欧亚类禽型H1N1猪流感病毒HA蛋白的表达及免疫原性评估
2019-03-29贾云慧许程志隋金钰吴运谱许榜丰陈艳杨焕良乔传玲陈化兰
贾云慧,许程志,隋金钰,吴运谱,许榜丰,陈艳,杨焕良,乔传玲,陈化兰
欧亚类禽型H1N1猪流感病毒HA蛋白的表达及免疫原性评估
贾云慧,许程志,隋金钰,吴运谱,许榜丰,陈艳,杨焕良,乔传玲,陈化兰
(中国农业科学院哈尔滨兽医研究所/兽医生物技术国家重点实验室/农业部动物流感重点开放实验室,哈尔滨 150069)
【目的】表达欧亚类禽型(eurasian avian-like,EA)H1N1猪流感病毒(SIV)的血凝素(HA)蛋白,并对其免疫原性进行测定。【方法】利用RT-PCR技术扩增病毒A/swine/Zhejiang/245/2013(H1N1)(ZJ245)的HA基因,将其克隆至真核表达载体pCAGGS中,获得重组质粒pCAGGS-HA(ZJ245),将其转染293T细胞,采用间接免疫荧光(IFA)检测和Western blot检测HA蛋白在体外的表达;将重组质粒pCAGGS-HA(ZJ245)经肌肉注射途径、以100 µg/只剂量免疫16只6周龄BALB/c小鼠(I组和II组),间隔3周后进行加强免疫(二免);同等数量的小鼠以相同方式注射100 µL无菌PBS作为非免疫对照组(III组和IV组)。首免和二免每周采血,分别采用血凝抑制(HI)试验和病毒中和(VN)试验两种方法测定不同类型的血清抗体效价;二免2周后,其中两组小鼠(I组和III组)用50 µL(106.0EID50)的ZJ245病毒经滴鼻感染途径进行攻毒,另外两组(II组和IV组)用A/swine/Heilongjiang/44/2009(H1N1)(HLJ44)病毒进行攻毒。攻毒后14 d内每天观察小鼠的临床症状、统计发病与死亡情况,且每天称量小鼠体重,在体重下降比率超过25%时判定为小鼠死亡。攻毒后第3 天,每组随机剖杀3只小鼠,分别采集脑、鼻甲、肺、脾和肾等脏器,匀浆处理后通过接种10日龄的非免疫鸡胚测定脏器的病毒含量。通过小鼠体重变化以及脏器滴定的病毒含量评估重组质粒pCAGGS-HA(ZJ245)的免疫保护效果。【结果】经酶切鉴定和测序验证表明ZJ245的HA基因的已正确克隆至真核表达质粒pCAGGS中获得重组质粒pCAGGS-HA(ZJ245),经体外转染293T细胞后,IFA和Western blot证实了病毒的HA蛋白能够正确表达,并具有良好的生物学活性;小鼠的免疫与攻毒试验结果表明,重组质粒pCAGGS-HA(ZJ245)首免后一周可检测到针对同源病毒ZJ245的低水平HI和VN抗体,加强免疫后抗体水平明显升高,HI抗体达到76.88、VN抗体达到152.5;同时针对异源病毒HLJ44也产生了较低水平的HI和VN抗体。106.0EID50的同源病毒ZJ245攻毒时,与非免疫组小鼠相对比,重组质粒pCAGGS-HA(ZJ245)的免疫完全阻止了因病毒攻击而导致的小鼠体重下降以及肺脏和鼻甲内病毒的复制;106.0EID50的异源病毒HLJ44攻毒时,相比非免疫组小鼠,质粒免疫组小鼠体重的下降比率、以及肺脏和鼻甲内的病毒滴度均显著降低(<0.0001、<0.001、<0.05)。【结论】重组质粒pCAGGS-HA(ZJ245)能够有效表达病毒HA蛋白,免疫后可使小鼠获得完全抵抗ZJ245感染及部分抵御HLJ44感染的免疫保护力,表明重组质粒 pCAGGS-HA(ZJ245)具有良好的免疫原性。
欧亚类禽型H1N1猪流感病毒;HA蛋白;重组质粒;免疫原性;DNA疫苗
0 引言
【研究意义】猪流感(swine influenza, SI)是由猪流感病毒(swine influenza virus, SIV)引起的一种急性、高度接触性呼吸道传染病,临床以发热、咳嗽、喷嚏和食欲下降等为主要特征。SI普遍存在于规模化养殖场中,虽单独感染引起的死亡率不高,但以该病为诱因引起的其他病原的混合感染,使病情复杂且加重,最终导致猪的死亡率上升,严重危害养猪业的健康发展。【前人研究进展】目前猪群中流行的SIV主要是H1N1、H1N2和H3N2[1-2],其中H1N1又分为经典型、类人型、类禽型和2009年造成全球人流感大流行的H1N1病毒(pdm H1N1/2009)等不同基因谱系[3]。pdm H1N1/2009的HA基因来源于经典型H1N1,NA基因来自源于欧亚类禽型H1N1(eurasian avian-like H1N1, EA H1N1)病毒[4]。EA H1N1于1979年首次于欧洲猪群中被分离出,是由H1N1亚型禽流感病毒突破种间屏障传染给猪的[5],目前,EA H1N1在欧洲和亚洲的多个国家的猪群中广泛流行[6-7];中国于1993年首次分离出该病毒,与经典型H1N1共同广泛流行于猪群中[8]。近年来,SI病原学监测结果表明,EA H1N1已成为我国猪群中的主要流行毒株[9];该毒株与经典型H1N1 SIV相比,具有更多的选择优势[10],且EA H1N1感染人的情况在欧洲和我国均时有发生[11-13]。疫苗免疫是防制SI发生与流行的重要措施,基于EA H1N1 SIV研制的灭活疫苗[14- 16]及重组腺病毒载体疫苗[17]均证实具有良好的免疫保护效果。【本研究切入点】HA是流感病毒的一种重要表面糖蛋白,其中HA蛋白在介导受体结合、膜融合、病毒粒子包装及致病性方面发挥着重要作用[4],该蛋白是宿主获得性免疫应答识别的主要抗原,具有刺激机体产生中和抗体、阻止病毒感染的能力[18]。DNA疫苗是将含有目的基因的重组表达质粒导入机体内,由宿主细胞合成抗原蛋白,进而诱导有效的体液免疫和细胞免疫应答,当前已成为流感基因工程疫苗研发的一个重要方向[19]。在我国,用于H5亚型高致病性禽流感防控的DNA疫苗已研制成功,并率先获得了生产许可。【拟解决的关键问题】本研究拟以EA H1N1 SIV毒株ZJ245的HA 蛋白作为靶抗原,构建真核表达质粒,制备DNA疫苗,并在BALB/c小鼠体内评估其免疫原性及对不同抗原型H1N1 SIV的免疫保护效果。
1 材料与方法
1.1 病毒、细胞及实验动物等
EA H1N1毒株A/swine/Zhejiang/245/2013 (ZJ245)[20]和pdm H1N1/2009毒株A/swine/Heilongjiang/44/2009 (HLJ44)[21]均由农业部动物流感重点开放实验室分离、鉴定并保存;鸡抗ZJ245病毒的多克隆血清由农业部动物流感重点开放实验室制备、保存;真核表达载体pCAGGS、293T细胞由农业部动物流感重点开放实验室保存;6周龄雌性BALB/c小鼠购自北京维通利华实验动物技术有限公司。
1.2 主要试剂
限制性内切酶I和Ⅱ购自New England Biolabs公司;RNA提取试剂盒购自天根生化科技有限公司、胶回收试剂盒购自OMEGA公司、质粒提取试剂盒购自QIAGEN公司;胎牛血清购自ExCell公司;DMEM和Opti-MEM培养基购自Gibco公司;异硫氰酸荧光素标记羊抗鸡 IgG 抗体(IgG-FITC)购自SIGMA公司、IRDye800CW羊抗鸡IgG抗体购自LI-COR公司;DNA分子Marker购自TaKaRa公司;感受态细胞DH5α购自TaKaRa公司;转染试剂Lipofectamine 3000购自Invitrogen公司;受体破坏酶 (Receptor destroying enzyme,RDE)购自日本生研;鸡抗ZJ245病毒的多克隆血清由农业部动物流感重点开放实验室制备。
1.3 病毒RNA提取及HA基因的扩增与鉴定
按照RNA提取试剂盒说明书进行病毒RNA的提取,以Uni12引物(序列为:5′-AGCAAAAGCAGG-3′)按照M-MLV说明书进行反转录,以获得的cDNA为模板,以HA基因的特异性引物扩增目的基因的开放阅读框(ORF)。引物:HAF: 5′-GCAATG GAAACAAAACTATTTGTATTA-3′、HAR: 5′-GCATTAAATGCATACTCTGCATTGC-3′(上游引物HAF带有I酶切位点,下游引物HAR带有Ⅱ酶切位点)。PCR程序为:95℃预变性5 min,94℃变性1 min,55℃退火40 s,72℃延伸2 min,循环扩增35次,72℃终延伸10 min。PCR产物经电泳、胶回收目的片段备用。
1.4 表达HA基因的重组表达质粒构建与鉴定
应用I和Ⅱ对HA基因片段进行酶切处理后与进行相应双酶切处理的pCAGGS质粒大片段连接,转化感受态细胞DH5α,提取质粒,进行I和Ⅱ双酶切鉴定,并对重组质粒中的HA基因进行测序验证,将构建的重组质粒命名为pCAGGS-HA (ZJ245)。
1.5 重组质粒pCAGGS-HA(ZJ245)的瞬时表达及检测
1.5.1 IFA检测 按照Lipofectamine 3000说明书要求,将重组质粒pCAGGS-HA(ZJ245)转染293T细胞,同时转染质粒pCAGGS为载体对照,并设立空白对照。转染后细胞,于37℃、5%CO2条件下培养,48 h后,用70%的冰乙醇4℃固定20 min,PBST洗3次后,以鸡抗ZJ245病毒的多克隆血清作为一抗(1﹕100),37℃作用2 h,PBST洗3次后,再以羊抗鸡IgG 抗体(IgG-FITC)作为二抗(1﹕500),室温作用45 min,PBST洗3次后,于荧光显微镜下观察。
1.5.2 Western blot检测 用重组质粒pCAGGS- HA(ZJ245)转染293T细胞,48 h后收集细胞,裂解后进行SDS-PAGE电泳,将蛋白转移至聚偏二氟乙烯(PVDF)膜,5%脱脂乳封闭2 h;洗膜3次后,以鸡抗ZJ245的多克隆血清作为一抗(1﹕100),室温孵育2 h;洗膜3次后,以IRDye800CW羊抗鸡IgG抗体作为二抗(1﹕5 000),室温孵育1 h;洗膜3次后,经红外激光成像系统扫膜进行Western blot检测。
1.6 小鼠的免疫及攻毒试验
将32只6周龄雌性BALB/c小鼠随机分为4组,每组8只。两个免疫组I、II组经腿部肌肉多点注射重组质粒pCAGGS-HA(ZJ245),免疫剂量为100 µg/只;剩余两组(III和IV)作为非免疫组,以相同的方式注射100 µL无菌PBS。间隔3周后进行第二次免疫,各组小鼠于免疫后每周采血,分离血清,测定抗HA蛋白的抗体。
二免2周后进行攻毒,采用滴鼻途径对I和III组小鼠用50 µL(106.0EID50)的ZJ245病毒进行攻毒, II和IV组小鼠用50 µL(106.0EID50)HLJ44病毒进行攻毒。攻毒后14 d,每天观察试验小鼠的临床症状,统计发病和死亡情况,并每天称量小鼠体重,体重下降比率超过25%的小鼠判定为死亡。攻毒后第3 天,每组随机剖杀3只小鼠,分别采集脑、鼻甲、肺、脾和肾等脏器,进行脏器的病毒含量测定。
1.6.1 HI抗体的测定 为了去除小鼠血清中非特异性血凝抑制因子,采用RDE对血清样品进行处理,以ZJ245和HLJ44为HI抗原,采用微量HI方法测定,以完全抑制4 HAU抗原的血清最高稀释倍数作为HI效价。
1.6.2 VN抗体的测定 首先测定病毒ZJ245和HLJ44的TCID50,将病毒适当稀释备用。将RDE处理过小鼠血清进行2倍倍比稀释,按照每孔50 µL终浓度为1﹕20、1﹕40等作倍比稀释,至1﹕10 240。将稀释后的血清样品与100TCID50的病毒混合,37℃作用1 h,然后将其接种于汇合度80%的MDCK单层细胞,37℃、5% CO2条件下继续培养,期间观察细胞病变情况,72 h后测定细胞上清的HA活性,最终以完全抑制红细胞凝集的最高血清稀释倍数作为中和效价。
1.6.3 脏器病毒测定 攻毒后第3 天,采取小鼠的脑、鼻甲、肺、脾和肾等组织,匀浆后离心取上清液,10倍倍比稀释后,将各稀释度接种至10日龄的非免疫鸡胚,每一稀释度接种3枚,0.1 mL/枚,72 h后测定鸡胚尿囊液的HA活性,按照Reed-Muench法计算结果。
1.6.4 数据统计分析 应用软件GraphPad Prism 6.0对免疫组与非免疫组的抗体滴度、病毒滴度采用Two way ANOVA进行统计分析,对其体重变化应用t-test进行统计分析。<0.05表示统计学差异显著,<0.01表示差异极显著。
2 结果
2.1 重组质粒pCAGGS-HA(ZJ245)的构建与鉴定
以病毒ZJ245的RNA为模板,RT-PCR扩增得到HA基因的ORF,大小为1 701bp(图1-A)。重组质粒pCAGGS-HA(ZJ245)经I和Ⅱ双酶切后,可得到两个条带,大小分别与空质粒pCAGGS和插入基因HA的大小相符(图1-B)。通过对重组质粒中的HA基因进行测序,发现与之前测得的序列完全一致,表明重组质粒pCAGGS-HA(ZJ245) 构建正确。
2.2 重组质粒表达的HA的IFA与Western blot检测
IFA检测结果表明,转染pCAGGS-HA(ZJ245)的293T细胞可观察到明显的绿色荧光(图2-A),对照组细胞未观察到荧光(图2-B)。Western blot结果显示,转染pCAGGS-HA(ZJ245)的293T细胞样品在72kD处有目的条带(图3)。表明HA蛋白在真核细胞中能正确表达,并具有良好的生物学活性。
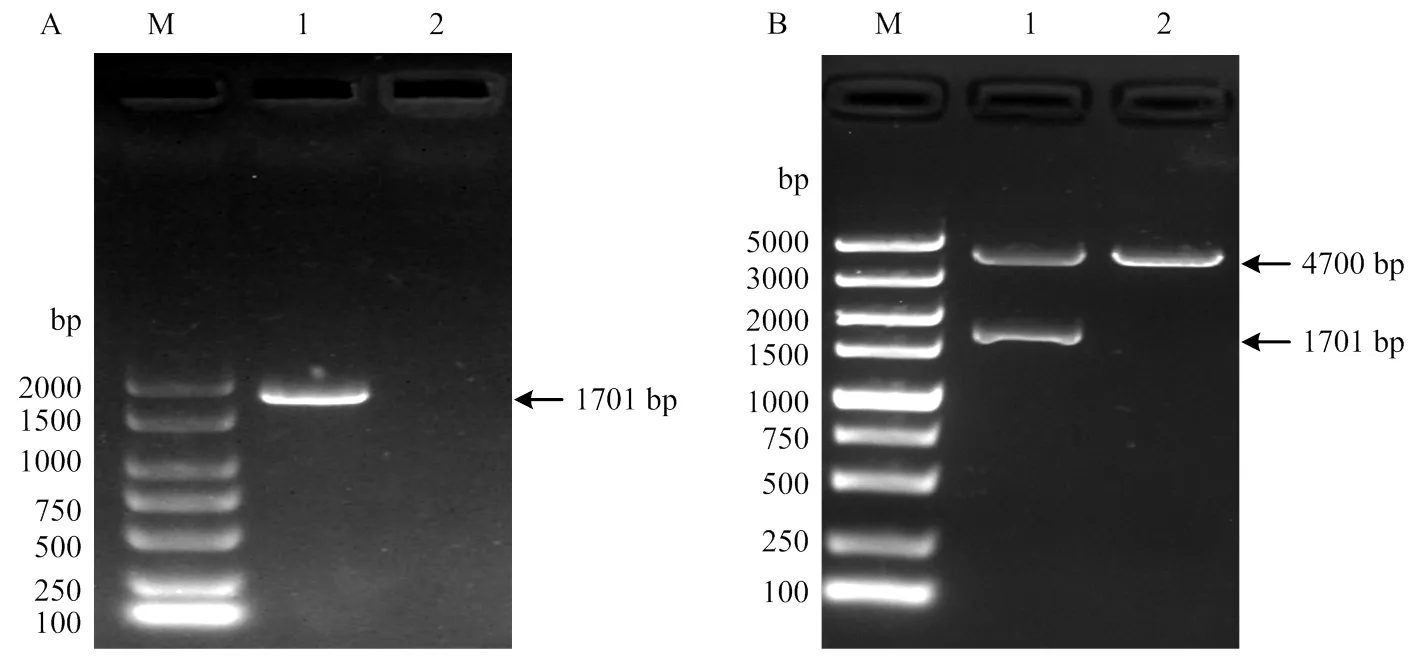
(A)M:DL 2000 DNA 相对分子质量标准;1:HA基因;2:水对照。(B)M:DL 5000 DNA 相对分子质量标准;1:pCAGGS-HA(ZJ245)酶切鉴定;2:pCAGGS酶切鉴定

A:pCAGGS-HA转染293T细胞;B:pCAGGS转染293T细胞
2.3 重组质粒pCAGGS-HA(ZJ245)免疫小鼠诱导产生的抗体水平
为了评价重组质粒pCAGGS-HA(ZJ245)的免疫原性,将pCAGGS-HA(ZJ245)免疫BALB/c小鼠,一免3周后进行二免,首免和二免后每周对各组小鼠采血,分离血清,进行RDE处理后,分别以ZJ245和HLJ44作为检测抗原,采用HI试验和VN试验检测抗体效价。pCAGGS-HA(ZJ245)免疫组,利用ZJ245作为HI抗原,首免后1周可检测到较低水平的HI抗体,效价为10.63,二免后1周HI抗体显著升高,达到76.88(<0.0001);用HLJ44作为HI抗原,首免后1周检测不到HI抗体,二免后1周HI抗体略有升高,为15.63(图4-A)。首免后1周可检测到抗ZJ245的VN抗体,效价为21.87,二免后1周VN抗体效价显著提升(<0.01),达到152.5;首免后1周可检测到抗HLJ44的VN抗体,效价为13.13,二免后1周抗体略有升高,效价为32.5(图4-B)。
图4 pCAGGS-HA(ZJ245)免疫小鼠的HI抗体效价(A)和VN抗体效价(B)
2.4 重组质粒对小鼠的免疫保护效果
根据小鼠攻毒后的体重变化情况及脏器病毒含量滴定结果来评估pCAGGS-HA(ZJ245)的免疫保护效果。
2.4.1 小鼠体重变化 用同源H1N1病毒ZJ245攻毒后,pCAGGS-HA(ZJ245)免疫组小鼠未出现体重下降,而非免疫组小鼠在攻毒后体重出现下降(最大下降比率达4.39%),攻毒后免疫组与非免疫组之间小鼠体重变化差异非常显著(<0.0001)(图5-A);而当采用异源H1N1病毒HLJ44攻毒时,非免疫组小鼠体重出现了明显下降(最大下降比率达16.19%),而pCAGGS-HA(ZJ245)免疫组小鼠的体重在攻毒后3 d内体重略有下降,之后缓慢上升,二者体重变化差异非常显著(<0.0001)(图5-B)。
2.4.2 脏器病毒测定 ZJ245攻毒时,免疫组和非免疫组小鼠的脑、肾脏和脾脏均未滴定到病毒(统计分析时赋值为100.5EID50);非免疫组小鼠的肺脏和鼻甲中的病毒含量较高,滴度分别为 106.5EID50和 102.75EID50,而在pCAGGS-HA(ZJ245)免疫组小鼠的肺脏与鼻甲中均未检测到病毒(<0.0001、<0.0001)(图6-A)。类似的,HLJ44攻毒时,免疫组和非免疫组小鼠的脑、肾脏和脾脏也未滴定到病毒;非免疫组小鼠的肺脏和鼻甲中均检测到高滴度的病毒(分别为107.64EID50和106.67EID50),与非免疫组相比,pCAGGS- HA(ZJ245)免疫组小鼠的肺脏和鼻甲中病毒含量均明显降低,分别为103.98EID50和104.33EID50(<0.001、<0.05)(图6-B)。

图5 ZJ245和HLJ44攻毒后小鼠的体重变化

图6 ZJ245(A)和HLJ44(B)攻毒后小鼠的脏器滴定
3 讨论
EA H1N1亚型SIV已经在欧洲和亚洲的许多国家的猪群中传播[7, 22],近年来的SI病原学和血清学监测结果显示当前EA H1N1 SIV已成为国内猪群中的主要流行毒株[9]。EA H1N1 SIV 不但能够造成猪的感染,并且能够作为基因供体,与其他基因型及亚型毒株之间形成新的基因重配毒株。自2009年以来,EA H1N1 SIV与pdm2009/H1N1、H1N2、H3N2病毒的重配型毒株多次在猪群中被检测到[23-25]。因此,该亚型病毒给畜牧业和人类公共卫生安全产生潜在威胁[9]。疫苗免疫作为流感防控的重要手段,SI灭活疫苗已发挥了重要的作用,但此类疫苗对新出现的抗原变异毒株无法提供有效保护,同时又会因为感染毒株与疫苗毒株抗原性的差异进一步导致动物呼吸道疾病的发生,加重病理损伤,这种情况被称之为疫苗相关增强呼吸系统疾病(vaccine-associated enhanced respiratory disease,VAERD)[26]。因此,加快对安全、高效新型基因工程疫苗的研发,为更好的防制SI具有重要的意义。
DNA疫苗能够同时诱导机体产生体液免疫应答和细胞免疫应答,具备产生长期免疫应答和交叉保护抗体的能力[27]。DNA疫苗的一系列优势,奠定了其在通用型流感疫苗的研制方面具有巨大潜力和良好前景。真核表达载体pCAGGS作为一种DNA疫苗载体,已成功应用于DNA疫苗的研究,并证实了其良好的免疫效果[28-29]。HA蛋白是流感病毒的主要保护性抗原,本研究选取EA H1N1 SIV毒株ZJ245的HA基因作为靶抗原,构建了重组表达质粒pCAGGS- HA(ZJ245),经体外活性检测,证实HA蛋白得到正确表达,进而制备DNA疫苗,通过免疫BALB/c小鼠评估了重组质粒的免疫原性及对不同抗原型H1N1 SIV的免疫保护效果。
针对流感病毒HA蛋白产生的抗体是机体抵抗病毒感染的主要保护性抗体,研究发现HI抗体水平达到40或者40以上时即可对同源病毒的攻击提供完全保护[30]。本研究中,pCAGGS-HA(ZJ245)首免及二免后每周测定HI和VN抗体结果发现二免后2周,免疫组小鼠针对ZJ245的HI抗体水平达到61.87,VN抗体比HI抗体出现的早,且高于同期的HI水平。攻毒保护试验中,分别通过体重变化和小鼠脏器病毒含量测定评价疫苗的免疫保护效果。结果表明,pCAGGS-HA(ZJ245)两次免疫后,免疫组小鼠能够对EA H1N1 SIV毒株ZJ245的感染产生完全的免疫保护能力。已有研究证实由EA H1N1 SIVs感染引起的机体免疫应答能够抵抗含有CS H1N1 SIV的HA基因的北美H1N1 SIVs的感染,并对pdm H1N1/2009病毒的感染提供一定的交叉保护[31]。本研究中,也评估了pCAGGS-HA(ZJ245)免疫对pdm H1N1/2009毒株HLJ44的攻毒保护力,结果显示,免疫组小鼠在一免后3周可产生针对HLJ44抗原的较低水平的HI抗体,VN抗体在一免后1周即可检测到,但两者均低于同期针对ZJ245的HI和VN抗体,表明EA H1N1和pdm H1N1/2009病毒抗原性的不同,且它们之间存在部分血清学交叉反应。HLJ44攻毒后,免疫组小鼠的体重下降水平以及肺脏、和鼻甲内的病毒含量均显著低于非免疫组,由此可见pCAGGS-HA(ZJ245)免疫在一定程度上对pdm H1N1/2009 SIVs提供了交叉保护。BRAUCHER等[32]通过对一株表达pdm H1N1/2009流感病毒HA蛋白复制缺陷型腺病毒的免疫原性研究发现,重组腺病毒诱导小鼠产生的细胞免疫应答在清除同源 pdm H1N1/2009病毒感染与抵御异源H1N2亚型SIV交叉保护方面起到了重要作用。本研究pCAGGS-HA (ZJ245)的免疫在低水平抗体出现时依然能够诱导对异源病毒攻击有效的交叉保护,经分析认为DNA疫苗的免疫保护效果不单单依赖于体液免疫应答,细胞免疫应答也具有关键作用。因此,在后续研究中会对该疫苗诱导的细胞免疫应答水平开展系统的检测,并进一步评估该DNA疫苗对猪的免疫保护效果。
4 结论
构建的重组质粒pCAGGS-HA(ZJ245)可有效表达EA H1N1 SIV 的HA蛋白,两次免疫能够有效诱导机体产生针对同源EA H1N1 SIV的高水平的HI和VN抗体,对同源EA H1N1 SIV的感染提供完全保护,对异源pdm H1N1/2009 SIV的感染也具有一定的交叉免疫保护效果。
[1] BROWN I. The epidemiology and evolution of influenza viruses in pigs., 2000, 74(1/2): 29-46.
[2] WEBBY R J, WEBSTER R G. Emergence of influenza A viruses., 2001, 356(1416): 1817-1828. doi:10.1098/rstb.2001.0997.
[3] BAUDON E, PEYRE M, PEIRIS M, COWLING B J. Epidemiological features of influenza circulation in swine populations: A systematic review and meta-analysis., 2017, 12(6): e179044.doi: 10.1371/journal.pone.0179044.
[4] NEUMANN G, NODA T, KAWAOKA Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus., 2009, 459(7249): 931-939.doi: 10.1038/nature08157.
[5] SCHULTZ U, FITCH W M, LUDWIG S, MANDLER J, SCHOLTISSEK C. Evolution of pig influenza viruses., 1991, 183(1): 61-73.
[6] LIU J, BI Y, QIN K, FU G, YANG J, PENG J, MA G, LIU Q, PU J, TIAN F. Emergence of European avian influenza virus-like H1N1 swine influenza A viruses in China., 2009, 47(8): 2643-2646. doi: 10.1128/JCM.00262-09.
[7] VINCENT A, AWADA L, BROWN I, CHEN H, CLAES F, DAUPHIN G, DONIS R, CULHANE M, HAMILTON K, LEWIS N, MUMFORD E, NGUYEN T, PARCHARIYANON S, PASICK J, PAVADE G, PEREDA A, PEIRIS M, SAITO T, SWENSON S, VAN REETH K, WEBBY R, WONG F, CIACCI-ZANELLA J. Review of influenza A virus in swine worldwide: a call for increased surveillance and research., 2014, 61(1): 4-17.doi: 10.1111/zph.12049.
[8] GUAN Y, SHORTRIDGE K F, KRAUSS S, LI P H, KAWAOKA Y, WEBSTER R G. Emergence of avian H1N1 influenza viruses in pigs in China., 1996, 70(11): 8041-8046.
[9] YANG H, CHEN Y, QIAO C, HE X, ZHOU H, SUN Y, YIN H, MENG S, LIU L, ZHANG Q, KONG H, GU C, LI C, BU Z, KAWAOKA Y, CHEN H. Prevalence, genetics, and transmissibility in ferrets of Eurasian avian-like H1N1 swine influenza viruses., 2016, 113(2): 392-397.doi: 10.1073/pnas.1522643113.
[10] CAMPITELLI L, DONATELLI I, FONI E, CASTRUCCI M R, FABIANI C, KAWAOKA Y, KRAUSS S, WEBSTER R G. Continued evolution of H1N1 and H3N2 influenza viruses in pigs in Italy., 1997, 232(2): 310-318.doi: 10.1006/viro.1997.8514.
[11] DE JONG J C, PACCAUD M F, DE RONDE-VERLOOP F M, HUFFELS N H, VERWEI C, WEIJERS T F, BANGMA P J, VAN KREGTEN E, KERCKHAERT J A, WICKI F, WUNDERLI W. Isolation of swine-like influenza A (H1N1) viruses from man in Switzerland and the Netherlands., 1988, 139(4): 429-437.
[12] WANG D Y, QI S X, LI X Y, GUO J F, TAN M J, HAN G Y, LIU Y F, LAN Y, YANG L, HUANG W J, CHENG Y H, ZHAO X, BAI T, WANG Z, WEI H J, XIAO N, SHU Y L. Human infection with Eurasian avian-like influenza A(H1N1) virus, China., 2013, 19(10): 1709-1711.doi: 10.3201/eid1910. 130420.
[13] YANG H, QIAO C, TANG X, CHEN Y, XIN X, CHEN H. Human infection from avian-like influenza A (H1N1) viruses in pigs, China., 2012, 18(7): 1144-1146. doi:10.3201/ eid1807.120009.
[14] SUI J, YANG D, QIAO C, XU H, XU B , WU Y, YANG H, CHEN Y, CHEN H. Protective efficacy of an inactivated Eurasian avian-like H1N1 swine influenza vaccine against homologous H1N1 and heterologous H1N1 and H1N2 viruses in mice., 2016, 34(33): 3757-3763.doi:10.1016/j.vaccine.2016.06.009.
[15] RUAN B, WEN F, GONG X, LIU X M, WANG Q, YU L X, WANG S Y, ZHANG P, YANG H M, SHAN T L, ZHENG H, ZHOU Y J, TONG W, GAO F, TONG G Z, YU H. Protective efficacy of a high-growth reassortant H1N1 influenza virus vaccine against the European avian-like H1N1 swine influenza virus in mice and pigs., 2018, 222: 75-84.doi: 10.1016/j.vetmic. 2018.07.003.
[16] LIU L, LU J, ZHOU J, LI Z, ZHANG H, WANG D, SHU Y. Construction and comparison of different source neuraminidase candidate vaccine strains for human infection with Eurasian avian-like influenza H1N1 virus., 2017, 19(12): 635-640.doi: 10.1016/j.micinf.2017.08.004.
[17] WU Y, YANG D, XU B, LIANG W, SUI J, CHEN Y, YANG H, CHEN H, WEI P, QIAO C. Immune efficacy of an adenoviral vector-based swine influenza vaccine against antigenically distinct H1N1 strains in mice., 2017, 147: 29-36. doi: 10.1016/j.antiviral.2017.09.009.
[18] SHVARTSMAN D E, KOTLER M, TALL R D,ROTH M G, HENIS Y I. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts., 2003, 163(4): 879-888. doi: 10.1083/jcb.200308142.
[19] TREGONING J S, KINNEAR E. Using plasmids as DNA vaccines for infectious diseases., 2014, 2(6):1-16.doi: 10.1128/microbiolspec.PLAS-0028-2014.
[20] 徐汇洋,许榜丰,陈艳,隋金钰,杨焕良,尹航,杨大为,乔传玲,陈化兰. 一株H1N1猪流感病毒的进化分析与分子特征[J]. 中国农业科学, 2015(15): 3071-3078. doi:10.3864/j.issn.0578-1752. 2015.15.018.
XU H Y, XU B F, CHEN Y, SUI J Y, YANG H L, YIN H, YANG D W, QIAO C L, CHEN H L. Phylogenetic analysis and molecular characteristics of an H1N1subtype swine influenza virus., 2015(15): 3071-3078. (in Chinese). doi: 10.3864/ j.issn.0578-1752.2015.15.018.
[21] CHEN Y, ZHANG J, QIAO C, YANG H, ZHANG Y, XIN X, CHEN H. Co-circulation of pandemic 2009 H1N1, classical swine H1N1 and avian-like swine H1N1 influenza viruses in pigs in China.2013, 13: 331-338.doi: 10.1016/j.meegid. 2012.09.021.
[22] PENSAERT M, OTTIS K, VANDEPUTTE J, KAPLAN M M, BACHMANN P A. Evidence for the natural transmission of influenza A virus from wild ducts to swine and its potential importance for man., 1981, 59(1): 75-78.
[23] DUCATEZ M F, HAUSE B, STIGGER-ROSSER E, DARNELL D, CORZO C, JULEEN K, SIMONSON R, BROCKWELL-STAATS C, RUBRUM A, WANG D, WEBB A, CRUMPTON J C, LOWE J, GRAMER M, WEBBY R J. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States., 2011, 17(9): 1624-1629. doi:10.3201/ eid1709.110338.
[24] QIAO C, LIU L, YANG H, CHEN Y, XU H, CHEN H. Novel triple reassortant H1N2 influenza viruses bearing six internal genes of the pandemic 2009/H1N1 influenza virus were detected in pigs in China., 2014, 61(4): 529-534. doi: 10.1016/j.jcv. 2014.10.014.
[25] WATSON S J, LANGAT P, REID S M, LAM TT, COTTEN M, KELLY M, VAN REETH K, QIU Y, SIMON G, BONIN E, FONI E, CHIAPPONI C, LARSEN L, HJULSAGER C, MARKOWSKA- DANIEL I, URBANIAK K, DÜRRWALD R, SCHLEGEL M, HUOVILAINEN A, DAVIDSON I, DÁN Á, LOEFFEN W, EDWARDS S, BUBLOT M, VILA T, MALDONADO J, VALLS L, ESNIP3 CONSORTIUM, BROWN IH, PYBUS OG, KELLAM P.Molecular epidemiology and evolution of influenza viruses circulating within European swine between 2009 and 2013., 2015, 89(19): 9920-9931. doi: 10.1128/JVI.00840-15.
[26] RAJÃO D S, CHEN H, PEREZ D R, SANDBULTE M R, GAUGER P C, LOVING C L, SHANKS G D, VINCENT A. Vaccine-associated enhanced respiratory disease is influenced by Haemagglutinin and Neuraminidase in whole inactivated influenza virus vaccines.irology, 2016, 97(7): 1489-1499. doi:10.1099/jgv.0. 000468.
[27] PILLET S, KOBASA D, MEUNIER I, GRAY M, LADDY D, WEINER D B, VON MESSLING V, KOBINGER G P. Cellular immune response in the presence of protective antibody levels correlates with protection against 1918 influenza in ferrets., 2011, 29(39): 6793-6801. doi: 10.1016/j.vaccine.2010.12.059.
[28] JIANG Y, YU K, ZHANG H, ZHANG P, LI C, TIAN G, LI Y, WANG X, GE J, BU Z, CHEN H. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with codon optimized HA gene in a pCAGGS plasmid vector., 2007, 75(3): 234-241. doi:10.1016/j.antiviral.2007.03.009.
[29] PING X, HU W, XIONG R, ZHANG X, TENG Z, DING M, LI L, CHANG C, XU K. Generation of a broadly reactive influenza H1 antigen using a consensus HA sequence., 2018, 36(32 Pt B): 4837-4845. doi:10.1016/j.vaccine.2018.06.048.
[30] MURPHY B R, CLEMENTS M L. The systemic and mucosal immune response of humans to influenza A virus.1989, 146: 107-116.
[31] DE VLEESCHAUWER A R, VAN POUCKE S G, KARASIN A I, OLSEN CW, VAN REETH K. Cross-protection between antigenically distinct H1N1 swine influenza viruses from Europe and North America., 2011, 5(2): 115-122. doi: 10.1111/j.1750-2659.2010.00164.x.
[32] BRAUCHER D R, HENNINGSON J N, LOVING C L, VINCENT A L, KIM E, STEITZ J, GAMBOTTO A A, KEHRLI ME J R. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs., 2012, 19(11): 1722-1729. doi:10.1128/CVI.00315-12.
Immunogenicity Evaluation of Eukaryotic Expressing Plasmids Encoding HA Protein of Eurasian Avian-like H1N1 Swine Influenza Virus
JIA YunHui, XU ChengZhi, SUI JinYu, WU YunPu, XU BangFeng, CHEN Yan, YANG HuanLiang, QIAO ChuanLing, CHEN HuaLan
(Animal Influenza Key Laboratory of the Ministry of Agriculture/State Key Laboratory of Veterinary Biotechnology/Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069)
【Objective】This study aimed to construct the recombinant plasmid expressing HA gene of Eurasian avian-like H1N1 (EA H1N1) swine influenza virus (SIV) and then evaluate its immunogenicity in mice. 【Method】HA gene of A/swine/Zhejiang/ 245/2013(H1N1) (ZJ245) was amplified by RT-PCR, and inserted into an eukaryotic expression vector pCAGGS. The recombinant plasmid, designated as pCAGGS-HA(ZJ245), was transfected into 293T cells, and the expressed HA protein was identified by indirect fluorescence assay (IFA) and western blot. In order to evaluate the immunogenicity of the recombinant plasmid pCAGGS- HA(ZJ245), sixteen six-week-old female BALB/c mice were immunized with 100 µg of the recombinant plasmid by intramuscular injection, and then were boosted once with a 3-week interval. Another group of sixteen mice received 100 µL of phosphate-buffered saline (PBS) were used as unvaccinated control. Serum samples were collected every week after prime and boost immunization in order to detect the hemagglutinin inhibition (HI) antibodies, virus neutralization (VN) antibodies, respectively. Two weeks after the boost immunization, pCAGGS-HA(ZJ245)-immunized and PBS-inoculated mice were intranasally challenged with 50 µL(106.0EID50) of the homologous ZJ245 and heterologous A/swine/Heilongjiang/44/2009(H1N1) (HLJ44), respectively. All mice per group were monitored daily for clinical signs of infection and body weight changes for two weeks. The mice that lost more than 25% of their initial body weight were euthanized on humane ground. On day 3 post-challenge three mice per group were euthanized and their organs including brain, nasal turbinate, lung, spleen and kidney were collected for virus titration in eggs.Immune efficacy of the recombinant plasmid pCAGGS-HA(ZJ245) was evaluated by body weight loss and virus replication titer in mice, respectively.【Result】The recombinant plasmid pCAGGS-HA(ZJ245) was constructed by inserting HA gene of ZJ245 virus and verified by restriction endonuclease analysis and plasmid sequencing. IFA and western blot analysis confirmed that the HA protein could be correctly expressed by the recombinant plasmid pCAGGS-HA(ZJ245) and had a good biological activity in vitro. Immunization and challenge trial indicated that low levels of HI and VN antibody against the homologous ZJ245 virus were initially detected one week after the first immunization, and significantly increased after the second immunization, with the HI titer of 76.88 and the VN titer of 152.5, respectively. Meanwhile, low levels of HI and VN antibodies against the heterologous HLJ44 virus were also detected. Compared with PBS-inoculated mice, the weight loss and viral replication rate of the pCAGGS-HA(ZJ245)-vaccinated mice when challenged with 106.0EID50of the homologous ZJ245 virus were completely inhibited. When challenged with 106.0EID50of the heterologous HLJ44 virus, the extent of weight loss and viral titer of the challenge virus detected in the pCAGGS-HA(ZJ245)- vaccinated mice were significantly lower than those in the PBS-inoculated mice (<0.0001,<0.001,<0.05).【Conclusion】The recombinant plasmid pCAGGS-HA(ZJ245) could efficiently express HA protein, and provided complete protection for the immunized mice against the homologous ZJ245 virus infection and partial cross-protection against the heterologous HLJ44 virus infection, which indicated that the recombinant plasmid pCAGGS-HA(ZJ245) had good immunogenicity.
Eurasian avian-like H1N1 swine influenza virus; hemagglutinin protein; recombinant plasmid; immunogenicity; DNA vaccine
10.3864/j.issn.0578-1752.2019.05.014
2018-09-03;
2019-01-15
国家重点研发计划项目(2017YFD0500604)、黑龙江省自然基金项目(C2018072)
贾云慧,Tel:15004602635;E-mail:jiayunhui1993@163.com。通信作者乔传玲,Tel:0451-51051686;E-mail:qiaochuanling@caas.cn
(责任编辑 林鉴非)
