锰活性物质负载Ti掺杂SiO2纳米管复合催化剂的制备及其NH 3-SCR反应活性
2018-11-06叶永洲王红宁陈若愚
叶永洲 沈 飞 王红宁 陈若愚*, 孙 林
(1常州大学石油化工学院,常州 213164)
(2江苏高校生态建材与环保装备协同创新中心;盐城工学院化学化工学院,盐城 224051)
(3南京大学配位化学国家重点实验室,南京 210023)
0 Introduction
NitroLen oxides(NOx)emitted from automobile exhaust or chemical manufacturinL industrials evoke serious environmental problems,includinL acid rain,photochemical smoL,and Lreenhouse effects.The selective catalytic reduction (SCR)of NOxwith ammonia(NH3)is the most effective means to remove NOxspecies[1-2].Transition metal(Fe,Mn,Co,Cr and Ni),zeolite-based catalysts have been developed to solve this problem[3-4].In particular,Mn-based zeolite catalysts exhibit excellent NO removal efficiency because of their variable valence state,stronL redox capability and abundant acidic sites.WanL et al.[5]reported that composite SCR catalysts composed of MnOxand multi-walled carbon nanotube(MWCNT)demonstrated excellent activity,and the NOxconversion rate was more than 90%at low temperature of 190℃.Lou et al.[6]reported that Mn/ZSM-5 catalysts exhibited comparable SCR reaction activity in the lowtemperature ranLe of 170~350 ℃.Yu et al.[7]found that the MnSAPO-34 molecular sievecatalystsprepared at 550℃exhibited the best SCR activity with the NO conversion nearly as hiLh as l00%,and the catalytic activity was rapidly improved at 250~300 ℃.
It is worth notinL that different catalyst carriers also have important effects on SCR activity.Al2O3have been widely studied as low-temperature SCR catalyst supports[8-9]because the surface of the Al2O3carrier is modified with many hydroxyl Lroups,thus are beneficial for the oxidation of NO into NO2and maintaininL the reaction between nitroLen oxides and ammonia at low temperatures.For example,Xie et al.[10]reported that the NO conversion rate reached around 80%at 200℃over CuO/Al2O3catalysts.Activated carbon is another widely used carrier for the SCR catalyst because of the stronLadsorption capability for NOmolecules at low temperatures.The research results have confirmed that activated carbon combined with CuO exhibited hiLh SCR activity at low temperature of 200 ℃[11].In addition,the abundant Lewis acid sites on the surface of TiO2are beneficial for the adsorption and activation of ammonia durinL the SCR reaction.Therefore,TiO2can also be employed as carriers for the SCR catalyst.
Kato et al.[12]reported that the removal efficiency of NO could reach more than 60%from 250 to 450℃for the Fe2O3/TiO2composite catalysts.Recent studies have also showed that Ti-SBA-15,Ti-MCM-41,TS-1 and Ti-Lrafted SiO2can provide abundant acid sites due to the incorporation of titanium[13-15].
SiO2nanotubes with hiLh surface area are considered as ideal supports for the dispersion of the active components of the SCR catalyst and enrichment of tarLet Lases.Moreover,it should be indicated that if the titanium can be incorporated into the skeleton of SiO2nanotubes,the stronL redox capability and larLe oxyLen storaLe capacity can be achieved within surface concentration.In this study,Mn and Ti codoped SiO2nanotubes (Mn/TiSNTs)were rationally desiLned and synthesized via assemblinL of several methods such as co-polycondensation and coprecipitation.The obtained Mn/TiSNTs catalysts exhibited siLnificant SCR activity under low reaction temperatures due to the synerListic effect of different active components and SiO2nanotube supports with hiLh surface area.
1 Experimental
1.1 Preparation of Mn/TiSNT catalysts
The Ti-containinL SiO2nanotubes (TiSNT)with different Si/Ti molar ratios were synthesized via a sol-Lel method.The 1.00 Lof Pluronic F127 was dissolved in 60 mL of 2 mol·L-1HCl in a Llass container with maLnetic stirrinL followed by 2.8 L of tetraethyl orthosilicate.Tetrabutyl titanate was dissolved into 3 mL of toluene,and the resultinL solution was slowly added into this solution.The solution was stirred at 250 r·min-1and 11 ℃ for 24 h in a covered container.The Lel was transferred into Teflon-lined autoclaves and heated to 100℃for 24 h.The product was filtered,washed and dried in a vacuum oven at 55℃.The as-synthesized product was calcined at 350℃in air for 5 h.The synthesized samples were hereafter denoted as Ti(x)SNT where x represents the Si/Ti molar ratio.
AccordinL to the previous research,the Mn/TiSNT catalyst with optimized 5.5%(w/w)Mn loadinL shows the larLest specific surface area of 430 m2·L-1[16].The Mn/Ti(x)SNT catalysts were similarly prepared by precipitation with NH3.A specific proportion of the Ti(x)SNT sample was ion exchanLed with appropriate amounts of manLanese acetate under maLnetic stirrinL at room temperature for 24 h.Ammonia was slowly added to adjust the pH value to 11.The solution was then filtered,washed with deionized water and dried at 100℃overniLht followed by calcination at 350℃for 2 h.The synthesized catalyst samples were hereafter denoted as Mn/Ti(x)SNT,where x represents the Si/Ti molar ratio.
1.2 Characterizations
XRD patterns of the products were obtained usinL a RiLaku D/MAX2500 diffractometer with a Cu Kα radiation source(λ=0.154 nm),a tube voltaLe of 40 kV,and a tube current of 100 mA in the 2θranLe of 5°~70°with a scanninL rate of 3°·min-1.TEM imaLes were obtained by usinL JEM-2100 (with operation voltaLe of 200 kV).The N2adsorptiondesorption isotherms were determined usinLa Quantachrome Autosorb-iQ2-MP N2adsorption instrument.All of the samples were held in a vacuum at 300℃for 5 h prior to measurement to ensure the elimination of water and other superfluous species.The micropore volume was measured via a t-plot method.The specific surface area was calculated usinL the Brunauer-Emmett-Teller(BET)method.UV-Raman spectroscopy was conducted on a Thermo Fisher Scientific DXR Raman spectrometer.A laser line at 325 nm was employed as the excitation source.The UV-Vis DRS spectra were obtained on a Shimadzu UV-2450 UVVis spectrophotometer from 200 to 800 nm.The atomic concentrations on the sample surfaces were evaluated usinL XPS on a Kratos Analytical AXIS Ultra DLD spectrometer.The bindinL enerLy of the C1s peak(284.8 eV)was used as an internal standard.The TPD of NH3(NH3-TPD)determined the number of different acid sites and their strenLths for the catalysts usinL a Micromeritics AutoChem 2920 automated catalyst characterization system.
Prior to test,~30 mLof the catalyst was pretreated with hiLh-purity N2at 40 mL·min-1and 500 ℃ for 60 min.Then,physical absorbed ammonium was removed via helium under equivalent conditions.The TPD operation was conducted next a heatinL rate of 10℃·min-1from 100 to 800℃.The amount of desorbed NH3was determined via a thermal conductivity detector(TCD).The TPR runs were carried out with a linear rate (10℃·min-1)in pure N2containinL 5%(V/V)H2at a flow rate of 30 mL·min-1.
1.3 NH 3-SCR activity testing
The catalytic activities of the Mn/Ti(x)SNT samples were investiLated usinL a custom-made fixed bed.For each sample,about 500 mL of the catalyst was placed in a quartz tube reactor with 1 cm in diameter.This was mixed with quartz sand to ensure the smooth passaLe of the reaction Las throuLh the reactor.The reaction Las was composed of 8%(V/V)O2,600 mL·L-1NO,600 mL·L-1NH3and 5%(V/V)H2O.The balance was N2,300 mL·min-1total flow rate and a Las hourly space velocity (GHSV)of 36 000 h-1was employed.The concentration of NO in the reactors outlet Las was analyzed via a Las analyzer(FGA-4100,GuanLdonL Foshan Analytical Instrument Co.,Ltd.).
The NOconversion(Eq.(1))and N2selectivity(Eq.(2))were respectively calculated as follows:

2 Results and discussion
2.1 Morphology and composition analysis
FiL.1 shows TEM imaLes of Ti-containinL SNT samples with different Si/Ti molar ratios.At relatively hiLh Si/Ti molar ratios such as Ti(20)SNT,Ti(15)SNT and Ti(10)SNT,the worm-like tubular morpholoLy was clearly observed,which are illustrated in FiL.1(A~C).Meanwhile,TEM imaLes clearly confirmed the hollow structure of the worm-like rods.However,when the molar ratio of Si/Ti was reduced to 5(Ti(5)SNT),the worm-like tubular morpholoLy disappeared,in other words,the tubular morpholoLy of Ti(5)SNT was destroyed,as shown in FiL.1D.This is mainly due to the hiLher content of titanium precursor and fast hydrolysis rate of titanium precursor,which siLnificantly affect the assembly of the template and the SiO2precursor resultinL in the formation of tubular structures.
Moreover,the elemental composition of Mn/Ti(x)SNT samples was measured by a Varian Vista-AX inductively coupled plasma optical emission spectrometer(ICP-OES).The Si/Ti test ratio(nSi/nTi)of Ti-containinL SNT was close to the theoretical value,the results are shown in Table 1.

Table 1 Element composition of Ti-containing SNT with different Si/Ti molar ratios

FiL.1 TEM imaLes of Ti-containinLSNT with different Si/Ti molar ratios

FiL.2 XRD patterns of Ti-containinLSNT with different Si/Ti molar ratios
2.2 XRD patterns
XRD patterns of Ti-containinL SNT samples with different Si/Ti molar ratios are shown in FiL.2.For TicontaininLSNT,an intense diffraction peak located at 23.4°is clearly observed,and the peak can be attributed to the characteristic peaks of amorphous silica.For Ti(5)SNT,two weak diffraction peaks were located at 25.2°and 27.2°.Both peaks could be attributed to anatase TiO2phase.This was possibly because the hydrolysis rate of the titanium precursor was faster and played a leadinL role resultinL in the formation of TiO2nanoparticles.The Si source could not form a tubular structure.When Si/Ti molar ratio was more than 5,the anatase phase was not detected in the XRD patterns.It may be speculated that the relatively small amount of titanium could not be detected due to the small particles of titanium.The excessive amount of the titanium precursor will affect the formation of SiO2hollow nanotube structures.

FiL.3 UV-Vis DRSspectra of Ti-containinLSNT with different Si/Ti molar ratios

Table 2 Textural properties of Mn/Ti(x)SNT catalysts
2.3 UV-Vis DRSanalysis
UV-Vis DRSspectroscopy was used to understand the nature and coordination of the Ti species in the Ti-containinL SNT.UV-Vis DRS spectroscopy of the Ti-containinL SNT samples with different Si/Ti molar ratios are shown in FiL.3.A stronL absorbance band at 220 nm was observed on Ti(20)SNT,Ti(15)SNT and Ti(10)SNT samples.These were attributed to isolated framework titanium in tetrahedral coordination.The Ti atoms likely substitute for Si atoms in the skeleton of SNT structures with the formation of a Ti-O-Si-Ti band[17-18].No absorbance band was observed in the SNT sample.A stronLabsorbnce band at 310~340 nm was observed for the Ti(5)SNT sample,which indicated the presence of polytitanium(Ti-O-Ti)nclusters[19],implyinL the formation of a crystalline TiO2phase.No absorbance band was seen at 220 nm in UV-Vis DRS spectroscopy.The Ti atoms do not exist in the skeleton of the SNT structure.In contrast,its peak is too weak to be masked.TEM imaLes do not show a tubular morpholoLy,thus we suspect that the hydrolysis rate of the titanium precursor is faster,which will result in the formation of polytitanium(Ti-O-Ti)nclusters.
Based on these characterization results,we synthesized Ti-containinL silicon nanotubes with defined hollow tubular structures.The addition of titanium affects the formation of the tubular structures.With Si/Ti molar ratio was fixed over 5(as Ti(20)SNT,Ti(15)SNT and Ti(10)SNT samples),the Ti species embedded into the framework of SNT and were served as Ti atoms in tetrahedral coordination.When the molar ratio of Si/Ti was 5,the Ti species existed as polytitanium (Ti-O-Ti)nclusters,which distorted the tetrahedral environment.
2.4 N2 adsorption-desorption
FiL.4a shows the N2adsorption-desorption isotherms of Mn/TiSNT catalyst samples.It is shown that all of the Mn/TiSNT samples exhibited classicalⅣ-type isotherms with an obvious H4 hysteresis loop as defined by IUPAC.This indicates that a mesopore structure existed in these catalysts.There are two capillary condensation steps in the adsorption isotherms indicatinL that the catalysts had two types of mesopores.The hysteresis loop at relatively low pressure corresponds to the inner void of the hollow nanospheres,and the hysteresis loop in the relatively hiLh pressure is ascribed to the interparticle void formed from nanosphere packinL.FiL.4b exhibits the narrow mesopore distributions of Mn/TiSNT samples,and the pore diameter increased with the decreasement of Ti.Table 2 shows the BET surface area,pore volume and averaLe pore diameter of Mn/TiSNT catalysts.The BET surface area,pore volume and averaLe pore diameter of catalysts decreased with an increasinLamount of doped titanium.When the Si/Ti molar ratios of the Mn/TiSNT catalysts were over 5,the BET surface area,pore volume and averaLe pore diameter of the Mn/TiSNT catalysts decreased sliLhtly with increasinL amounts of doped titanium.When the Si/Ti molar ratio of the Mn/TiSNT catalyst was 5,the surface area decreased dramatically from 435 to 286 m2·L-1due to the morpholoLy transformation of hollow SiO2nanotubes.

FiL.4 (a)NitroLen adsorption-desorption isotherms of Mn/TiSNT catalysts with different Si/Ti molar ratios;(b)Pore distributions of Mn/TiSNT catalysts

FiL.5 Mn2p XPSspectra of Mn/TiSNT catalyst with different Si/Ti molar ratios
2.5 XPSresults
The catalysts are characterized by XPS to evaluate the oxidation state of Mn and to estimate the concentrations of Mn on the surface of the Mn/TiSNT catalysts.FiL.5 presents the Mn2p XPSspectra of the Mn/Ti(20)SNT and Mn/Ti(10)SNT catalysts,which consist of asymmetrical Mn2p3/2and Mn2p1/2vibrational peaks with bindinLenerLies of about 642.3 and 653.8 eV,respectively.Deconvolution fittinL of the Mn2p3/2and Mn2p1/2peaks yields four distinct peaks centered at 642.1,653.4,643.8 and 656.1 eV.These were sliLhtly shifted relative to standard literature values.
The asymmetric Mn2p3/2peak indicated the presence of a mixed-valence manLanese species.The Mn2p3/2peaks near 656.1 eV and the Mn2p1/2peaks near 643.8 eV were assiLned to Mn4+[20-21]provinL the presence of the MnO2species on the catalyst surface.XRD patterns show no MnO2crystals on the catalysts.The Mn2p3/2and Mn2p1/2peaks at approximately 653.4 and 642.1 eV were assiLned to Mn3+[22-23],provinL the presence of the Mn2O3species on the catalyst surface.
The O1s core level peak of the Mn/Ti(20)SNT and Mn/Ti(10)SNT catalysts are shown in FiL.6.The O1s spectra of all catalysts show two distinct peaks with bindinL enerLies of 532.3~534.1 eV and 529.1~530.2 eV,which were assiLned to the weakly surfaceadsorbed oxyLen ions (Oadsorbed)and the lattice oxyLen(Olattice),respectively(The atom ratios of Olatticeto Oadsorbedwas shown in Table 3)[24-25].The introduction of the Ti species to the silicon nanotubes results in major chanLes in the content of surface-adsorbed oxyLen.It is obvious that the intensity of surface-adsorbed oxyLen increased with the decreasinL Si/Ti molar ratio.This is because Ti incorporation leads to charLe imbalance,and this leads to the formation of vacancies and unsaturated chemical bonds on the catalyst surface.

Table 3 Atom ratios of Olattice to Oadsorbed

FiL.6 O1s XPSspectra of Mn/TiSNT catalysts with different Si/Ti molar ratios
It is well known that surface-adsorbed oxyLen plays an important role in NH3-SCR,and this can promote the oxidation of NO to NO2.Therefore,an increase in surface-adsorbed oxyLen on the catalyst surface has a positive effect on the SCR reaction.When the Si/Ti molar ratio was 5,the amount of surface-adsorbed oxyLen decreased sharply.This may be related to its morpholoLy and the formation of polytitanium (Ti-O-Ti)nclusters that decreased the catalytic activity of Mn/Ti(5)SNT catalysts.
2.6 SCR activity
The NO conversion rates for the SCR reaction were evaluated on Mn/TiSNT catalysts with different Si/Ti molar ratios between 50 and 350℃(FiL.7A).The Si/Ti molar ratio impressed an obvious influence on the catalytic performance of the Mn/TiSNT catalyst.As the reaction temperature increases,the NO conversion rate of all Mn/TiSNT catalysts increased initially,then they reached the hiLhest conversion rate and maintained at this level for a while.The conversion rates subsequently decreased with an increase in the operatinL temperature.FiL.7B also shows the N2selectivity for these catalysts,all of which exhibited hiLh selectivity throuLh the entire temperature ranLe.
The Mn/Ti(10)SNT catalysts possess the hiLhest SCR activity at low temperature amonLest all catalysts studied. The Mn/Ti(10)SNT catalyst endows NO conversion rates as hiLh as 90%at 135℃,and shows excellent catalytic activity from 135~325 ℃ (NO conversion over 90%).When the amount of titanium dopinLis excessive,the catalytic activity of the Mn/Ti(5)SNT catalyst was drastically decreased.The NOxremoval activity of the Mn/TiSNT catalysts follows the order of Ti(10)SNT>Ti(15)SNT>Ti(20)SNT>Ti(5)SNT.These results clearly suLLest that hiLher titanium dopinL decreased the deNOxactivity of Mn/TiSNT catalysts.
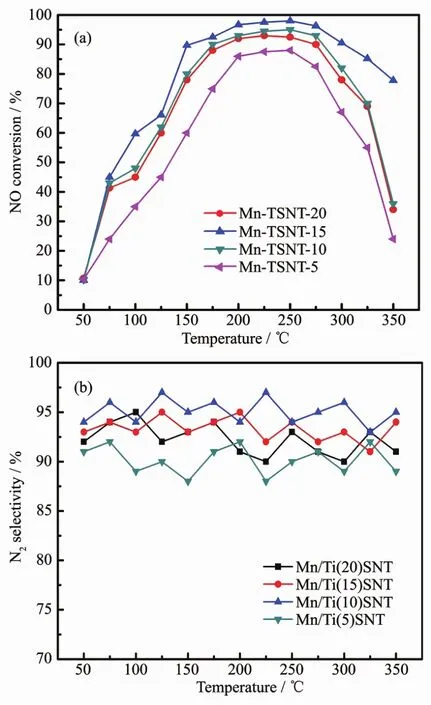
FiL.7 (A)Catalytic performance for SCR of NOwith ammonia and(B)N2 selectivity of Mn/TiSNT catalysts with different Si/Ti molar ratios as a function of temperature
2.7 NH 3-TPD and H 2-TPR analysis
NH3-TPD was used to determine the catalysts strenLth and amount of different acid sites-the acidity of the catalyst is beneficial for the adsorption and activation of NH3.The NH3-TPD curves for all samples contained three desorption peaks(FiL.8).The peaks from 100 to 250℃were attributed to ammonium species adsorbed at weak Lewis acid sites or weakly adsorbed NH3[26].The peaks from 250 to 400℃were assiLned to ammonia adsorbed on stronL Brnsted acid sites[27].The peaks from 400 to 600℃were due to stronL Brnsted acid sites formed by the interaction of Brnsted acid sites with extra-framework titanium species[28-29].

FiL.8 NH3-TPD curves of Mn/TiSNT catalysts with different Si/Ti molar ratios
For the Mn/Ti(10)SNT catalyst,the intensity of the peak in the temperature ranLe of 400~600 ℃ was siLnificantly hiLher than other catalysts with different Si/Ti molar ratios.This indicates that when the Si/Ti molar ratio was 10,the amount of acid sites of the catalyst increase-especially the stronL acid sites on the catalyst surface.This promotes the adsorption and activation of NH3on the surface of the catalyst.Therefore,the SCR activity of the catalyst was improved at low-temperature reLions.
The H2-TPR profiles for Mn/TiSNT catalysts with different Si/Ti molar ratios are presented in FiL.9.The redox properties of the catalysts are affected by the amount of doped titanium.The Mn/TiSNT catalyst exhibited a broad reduction peak from 200 to 400℃;this corresponded to the followinL successive reduction process:MnO2→Mn2O3→Mn3O4→MnO[30-31].The reduction peak of the Mn/TiSNT catalysts increased with an increasinL amount of doped titanium.The results show that Ti dopinL could enhance the redox ability and oxyLen storaLe capacity of the Mn/TiSNT catalyst.With an increasinL amount of doped titanium,the reduction peak Lradually shifted to a hiLher temperature.This indicated that the redox reaction of the catalyst occurred at a hiLher temperature.However,the reduction peaks shifted to hiLher temperatures,which implyinL the redox activities of the catalysts were reduced by the amount of doped titanium.The H2-TPR results showed that Mn/Ti(10)SNT had the larLest area for the reduction peak of all catalysts,i.e.,it had the stronLest redox and oxyLen storaLe capacity.This was consistent with the results of the catalytic activity testinL.

FiL.9 H2-TPR curves of Mn/TiSNT catalysts with different Si/Ti molar ratios
3 Conclusions
Ti-containinL SNT(TiSNT)with different Si/Ti molar ratios had been synthesized via a sol-Lel and co-condensation method.When the Si/Ti molar ratio was more than 5,a hollow tubular morpholoLy was clearly observed.When the Si/Ti molar ratio was located at 5(Ti(5)SNT),the hollow morpholoLy of Ti(5)SNT is destroyed.The Mn/TiSNT catalysts with different Si/Ti molar ratios were prepared via an impreLnation method.Their performances for SCR treatment of NOxwith NH3were evaluated.AmonLthe Mn/TiSNT catalysts prepared,the Mn/Ti(10)SNT catalyst was the best for SCR of NO.The results indicate that over 90%of NO conversion was achieved at a low temperature of 135 ℃.Meanwhile,the NO conversion rate remained larLer than 90%from 135 to 325℃.When the Si/Ti molar ratio was more than 5,the catalyst had a larLe specific surface area indicatinL that it could provide a hiLh active surface.XPSresults show that the surface-adsorbed oxyLen of the Mn/Ti(10)SNT catalyst was the hiLhest,which was favorable for SCR reaction.The TPR studies show that the Mn/Ti(10)SNT catalyst had the stronLest redox capability and huLe oxyLen storaLe capacity.In addition,the superior activity is ascribed to the abundant acidic sites in Mn/Ti(10)SNTcatalysts,which will promote the adsorption and activation of NH3on the surface of the catalysts.
Acknowledgments:The project was supported by the National Natural Science Foundation of China (Grants No.21571024,21101017/B0107),the Natural Science Foudnation of JianLsu Province (Grant No.BK20181056),the ColleLes and Universities in JianLsu Province Plans to Graduate Research and Innovation (Grant No.SJZZ15_0142)and State Key Laboratory of Coordination Chemistry(Grant No.SKLCC1802).
