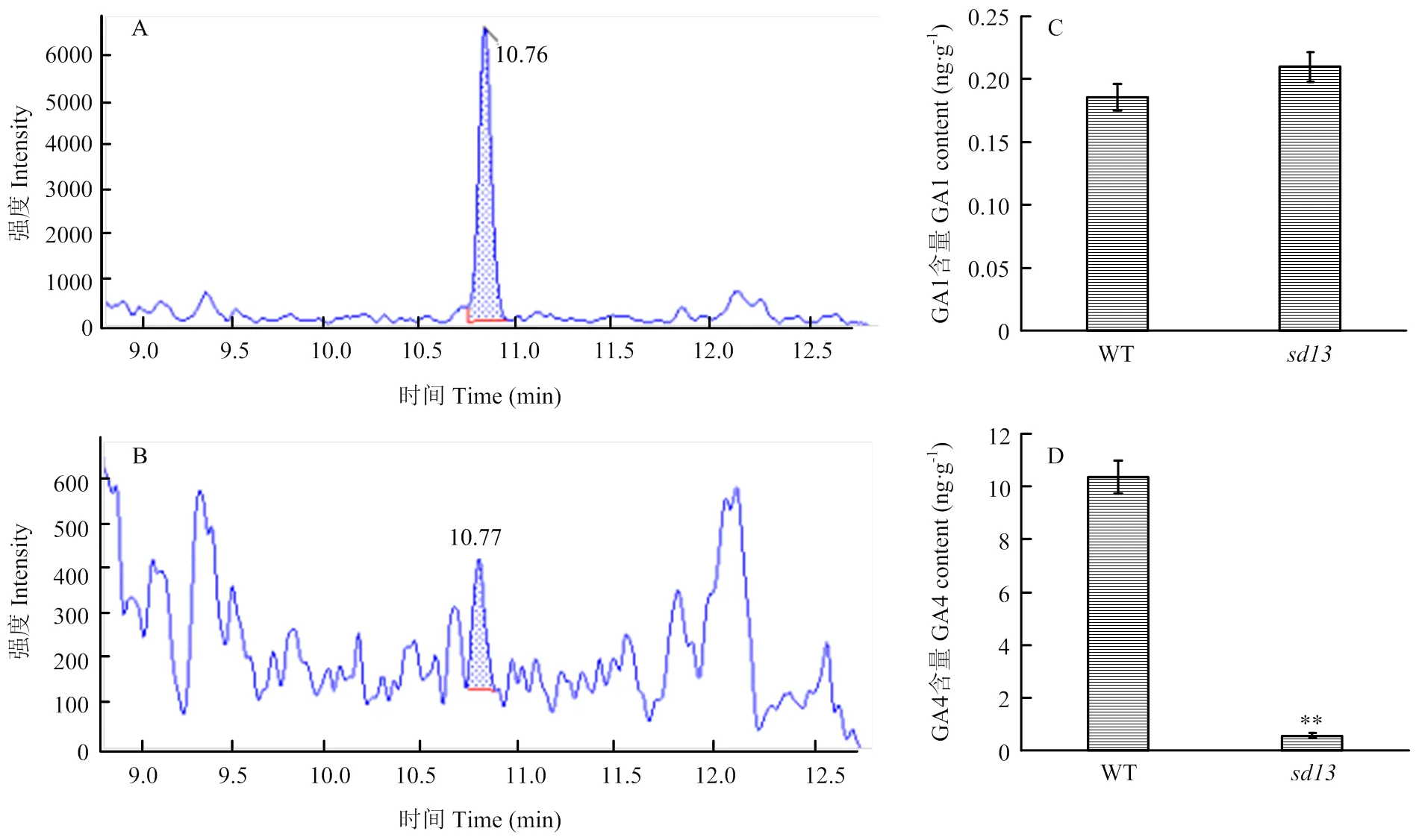
A:野生型水稻GA4检测峰图;B:突变体水稻GA4检测峰图;C:野生型和突变体水稻中GA1含量;D:野生型和突变体水稻中GA4含量
2.5 GA对水稻分蘖的影响
赤霉素(GA)和独脚金内酯(SL)通常参与调控植物的枝条分枝或分蘖[25],并且GA和SL之间存在着竞争DELLA蛋白结合位点的关系[26]。转录组测序分析韦恩图显示,野生型和突变体有12个共同对GA应答的基因(图9-A),利用GO注释获得这12个基因的功能(表3)。有趣的是,最后3个基因、和在突变体中也下调表达。是D14蛋白,作为独脚金内酯(SL)的特异性受体,可与赤霉素受体GID1竞争DELLA蛋白结合位点,调控植物的生长发育[27]。qRT-PCR结果显示(图9-B),与野生型相比,在突变体中下调表达,差异极显著。野生型和突变体喷施GA后,均上调表达,其中野生型上调表达较高,差异极显著(<0.01),而突变体中虽然也出现了上调表达,但与喷施GA之前相比,差异不显著。

表3 野生型和突变体共表达的12个GA应答基因

图9 差异基因韦恩图及D14在野生型和突变体茎中的相对表达量
3 讨论
3.1 OsGA13ox异常表达使GA4含量减少是突变体半矮化的原因
许多研究表明水稻的矮化及半矮化与赤霉素合成或信号转导受阻有关,Itoh等[29]研究表明水稻突变是突变体矮化的原因,而Lo等[30]发现超表达水稻也会出现矮化等现象。这些现象产生的原因是GA3ox氧化酶是控制合成GA1和GA4的关键因子,而GA2ox氧化酶可以使GAs钝化失活[31]。本研究发现一株半矮化突变体,其株高变矮,分蘖增多,且花发育异常,结实率低。该突变体对外源GA敏感,并且喷施GA3能使表型得到一定程度的恢复,说明该突变体为功能缺失型突变体。赤霉素的合成主要受内根-贝壳杉烯合成酶(KS)、内根-贝壳杉烯19-氧化酶(KO)、GA-13-羟化酶(GA13ox)及GA-20-氧化酶(GA20ox)等酶的统一调控(图10)。已有研究表明,在拟南芥[32-33]和水稻[34]中,GA1的生物活性低于GA4,而GA13ox作为调控GA4和GA1的枢纽,对植物体内赤霉素的相对稳定具有十分重要的作用。MAGOME等[24]发现在水稻中超表达可导致水稻半矮化,而GA13突变体却表现出植株增高,穗茎节间变长。造成这种现象的原因是超表达致使活性更高的GA4富集减少,突变体则相反,由此可说明在控制植物体内GA4和GA1的相对稳定发挥着重要的作用。本研究转录组测序和qRT-PCR结果显示,与WT相比,半矮化突变体表达上调,使活性更高的GA4含量减少,破坏GA4和GA1之间的相对平衡,这与前人研究结论相似,再次证明了GA13ox对植物体内赤霉素的相对平衡是至关重要的。
3.2 sd13的突变基因可能是能够抑制OsGA13ox基因表达的转录因子
通常基因的转录水平受转录因子的调节,Zhang等[35]研究发现水稻转录因子OsWRKY71是赤霉素信号转导的抑制子,而OsGAMYB是GA诱导型转录激活因子,其启动子中含有W盒功能性保守基序TGAC,OsWRKY71可以与该基序特异性结合而抑制OsGAMYB的激活,导致GA信号的转导受到阻碍。Zhang等[36]又发现也可以抑制赤霉素信号的转导。Guo等[37]研究表明拟南芥是编码具有抑制活性的含B3结构域的转录因子,突变体致使拟南芥出现矮化等现象,同时发现赤霉素(GA)失活基因的表达显著增加;并且验证了突变体中、和3种GA生物合成基因表达减少,导致内源GA4富集明显降低。外源GA处理不仅可以诱导拟南芥表达,而且能够部分缓解突变体的矮化程度,说明能够抑制植物体内赤霉素失活基因的表达。因此,推测本研究中的异常表达可能与具有抑制活性的转录因子突变有关。利用植物转录因子数据库(http://planttfdb.cbi.pku.edu.cn)对转录起始位点前500位和后100位启动子序列进行基序预测,结果如表4所示。除此之外,据报道,茉莉酸(JA)可以通过强烈抑制和的转录拮抗GA的合成[38],说明高水平JA可能拮抗茎中GA的生物合成。所以与相关的转录因子是否突变有待于进一步深入研究,并且探索茉莉酸合成基因或其他基因是否与的异常表达有关。

图10 植物体内赤霉素合成途径[31]
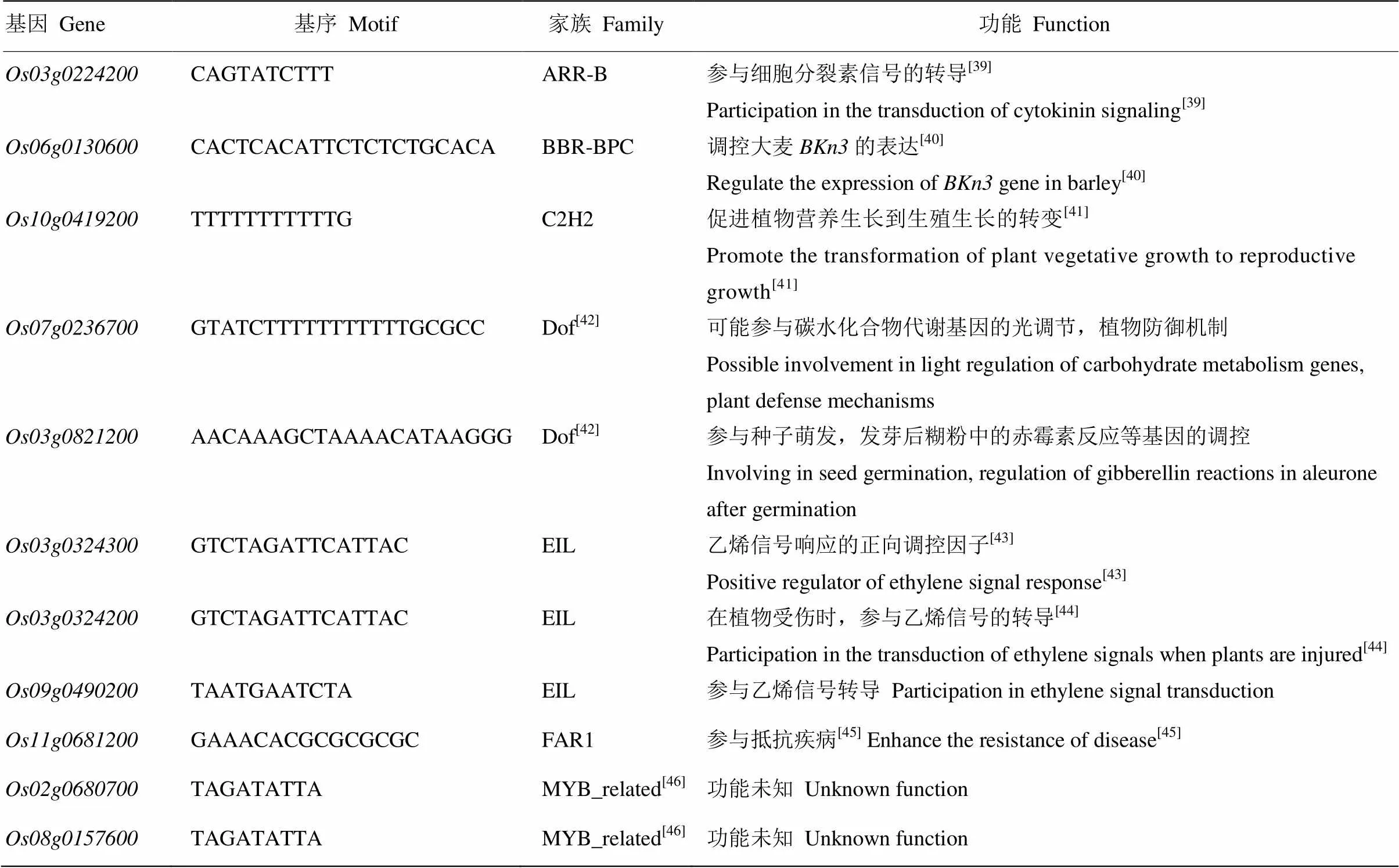
表4 OsGA13ox启动子序列中的基序预测结果
3.3 GA可能通过调控D14的表达间接调控水稻分蘖
拟南芥突变体拥有更多的分枝,超表达的水稻也表现出分蘖增多[30],而水稻突变体中赤霉素合成基因上调表达使得分蘖减少[47],说明GA能够抑制水稻分蘖。Ito等[25]已证明外源GA能够使水稻分蘖异常突变体得到恢复。除赤霉素之外,独脚金内酯(SL)也能够抑制枝条分枝或者分蘖[48],且SL的合成受GA信号调控[49]。D14蛋白为SL的特异性受体,二者能够和DELLA蛋白结合形成复合物,从而调控下游基因的表达,间接调节枝条分枝或者分蘖。本研究发现,与WT相比,突变体的下调表达,而作为SL的特异性受体,下调表达会使SL信号减弱,从而使分蘖增多,这与突变体的分蘖增多相符合。外源喷施GA使上调表达,会增强SL信号从而使分蘖减少,而WT喷施GA后分蘖减少也验证了这一猜测。本研究推测GA可能通过的表达间接调控植物体的枝条分枝或分蘖。
4 结论
半矮化突变体是由异常表达导致GA4富集减少引起的;赤霉素可能通过间接调控水稻的分蘖。
[1] Spielmeyer W, Ellis M H, Chandler P M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene., 2002, 99(13): 9043-9048.
[2] Peng J, Richards D E, Hartley N M, Murphy G P, Devos K M, Flintham J E, Beales J, Fish L J, Worland A J, Pelica F. ‘Green revolution’ genes encode mutant gibberellin response modulator., 1999, 400: 256-261.
[3] YAMAMURO C, IHARA Y, WU X, NOGUCHI T, FUJIOKA S, TAKATSUTO S, ASHIKARI M, KITANO H, MATSUOKA M. Loss of function of a rice brassinosteroid insensitive 1 homolog prevents internode elongation and bending of the lamina joint., 2000, 12: 1591-1606.
[4] HONG Z, UEGUCHI-TANAKA M, FUJIOKA S, TAKATSUTO S, YOSHIDA S, HASEGAWA Y, ASHIKARI M, KITANOH, MATSUOKA M. The Rice brassinosteroid-deficient dwarf2 mutant defective in the rice homolog ofDIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone., 2005, 17: 2243-2254.
[5] ARITE T, IWATA H, OHSHIMA K, MAEKAWA M, NAKAJIMA M, KOJIMA M, SAKAKIBARA H, KYOZUKA J. DWARF10, an RMsI/MAX4/DAD1 ortholog, controls lateral bud out-growth in rice., 2007, 51(6): 1019-1029.
[6] ISHIKAWA S, MAEKAWA M, ARITE T, ONISHI K, TAKAMURE I, KYOZUKA J. Suppression of tiller bud activity in tillering dwarf mutants of rice., 2005, 46(1): 79-86.
[7] FLEET C M, SUN T P. A DELLAcate balance: the role of gibberellin in plant morphogenesis., 2005, 8(1): 77-85.
[8] 谈心, 马欣荣. 赤霉素生物合成途径及其相关研究进展. 应用与环境生物学报, 2008, 14(4): 571-577.
Tan X, Ma X R. Advance in research of gibberellin biosynthesis pathway., 2008, 14(4): 571-577. (in Chinese)
[9] Thomas S G, Sun T P. Update on gibberellin signaling. A tale of the tall and the short., 2004, 135: 668-676.
[10] Yamaguchi S, Sun T p, Kawaide H, Kamiya Y. The GA2 locus ofencodes ent-kaurene synthase of gibberellin biosynthesis., 1998, 116: 1271-1278.
[11] Helliwell CA, Poole A, Peacock W J, Dennis E S.ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis., 1999, 119(2): 507-510.
[12] MARGIS-PINHEIRO M, ZHOU X R, ZHU Q H, DENNIS E S, UPADHYAYA N M. Isolation and characterization of a Ds-tagged rice (L.) GA-responsive dwarf mutant defective in an early step of the gibberellin biosynthesis pathway., 2005, 23(12): 19-33.
[13] Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M. A rice semi-dwarf gene, Tan-ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase., 2004, 54(4): 533-547.
[14] Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin., 2005, 437(7059): 693-698.
[15] GOMI K, SASAKI A, ITOH H, UEGUCHI-TANAKA M, ASHIKARI M, KITANO H, MATSUOKA M. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice., 2004, 37(4): 626-634.
[16] Sun T P. Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development., 2010, 154(2): 567-570.
[17] 尹昌喜. 细胞质雄性不育水稻包穗的激素调控[D]. 南京: 南京农业大学, 2007: 6.
Yin C X. Plant hormone regulation on panicle enclosure in cytoplasmic male sterile rice[D]. Nanjing: Nanjing Agricultural University, 2007: 6. (in Chinese)
[18] 张玲. 水稻矮化并花发育异常突变体()的基因定位与候选基因分析[D]. 重庆: 西南大学, 2015: 6.
Zhang L. Gene mapping and candidate gene analysis of()mutants in rice () [D]. Chongqing: Southwestern University, 2015: 6. (in Chinese)
[19] Wang Y P, Tang S Q, Wu Z F, SHI Q H, WU Z M. Phenotypic analysis of a() mutant in rice (L.) and characterization of candidate genes., 2018, 5(17): 1057-1065.
[20] Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, Leyser O. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching inand pea., 2003, 17: 1469-1474.
[21] Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, Yi W, Zhao L, Ma H, He Y, Wu Z, Melcher K, Qian Q, Xu H E, Wang Y, Li J. DWARF 53 acts as a repressor of strigolactone signalling in rice., 2013, 504(7480): 401-405.
[22] Young M D, Wakefield M J, Smyth G K, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias., 2010, 11(2): R14.
[23] Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment., 2008, 36: D480-D484.
[24] Magome H, Nomura T, Hanada A, Takeda-Kamiya N, Ohnishi T, Shinma Y, Katsumata T, Kawaide H, Kamiya Y, Yamaguchi S. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice., 2013, 110(5): 1947-1952.
[25] Ito S, Yamagami D, Asami T. Effects of gibberellin and strigolactone on rice tiller bud growth., 2018, 43(3): 220-223.
[26] Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L. The rice HIGH-TILLERING DWARF1 encoding an ortholog ofMAX3 is required for negative regulation of the outgrowth of axillary buds., 2006, 48: 687-698.
[27] Nakamura H, Xue Y L, Miyakawa T,Hou F, Qin H M, Fukui K, Shi X, Ito E, Ito S, Park S H, Miyauchi Y, Asano A, Totsuka N, Ueda T, Tanokura M, Asami T. Molecular mechanism of strigolactone perception by DWARF14., 2013, 4: 2613.
[28] Kim S K, Park H Y, Jang Y H, Lee K C, Chung Y S, Lee J H, Kim J K. OsNF-YC2 and OsNF-YC4 proteins inhibit flowering under long-day conditions in rice., 2016, 243(3): 563-576.
[29] Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M. Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice., 2001, 98(15): 8909-8914.
[30] Lo S F, Yang S Y, Chen K T, HSING YI, ZEEVAART JA, CHEN L J, YU S M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice., 2008, 20(10): 2603-2618.
[31] Peter H, Valerie S. A century of gibberellin research., 2015, 34: 740-760.
[32] Cowling R J, Kamiya Y, Seto H, Harberd N P. Gibberellin dose-response regulation of GA4 gene transcript levels in., 1998, 117(4): 1195-1203.
[33] Talon M, Koornneef M, Zeevaart J A. Endogenous gibberellins inand possible steps blocked in the biosynthetic pathways of the semidwarf GA4 and GA5 mutants., 1990, 87(20): 7983-7987.
[34] Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin., 2007, 19(7): 2140-2155.
[35] Zhang Z L, Xie Z, Zou X, Casaretto J, Ho T H, Shen Q J. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells., 2004, 134(4): 1500-1513.
[36] Zhang Z L, Shin M, Zou X, Huang J, Ho T H, Shen Q J. A negative regulator encoded by a rice WRKY gene represses both abscisic acid and gibberellins signaling in aleurone cells., 2009, 70(1/2): 139-151.
[37] Guo X, Hou X, FANG J, WEI P, XU B, CHEN M, FENG Y, CHU C.The rice GERMINATION DEFECTIVE 1, encoding a B3 domain transcriptional repressor, regulates seed germination and seedling development by integrating GA and carbohydrate metabolism., 2013, 75(3): 403-416.
[38] Heinrich M, Hettenhausen C, Lange T, Wünsche H, Fang J, Baldwin I T, Wu J. High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth ofstems., 2013, 73(4): 591-606.
[39] Ito Y, Kurata N. Identification and characterization of cytokinin-signalling gene families in rice., 2006, 382: 57-65.
[40] Santi L, Wang Y, Stile M R, Berendzen K, Wanke D, Roig C, Pozzi C, Müller K, Müller J, Rohde W, Salamini F. The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene Bkn3., 2003, 34(6): 813-826.
[41] Wu C, You C, Li C, Long T, Chen G, Byrne M E, Zhang Q. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice., 2008, 105(35): 12915-12920.
[42] Lijavetzky D, Carbonero P, Vicente-Carbajosa J. Genome-wide comparative phylogenetic analysis of the rice andgene families., 2003, 1: 17.
[43] Mao C, Wang S, Jia Q, Wu P.OsEIL1, a rice homolog of theEIN3 regulates the ethylene response as a positive component., 2006, 61(1): 141-152.
[44] Hiraga S, Sasaki K, Hibi T, Yoshida H, Uchida E, Kosugi S, Kato T, Mie T, Ito H, Katou S, Seo S, MATSUI H, OHASHI Y, MITSUHARA I. Involvement of two rice ETHYLENE INSENSITIVE3-LIKE genes in wound signaling., 2009, 282(5): 517-529.
[45] Rice Chromosomes 11 and 12 Sequencing Consortia. The sequence of rice chromosomes 11 and 12, rich in disease resistance genes and recent gene duplications., 2005, 3: 20.
[46] Kirik V, Baumlein H. A novel leaf-specific myb-related protein with a single binding repeat., 1996, 183(1/2): 109-113.
[47] Li W Q, Wu J G, Weng S L, ZHANG Y, ZHANG D, SHI C. Identification and characterization of dwarf 62, a loss-of-function mutation in DLT/OsGRAS-32 affecting gibberellin metabolism in rice., 2010, 232(6): 1383-1396.
[48] Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones., 2008, 455(7210): 195-200.
[49] Ito S, Yamaqami D, Umehara M, Hanada A, Yoshida S, Sasaki Y, Yajima S, Kyozuka J, Ueguchi-Tanaka M, Matsuoka M, Shirasu K, Yamaguchi S, Asami T. Regulation of strigolactone biosynthesis by gibberellin signaling., 2017, 174(2): 1250-1259.
Enrichment profile of GA4 is an important regulatory factor triggering rice dwarf
HUANG ShengCai1, WANG Bing1, XIE GuoQiang2, LIU ZhongLai2, ZHANG MeiJuan1, ZhANG ShuQing1, CHENG XianGuo1
(1Intitute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081;2Jiu Jiang Academy of Agricultural Sciences, Jiujiang 332101, Jiangxi)
【Objective】A dwarf rice mutant was generated by culturing rice embryo tissues and characterized to elucidate the reasons for leading to an occurence of semi-dwarf rice with more tiller number. It is expected that this study will provide a theoretical basis for scientifically cultivating rice varieties of lodging-resistant and high-yielding in overcoming the dwarf obstacle factors.【Method】In this study, both the rice dwarf mutant and wild type were phenotypically profiled, and the structural characteristics of flower and cell appearance of leaves were investigated by stereomicroscope and light microscopy; The differential gene expression profiles were analyzed by both the transcriptomics and qRT-PCR, and the sensitivity of the mutant to exogenous gibberellin was detected by spraying exogenous GA3; The enrichment profiles of gibberellin in the mutant were detected by a high performance liquid chromatography and a mass spectrometry. 【Result】Data showed that the mutant demonstrated a decrease of 56.59% in the average plant height and an increase of 47.44% in the effective tiller number compared with the wild type, respectively (<0.01). Observation showed that the mutant led to disappearance of the epidermis and revealed a smaller stamen accompanying a delayed development in the flower organs. Although the mutant has a higher effective tiller number, but significantly lowers the seed setting rate, which only accounts for 12.62% of that in the wild type. The length and the width of grains also are significantly reduced (<0.01). Stem longitudinal sections reveal that the mutant decreased the cell length of 23% compared with the wild type (<0.01). However, when the mutant was exposed to exogenous gibberellin, the plant height, effective tiller number, seed setting rate, seed size, epidermis and the stem cell length were obviously restored, indicating that the dwarf mutant possibly results from the shortage of GA’s synthesis in plant. Transcriptome sequencing showed that the mutant significantly up-regulated thegene, and exhibited an identical result with the qRT-PCR analyses. Since theOsGA13oxcontrols the conversion of the GA12 to the GA53, both of which are converted to the GA4 and the GA1, respectively. Particularly, the GA4 exhibits a higher activity than the GA1, suggesting that rice dwarf might be triggered by the reduction of GA4 enrichment in plant. Measurement confirmed that the accumulation of the GA4 in the mutant was decreased by 94.9% compared with the wild type. Additionally, as a specific receptor for SL (Strigolactone), thegene is involved in the SL signaling transduction in plant, and thus inhibits branching or tillering. The qRT-PCR showed that the mutant significantly down-regulated thegene compared to the wild type, however, both the wild type and the mutant significantly up-regulated after spraying GA3. The data suggested that the up-regulation of thegene might lower the effective tiller number, while the down-regulation of thegene possibly increase the effective tiller number. Statistical analyses demonstrates that the mutant significantly increased the effective tiller number, but both the wild type and mutant decreased the effective tiller number after spraying GA3, indicating that the expression profiles of thegene in rice might be modulated by GA, and thereby exert on the tiller number.【Conclusion】Semi-dwarfed rice mutant is likely caused by a decrease of GA4 enrichment because of abnormal expression of thegene, and GA might indirectly regulate rice tiller by affecting the expression of thegene
rice; semi-dwarf mutant;; GA4;; effective tiller
10.3864/j.issn.0578-1752.2019.05.002
2018-11-06;
2018-12-09
转基因重大专项(2016ZX08010005-9)
黄升财,E-mail:huangshengcai_123@163.com。 通信作者张树清,E-mail:zhangshuqing@caas.cn。通信作者程宪国,Tel:010-82105031;E-mail:chengxianguo@caas.cn
(责任编辑 李莉)
