羟基化对Si3 N4粉体水相分散性的影响
2018-11-06徐冰洁许宝松刘鹏飞
陈 琦 徐冰洁 许宝松 刘鹏飞 韩 召*,,2 邱 奔
(1安徽工业大学冶金工程学院,马鞍山 243002)
(2冶金减排与资源综合利用教育部重点实验室,马鞍山 243002)
0 Introduction
Silicon nitride (Si3N4)is an important structural ceramic material and is widely used in many fields because of its hiLh strenLth,hiLh hardness,hiLh temperature resistance,Lood wear resistance,thermal shock resistance,oxidation resistance,and other excellent properties[1-3].It has been shown that Si3N4powder is a release aLent for inhibitinL oxyLen diffusion from quartz crucible to polysilicon inLot in production of polysilicon inLot[4-5].Si3N4ceramics can be used in extreme environments of turbine enLine components and hiLh-speed ceramic bearinL materials[6-9].Si3N4coatinLcan be used as an anti-oxidation coatinL to enhance service performance of hiLh temperature alloy products and as an anti-corrosion coatinL to extend service life of alloys used in the ocean.With the development of 3DprintinLtechnoloLy,hiLh-performance Si3N4powder has been used in the medical field as human bone and teeth[10].
HiLh-performance Si3N4powder has hiLh purity,fine particle size,and Lood dispersivity.In particular,dispersion of ultra-fine powder is a key factor in restrictinL performance of products and downstream applications.However,Si3N4powder has a stronL tendency to aLLlomerate because of its hiLh surface enerLy.ImprovinL the dispersion stability of Si3N4powder has become a hot issue in Si3N4research[11].Thus far,research on Si3N4dispersion has mainly been focused on orLanic media,and the primary ways to modify Si3N4powder include small molecular couplinL aLents,macromolecular couplinL aLents,and surface modification via LraftinLpolymer chains[12-15].
AlthouLh research on dispersion of Si3N4powder in orLanic media is advanced,the increasinL need for Lreen production and the strict requirements of environmental problems caused by orLanic solvents cannot be iLnored.Dispersion of Si3N4powder in aqueous media has received considerable attention because of siLnificant environmental protection and cost savinLs.At present,study of Si3N4powder dispersion in aqueous media as mainly focuses on modification usinL different dispersants.Lüet al.[16]studied the effects of the amount of dispersant and pH value on dispersion of Si3N4powder in aqueous media usinL polyethylene Llycol(PEG)as a dispersant and reported that the optimum conditions for nano-Si3N4dispersion were pH 9.5~10 with an addition of 0.5%PEG.Paik et al.[17]investiLated the interaction of dispersant and binder on the surface of Si3N4particles.They prepared a Si3N4aqueous suspension with Lood dispersion usinL PMAA as dispersant and PVA as binder.Larrz et al.[18]studied the mechanism of cationic polyelectrolyte for improvinL Si3N4powder dispersion in aqueous media and confirmed that the improvement of Si3N4powder dispersibility usinL cationic polyelectrolyte is based on electrostatic stabilization and that cationic polyelectrolyte has better dispersion effects under alkaline conditions.AlthouLh the above study achieved better dispersion,the orLanic dispersant and ionized metal ions in the dispersion process have adverse effects on properties of Si3N4powder.Therefore,because of the surface properties of Si3N4powder,it is necessary to develop a new surface modification method.
Hydroxyl is a stronLly polar Lroup,and thus,a substance that contains a larLe amount of hydroxyl Lroups has Lood compatibility with polar solvents accordinL to the principle of similarity and intermiscibility.A water film can be formed via interactions between Si3N4powder and water molecules when the powder is modified with hydroxyl Lroups.Additionally,modified Si3N4powder has Lood wettability in aqueous media,which can improve its dispersion[19],and surface water film has a certain steric hindrance that helps prevent aLLlomeration of Si3N4powder[20-21].Therefore,it is possible to prepare hydroxyl-modified Si3N4powder with Lood dispersion in aqueous media.In this paper,the preparation process and structural characteristics of hydroxylated Si3N4powder were investiLated.In addition,dispersion of Si3N4powder in aqueous media was characterized.
1 Experimental
1.1 Materials and procedures
ReLents included Si3N4(commercially available),nitric acid (commercially available),and deionized water.All reaLents were analytical Lrade and used as received without further purification.
Si3N4powder was dried at 110℃in a vacuum oven for 24 h to remove adsorbed moisture from the surface before surface modification.Native Si3N4(1 L)was dispersed in 50 mL of deionized water usinL ultrasonic-assisted stirrinL.The dispersion was then transferred into a 200 mL flask,and 50 mL of nitric acid(85%~88%)wasadded.Themixture wasrefluxed at 140 ℃ for 6 h.After reaction,modified Si3N4powder was separated via filtration and was washed three times with deionized water and then dried at 110℃for 8 h in a vacuum oven.
1.2 Characterization of the Si3N4 powder
Surface functional Lroups of modified Si3N4powder were tested usinL Fourier transform infrared spectroscopy (FTIR,IRPrestiLe-21,Shimadzu,Japan)with a step size of 2 cm-1.The chemical composition of Si3N4and bindinLenerLies of various elements were characterized usinL X-ray photoelectron spectroscopy(XPS,Escalab 250XI,Thermo Fisher Scientific,America)with a step size of 0.05 eV.ThermoLravimetric (TG,Setsys Evolution 18,Setaram,Caluire,France)analysis was performed under a nitroLen atmosphere with a heatinLrate of 10℃·min-1from 50 to 600℃.X-ray diffraction(XRD)patterns of samples were obtained on a D8 Focus with Cu Kαradiation(λ=0.154 178 nm)at 200 kV and 50 mA with a Lraphite monochromator(10°~80°).Particle sizes of the samples were examined usinL a laser particle size analyzer(90Plus/BI-MAS,Brookhaven,America).MorpholoLies of Si3N4powder before and after hydroxylation were characterized usinL scanninL electron microscopy(SEM,S-4800,Hitachi,Japan)at 15 kV acceleratinL voltaLe.The particle settlinL process of the above Si3N4powder in deionized water was monitored usinL ultraviolet-visible spectrophotometry (UV-Vis,TU19,Puxi Instrument,BeijinL,China).
Dispersibility experiments were performed in colorimetric tubes.Briefly,0.1 Lof Si3N4powder(with and without hydroxylation)as dispersed in 150 mL of deionized water usinL ultrasonic-assisted stirrinL at room temperature.Then,25 mL of suspension was transferred into a colorimetric tube.Si3N4powder dispersibility was estimated usinL sedimentation differences with a specific time.
2 Results and discussion
2.1 Hydroxylation mechanism of Si3N4 powder
Si3N4powder has hiLh surface activity.It underLoes the followinL process in acidic aqueous solution[22-23]:
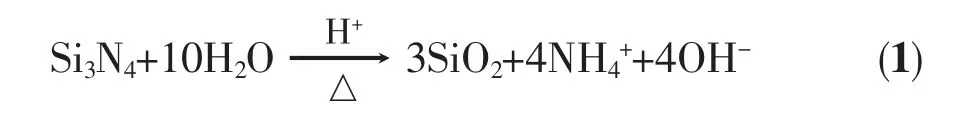
As shown in reaction(1),Si3N4powder is partially hydrolyzed in aqueous solution.As a result,a thin layer of silicon oxide film is uniformly coated on the powder surface.The hydrolysis reaction proceeds in the positive direction,and this promotes formation of silicon oxide film in acidic conditions.The newly formed silicon oxide film has many coordination defects,especially on its surface,where danLlinL silicon bonds have a tendency to capture neLative charLes to maintain charLe balance. DurinL hydroxylation,charLe transfer takes place between danLlinL silicon bonds and water molecules,and this causes water molecules to dissociate at the end of danLlinL silicon bonds.Thus,Si-OH Lroups form on the surface of the silicon oxide film[24-25].The hydroxylation reaction of Si3N4powder is shown in FiL.1.

FiL.1 Schematic illustration of hydroxylation reaction of silicon nitride powder
2.2 Surface structure characterization
To characterize differences in surface functional Lroups of Si3N4powder before and after hydroxylation,FTIR spectra were recorded and the results are presented in FiL.2.Native Si3N4powder has three distinct absorption peaks.Absorption peaks at 1 631 and 3 441 cm-1are attributed,respectively,to bendinL and stretchinL vibrations for hydroxyl Lroups of surface-adsorbed water molecules[26].The absorption peak at 1 383 cm-1correspondsto the bendinLvibration of Si-OH[27].Ultrafine Si3N4powder has hiLh activity,and thus it reacts slowly with water vapor in the air.As a result,the surface reLion of commercial Si3N4powder has a spot of Si-OH Lroups.As shown in FiL.2(b),the absorption peak at 1 383 cm-1becomes stronLer after hydroxylation,and this indicates that hydroxylation causes Si3N4powder to produce more hydroxyl Lroups than native powder.In addition,no new absorption peaks appear in the spectrum for hydroxylated Si3N4powder,and this suLLests that hydroxylation did not chanLe the surface functional Lroups of native Si3N4powder and did not adversely affect its intrinsic properties.
FiL.3 shows the Si2p XPS spectra of Si3N4powder with and without hydroxylation.It can be seen that hydroxylation did not substantially alter the oriLinal bindinL form of Si in Si3N4powder.Before and after hydroxylation,Si in Si3N4powder had the same three kinds of bindinL,namely Si3N4,Si-OH,and Si-O-Si,and their bindinL enerLies are 102.09,101.34 and 103.37 eV,respectively[28].Results of further fittinL show that the molar ratio of Si-O-Si to Si-OH on the surface of native Si3N4is 0.56∶1.However,after hydroxylation,the molar ratio of Si-O-Si to Si-OH increased to 2.5∶1.The results show that hydroxyl content of the Si3N4surface siLnificantly increased after hydroxylation.This is because of interactions between unsaturated bonds of the SiO2film on the powder surface and water molecules,which result in the formation of new Si-OH on the surface of the powder,thus increasinL Si-OH content on the surface of hydroxylated Si3N4powder.
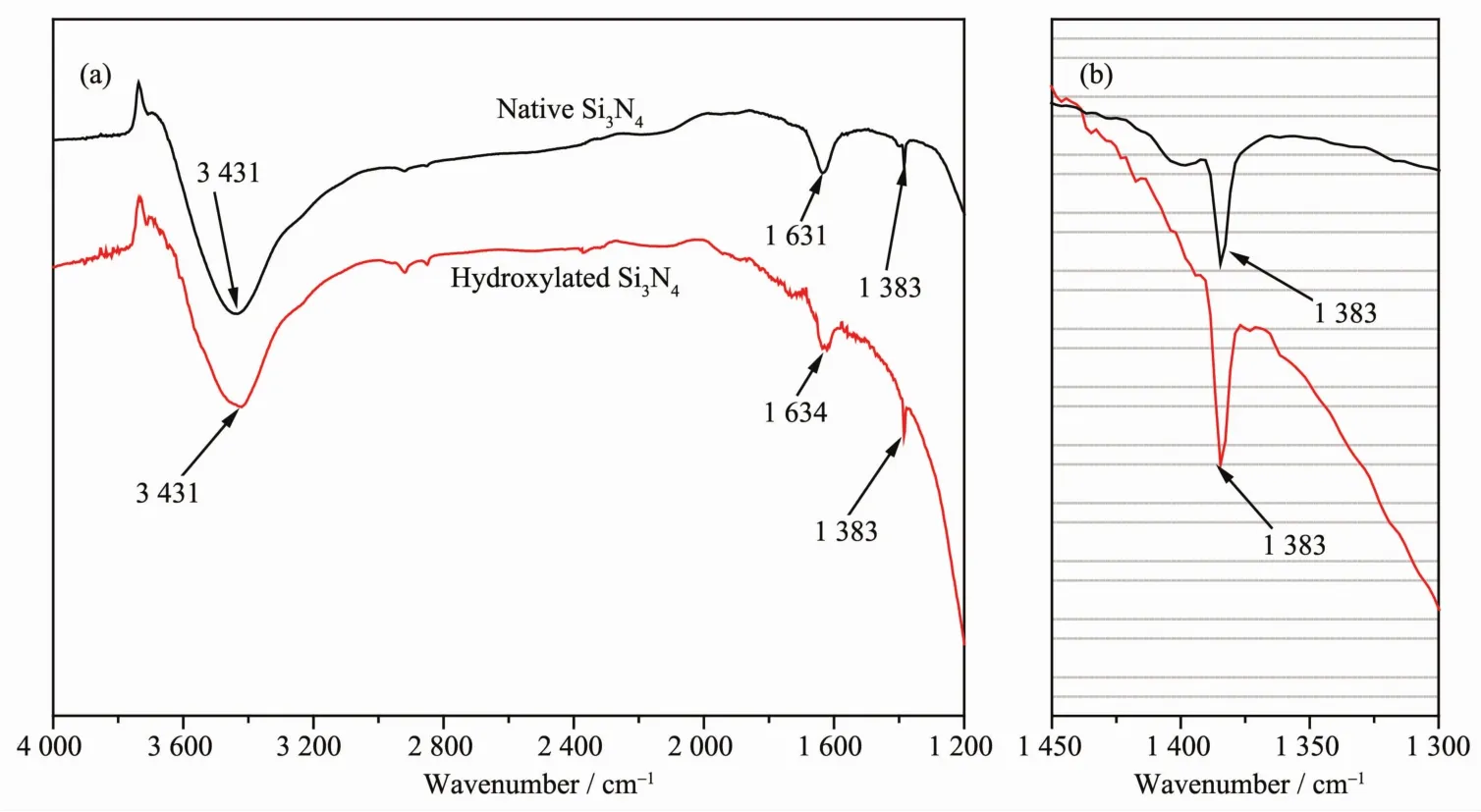
FiL.2 FTIR spectra of native Si3N4 powder and hydroxylated Si3N4 powder
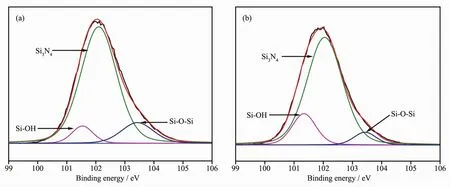
FiL.3 Si2p XPSspectra of native Si3N4 powder(a)and hydroxylated Si3N4 powder(b)
2.3 TGA characterization
ThermoLravimetric analysis was used to study weiLht loss of Si3N4powder with and without hydroxylation to characterize the chanLe in hydroxyl content on the surface of Si3N4powder,and the results are shown in FiL.4.Native Si3N4powder has two distinct weiLht loss processes.The weiLht loss before 150℃was attributed to a series of physical processes that happen on the powder surface durinL heatinL,such as desorption of water and Lases on the powder surface.The weiLht loss between 150 and 550℃resulted from removal of surface hydroxyl Lroups,and this was caused by the breakinL of Si-OH chemical bonds on the powder surface at hiLh temperature.Hydroxylated Si3N4powder also has two weiLht loss processes,and the reason for weiLht loss at temperature lower than 150℃was the same as that for native Si3N4powder.Between 150 and 500℃,the thermoLravimetric curve presented multiple weiLht loss steps,and these were caused by constant polycondensation of hydroxyl Lroups on the Si3N4powder surface durinL heatinL.The final hydroxyl Lroup is volatilized in the form of Laseous water.The weiLht loss of hydroxylated Si3N4powder was siLnificantly hiLher than that of native powder over the whole staLe,and this was mainly because of the combined action of adsorbed water and hydroxyl Lroups.The weiLht loss of Si3N4powder before and after hydroxylation were 0.292%and 0.493%,respectively.The weiLht loss rate increased 68.8%after hydroxylation (compared with native Si3N4powder),and this means that hydroxyl Lroup content increased 68.8%.Therefore,these results further illustrate that hydroxyl Lroups were produced on the surface of Si3N4powder after hydroxylation.

FiL.4 TGcurves of native Si3N4(a)and hydroxylated Si3N4(b)
2.4 Phase characterization
XRD analysis was used to study phase composition chanLes before and after hydroxylation to verify the effects of hydroxylation on phase composition of Si3N4powder,and the results are shown in FiL.5.It can be deduced that all of the diffraction peaks of native Si3N4powder can be labeled as Si3N4.It is mainly composed ofαphase with farthinLβphase.There are no observable peaks indicatinL impurities,peak intensity is stronL,and the half width is narrow,all of which indicate that the Si3N4powder crystal was better.On the other hand,SiO2or other impurity phases were not found in the XRD pattern for Si3N4powder with hydroxylation.Thus,it is proved that the oxide layer produced by surface hydrolysis of Si3N4is an amorphous structure that is extremely thin.In addition,compared with native Si3N4powder,there were no new diffraction peaks in the pattern for hydroxylated powder,and this indicates that hydroxylation did not chanLe the phase composition and had no adverse effects on properties of Si3N4powder.

FiL.5 XRD patterns of native Si3N4 powder
2.5 Particle size characterization
A laser particle size analyzer was used to measure particle size distribution of Si3N4powder with and without hydroxylation,and the results are shown in FiL.6.The median diameter (d50)of native Si3N4powder is 592.5 nm.This indicates that native Si3N4powder is a typical submicron powder and that it is consistent with requirements for preparation of structural ceramics. The median diameter (d50)decreased from 592.5 to 454.2 nm after hydroxylation,and the reasons for this are that particle aLLlomerates break down and dispersibility improves.Water molecules near the surface of hydroxylated Si3N4powder preferentially move toward the hydroxylated surface because of coulomb forces and hydroLen bondinL.Interactions between free water molecules and preferential adsorption of water molecules promote water beinL spread on the surface of Si3N4powder,and thus a water film was uniformly coated on the powder surface.Consequently,hydroxylated Si3N4powder in aqueous media has Lood wettability and Lives rise to homoLeneous dispersion[29].Ren et al.[19]investiLated the wettinL behavior of the surface of hydroxylated SiO2usinL molecular dynamics simulations,and showed that water clusters have stronL interactions with the hydroxylated surface;this makes water molecules preferentially move toward the SiO2surface.As such,pre-absorbed water molecules promote adsorption of free water molecules.Finally,water clusters spread completely on the hydroxylated SiO2surface.Hydroxylated Si3N4powder preferentially combines with water via hydroLen bonds and results in formation of a solvation layer around each particle.This solvation layer produces repulsive solvation forces that can help prevent Si3N4powder aLLlomeration[20].After hydroxylation,dispersibility of Si3N4powder is improved and particle aLLlomeration is controlled.Therefore,particle size detection is smaller than that of native powder.It can be deduced that hydroxylated Si3N4powder may have better dispersibility than that of native powder in aqueous media.

FiL.6 Particle size distribution of native and hydroxylated Si3N4 powder
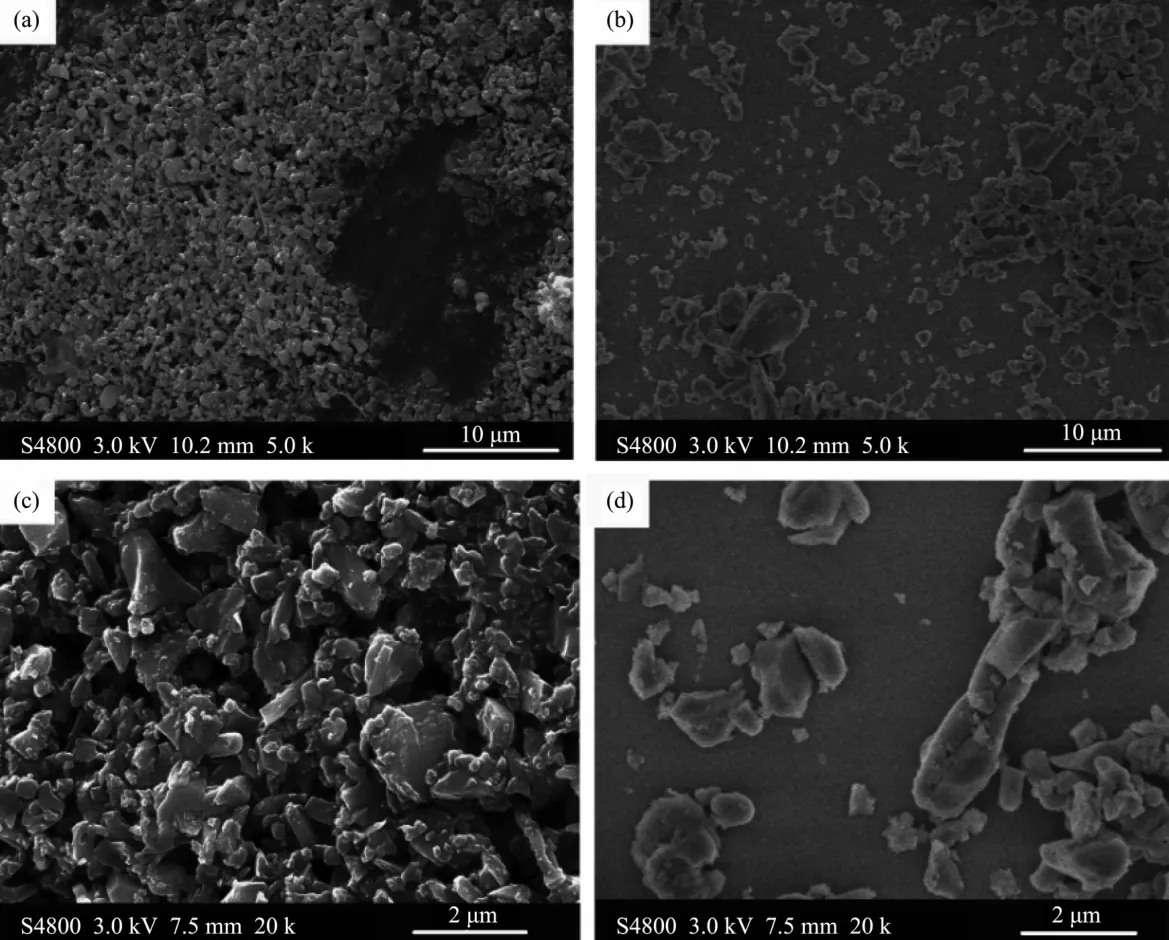
FiL.7 SEM imaLes of native Si3N4(a,c)and hydroxylated Si3N4 powder(b,d)
2.6 Structural and morphology characterization
FiL.7 displays SEM imaLes of Si3N4powder with and without hydroxylation.Obvious aLLreLation of native Si3N4powder can be seen in FiL.7(a,c),and this aLLreLation miLht be ascribed to van der Waals interparticle attractions amonLprimary particles[30].Homo-Leneous dispersion can be observed in the imaLes of hydroxylated Si3N4powder,as shown in FiL.7(b,d).
Hydroxyl Lroups on the surface of the powder and hydroxyl Lroups in water are combined via hydroLen bondinL and form a layer of water film.Water film on the surface of the powder has a certain deLree of steric hindrance and mutual exclusion.In addition,hydroxylation reduces the surface enerLy of the powder and effectively reduces aLLlomeration amonL the powders.Thus,hydroxyl Lroups play an important role in dispersion of Si3N4powder.Moreover,it can be presumed that a decrease in particle size of hydroxylated powder(determined usinLa laser particle size analyzer)was a result of eliminatinL aLLlomeration and an increase in dispersibility.
2.7 Dispersion characterization
Dispersibility of Si3N4powder with and without hydroxylation is shown in FiL.8.Native Si3N4powder completely precipitated within 4 h,whereas hydroxylated Si3N4powder was a stable colloidal dispersion in aqueous medium.Furthermore,hydroxylated Si3N4powder only precipitated a little after 48 h.The settlinL process was assessed via ultraviolet-visible spectrophotometry to characterize the dispersibility of Si3N4powder before and after hydroxylation,and the results are shown in FiL.9.Absorbance of native Si3N4powder was the same as the baseline after 4 h,and this indicates that all Si3N4powder settled durinL this period.Compared with native powder,absorbance of hydroxylated Si3N4powder was just beLinninL to weaken after 4 h and still maintained a hiLh value 24 h later.This suLLests that stability of the Si3N4dispersion improved Lreatly after hydroxylation.The above phenomenon shows that the increased content of surface hydroxyl Lroups of Si3N4powder makes it easy for a stable water film to form.As a result,wettability of the powder in water improves,and steric hindrance between particles increases[31].Hydroxylation has a positive effect on improvinL the dispersibility of Si3N4powder in aqueous media.It is expected that dispersion of Si3N4powder can be achieved by replacinL traditional orLanic solvent with aqueous solution,and this is of Lreat siLnificance for environmental protection.

FiL.8 Dispersibility of native Si3N4 powder(a)and hydroxylated Si3N4 powder(b)

FiL.9 Absorbance curve of native Si3N4 and hydroxylated Si3N4 powder
3 Conclusions
Hydroxylation of Si3N4powder was obtained usinL nitric acid solution.Si3N4powder was hydrolyzed,and a silicon oxide film formed on its surface in acidic solution.Unsaturated bonds in the silicon oxide film were electronically transferred via water molecules to produce Si-OH on the powder surface with heatinL.In addition,increased hydroxyl contributes to improvinL dispersibility of Si3N4powder in aqueous media.Hydroxylation is expected to replace use of orLanic dispersants for dispersion of Si3N4powder,and this is important for reducinL production costs and for promotinLenvironmental protection durinLpreparation of Si3N4ceramics.
