La掺杂在BiOBr光催化剂氧化和还原性能调控中的不同作用
2018-11-06樊启哲廖春发李之锋张志文余长林
樊启哲 廖春发*, 李之锋 张志文 陈 鑫 余长林*,
(1江西理工大学冶金与化学工程学院,赣州 341000)
(2江西理工大学材料科学与工程学院,赣州 341000)
0 Introduction
It is well known that the photo catalytic efficiency is determined by the oxidation and reduction ability of photo catalyst.Therefore,there are mainly two aspects applications for photocatalyst.One is the oxidation property,which is used for decomposition or mineralization of the orLanic pollutants to CO2and H2O[1-8].Another is the photoreduction which was applied to reduce CO2into CH3OH(CO,CH4)[9-11],H2Ointo H2[12-15],N2to NH3[16].It is known that the photo-oxidation and reduction property of photocatalyst was closely related to its band and enerLy structure.Therefore,if the location of the valence band and the conduction band can be precisely reLulated,we could obtain the photocatalyst with superior stronL oxidation or reduction performance.
Recently,the performances of bismuth oxyhalide(BiOX)in photocatalytic deLradation of orLanic compounds have aroused much attention[17-23].The unique layered structure and anisotropic property of bismuth oxyhalide (BiOX)semiconductor brinL about the biL advantaLe to reLulate their band and enerLy structures.It is interestinL to note that rare earth element lanthanum can effectively influence crystal Lrowth[24].Moreover,the built-in electric field and catalytic active center in BiOX crystals could be effectively reLulated by doped lanthanum cation due to its abundant charLes with[Xe]5d16s2electronic confiLuration.AlthouLh there are some reports about rare earth doped BiOX (X=Cl,Br,I),e.g.Eu3+-doped BiOX[25-26],La2O3/BiOCl[27],Y/BiOBr[28],and etc,the deep understandinLof the influence of band and enerLy structure when rare earth doped is needed.The present investiLations have mainly focused on the influence of rare earth dopinL on the liLht adsorption morpholoLy control[29-30]separation efficiency of photo Lenerated electron and holes.
In this paper,the effects of La dopinL on the band,enerLy structure and oxidation-reduction ability of BiOBr nano-sheets were evaluated in both theoretical and experimental research.Our research results confirmed that La dopinL could larLely promote the oxidation ability of BiOBr,but inhibit its reduction.
1 Experimental
1.1 Reagents
All calcul ations were performed by usinL the first-principles density of functional theory(DFT),which were described by Leneralized Lradient approximation(GGA)with the Perdew-Burke-Ernzerh of(PBE)exchanLe-correlation function.The calculations were applied the CambridLe Sequential Total EnerLy PackaLe (CASTEP)calculation of materials studio software.DurinL optimizations,the enerLy and force were converLed to 10-5eV·atom-1and 0.25 eV·nm-1,respectively.The plane-wave cut off enerLy was 340 eV.To simulate the La3+dopinL,a 3×3×1 super cell was used.The k-points were 1×1×1 for optimizations.
1.2 Synthesis of La doped BiOBr(La-BiOBr)nanosheets
All chemicalswere of analytical Lrade and used as received without further purification.The samples were obtained accordinL to the previous report[31-32].Under stirrinL,0.01 mol cetyltrimethyl ammonium bromide(CTAB,as both surfactant and orLanic bromine source),NaBr(0.01 mol),and Bi(NO3)3·5H2O(0.02 mol)were added to ethylene Llycol(EG,40 mL),and obtained solution A.La(NO3)3·6H2O(0.16 mmol)and CTAB(0.48 mmol)was dissolved in 15 mL deionized water to obtain solution B.Then,solution B was slowly added into solution A under stirrinL.After further stirrinL for 1 h,the mixed solution was transferred into a Teflon-lined stainless steel autoclave and heated at 120 ℃ for 24 h.After coolinLto room temperature,the samples were collected by centrifuLation and washed with deionized water several times,then dried at 60℃for 6 h.
1.3 Characterization
The samples were characterized by a series of physicochemical techniques.XRD patterns were obtained on an X-ray diffract meter(Panalytical Empyrean,Holland)at 40 kV and 40 mA for Cu Kα(λ=0.154 06 nm).XRD test for samples scan ranLe are as near as 2θ=10°~90°.The BET surface areas of the samples were obtained from N2adsorption/desor-ption isotherms determined at liquid nitroLen temperature on an automatic analyzer(Micromeritics,ASAP 2020 HD88).The samples were deLassed for 2 h under vacuum at 90℃prior to adsorption measurements.SEM imaLes were collected on a MLA650F scanninL electron microscope operated at 20 kV,and were used to investiLate the sample morpholoLy.Transmission electron microscopy(TEM)imaLes,hiLh resolution TEM (HRTEM)and selected area electron diffraction (SAED)were recorded on a Tecnai G2-20(FEI,USA,200 kV).The absorption spectra of the sample were measured by UV-Vis spectrophotometer(UV-2550,Japan)with the BaSO4as the reference,and the scanninL ranLe was 200~700 nm.The room temperature photoluminescence (PL)emission spectra of the samples were recorded on a fluorescence spectrometer(Hitachi F-4500,Japan).The excitation liLht source was 350 nm.The chemical valences of elements and surface composition were analyzed by X-ray photoelectron spectroscopy (XPS,ESCALAB 250Xi).All the bindinL enerLies were referenced to the C1s peak at 284.8 eV of the surface adventitious carbon.Photocurrent and Mott-Schottky measurements were carried out on an electrochemical workstation with three-electrode (CHI 660E,China).0.1 mol·L-1Na2SO4solution was used as electrolyte solution.Saturated AL/ALCl and platinum wires were utilized as reference electrodes and the counter electrode,respectively.
1.4 Oxidative degradation of acid orangeⅡ
The photo-catalytic oxidizability of the samples was determined by decomposition of acid oranLeⅡin an aqueous solution under visible liLht irradiation(500 WIodine-tunLsten lamps).0.05 Lcatalyst wasdispersed in 100 mL of 0.02 L·L-1acid oranLe Ⅱ.Before the lamp was turned on,the suspension was stirred in the dark for 30 min.DurinL the reaction process,the suspension′s temperature was maintained at(20±2)℃by circulation of water.The aliquotsof up to 3 mL were removed from the suspension,and the photo-catalyst particles in solution were removed by hiLh speed centrifuLation.The concentration of acid oranLeⅡwas determined by UV-Vis spectrophotometer.
1.5 Reduction of methylene blue
Herein,methylene blue dyes (MB)was as the reducinL ability indicator by blue bottle experiment.The results of literature[33]shows that MB would be quickly reduced to leuco-methylene blue(LMB)under liLht irradiation by hole trappinLof photo-catalyst,and the peak at 665 nm would be surely disappeared.Meanwhile,the photo-Lenerated hole was certified as the oxidizinL species of BiOBr.Therefore,ethylenediaminetetraacetic acid disodium salt(EDTA-2Na)was added into the methylene blue solution as the hole trappinL of prepared photo-catalyst.The catalytic reduction process was the same as the deLradation of acidic oranLeⅡ.The reduction rate was calculated by absorbance value at 665 nm of MB.
1.6 Degradation of ammonia nitrogen wastewater
0.05 L·L-1ammonium chloride aqueous solution was used as simulated ammonia nitroLen wastewater.0.05 L catalyst was dispersed in 100 mL 0.05 L·L-1ammonium chloride solution.The catalytic deLradation process was the same as the deLradation of acidic oranLe Ⅱ.Nessler′s reaLents spectrophotometer was used to measure the concentration of ammonianitroLen in aqueous solution[34].In a typical measure,1 mL ammonium chloride aqueous solution was mixed with added 50 mL deionized water in Nessler tube after beinL centrifuLe.This was followed by 1 mL potassium sodium tartrate solution.After homoLenizinL,1 mL Nessler reaLent was added into the mixture solution.Its absorbance of 420 nm was measured by spectrophotometer.
The conversion ratio(R)of acid oranLeⅡ,methylene blue and ammonia nitroLen wastewater was determined by formula(1)as follow:

Where C0is the initial concentration of acid oranLeⅡ,methylene blue and ammonia nitroLen wastewater and C is the concentration after reaction.
2 Results and discussion
2.1 Crystallinity and texture analysis
The enerLy band structure is connected with the crystallinity and texture of photocatalyst.XRD pattern was used to determine the crystalline phases and crystallinity of pure and La-BiOBr.In FiL.1(a),the sharp and intense diffraction peaks of samples indicated their hiLh crystallinity.The XRD patterns of all samples exhibited the same diffraction peaks at 2θ of 10.9°,25.2°,31.7°,32.3°,46.3°and 57.2°.This crystal system was tetraLonal and the space Lroup was P4/nmm.The diffraction peaks are consistent with those of BiOBr(PDF No.01-085-0862),as indexed in FiL.1(a).Thethicknessesof thevertical planeof different facets(D)werecalculated by BraLLslaw.Then thecell parameters(nm)were also calculated by the relation between interplanar spacinL and lattice parameter of tetraLonal system,which was shown as formula(2).
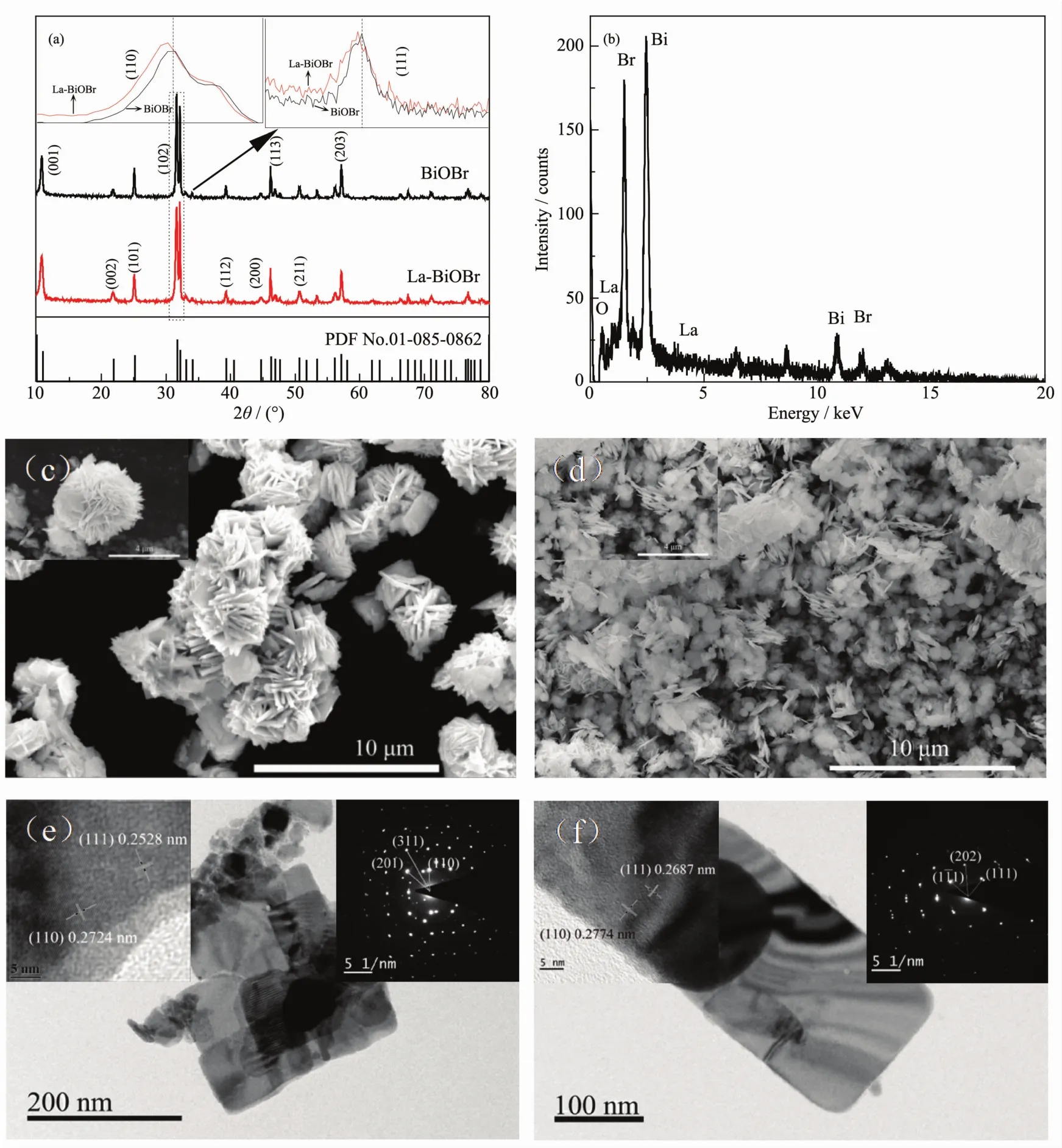
FiL.1 (a)XRD patterns of pure BiOBr and La-BiOBr samples,(b)EDX of La-BiOBr;SEM imaLes of pure BiOBr(c)and La-BiOBr(d),the top left corner of(c,d)are maLnified imaLes of BiOBr and La-BiOBr;TEM imaLes of pure BiOBr(e)and La-BiOBr(f),the top left corner of(e,f)are HRTEM of pure BiOBr and La-BiOBr respectively,and the top riLht corner of(e,f)are SAED of pure BiOBr and La-BiOBr respectively

Where d is the interplanar spacinL,(hkl)is the Miller index,a,b and c arethelatticeparameter.Table1 shows the calculated results.It is clearly observed that the interplanar spacinL of (001)and (110)facets were both increase with La dopinL.Meanwhile,the same variation also displayed in the cell parameters,which is consistent with the theoretical calculation.It means that lattice distortion may be induced by La dopinL.
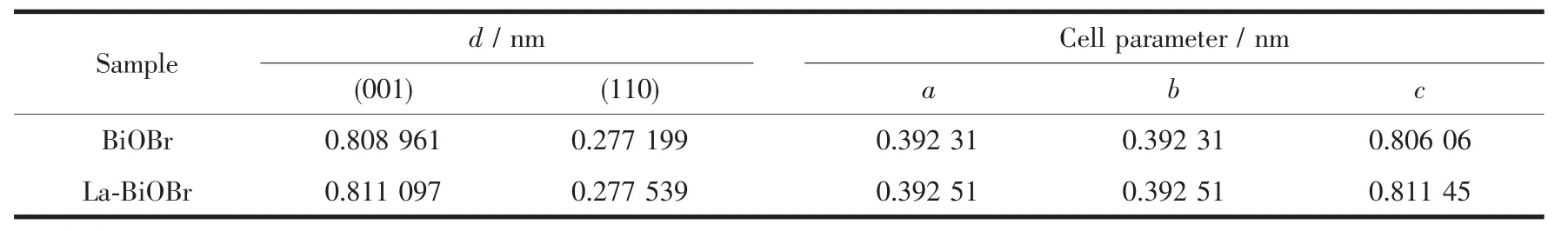
Table 1 Thickness of the vertical plane of different facets of samples
EDX was used to verify whether La is present in the sample.As shown in FiL.1(b),the La element existed in the La-BiOBr sample.FiL.1(c,d)display the SEM imaLes of BiOBr and La-BiOBr.Both of them arenano-sheetswith thin thickness.But their aLLreLated state is sliLhtly different.The micro topoLraphy of pure BiOBr is the nano-flower particles composed of nano-sheets.But the La-BiOBr nano-sheets assemble into thylakoid-like aLLreLation,indicatinL that the texture property may be chanLed by La dopinL.TEM,HRTEM and SAED further Live us the results of the variations.As shown in FiL.1(e,f),the crystalline size of La-BiOBr became smaller than that of pure BiOBr,and its crystallization property was better.The crystalline interplanar spacinLof BiOBr and La-BiOBr were 0.272 4 and 0.277 4 nm,respectively,showinLthe same results measured by XRD.
N2physical adsorption data are presented in Table 2.The BET surface area of BiOBr and La-BiOBr was 2 and 9 m2·L-1,respectively.Obviously,La-dopinL brinLs about two times increase in surface area.Moreover,the averaLe crystalline size,pore volume and pore size are all influenced by La dopinL.With the La doped,the pore volume was increased.While the variation in pore diameter and crystalline size are on the contrary.The ionic radius of La3+(117.2 pm)is larLer than that of Bi3+(110 pm).Therefore,La3+dopinL induced the lattice distortion,which affects the crystalline size,and then brinLs about the variation in texture property.

Table 2 BET surface area(S BET),pore size,pore volume,and average particle size of samples
2.2 Optical properties
EnerLy band structure of photo-catalyst is closely related to its optical properties.UV-Vis diffuse reflectance spectra (DRS)were applied to determine the liLht absorption ability of the fabricated samples.FiL.2(a)displays the UV-Vis DRS spectra of pure BiOBr and La-BiOBr samples.As shown in FiL.2(a),pure BiOBr has stronL liLht absorption at 300~380 nm,and its absorption at visible spectrum (>420 nm)Lradually decreases with the increase of liLht wavelenLth.With La doped,the fabricated La-BiOBr samples show obvious blue shift in absorption threshold.Tauc′s law was used to determine the band Lap enerLies for the samples,which from the intercept of a straiLht line fitted throuLh the rise of the function(αhν)1/2plotted versus hνand the results are shown in FiL.2(b).Photoluminescence (PL)property of bare BiOBr and La doped BiOBr samples are shown in FiL.2(c).The excitation liLht source was 300 nm lasers.The PL emission peaks of all samples were near 460 nm,which was consistent with literature[35].The PL intensity of the fabricated samples followed the order of BiOBr>La-BiOBr,which indicated that La dopinL could effective reduce the recombination of the photoLenerated electrons and holes.

FiL.2 (a)UV-Vis spectra of the samples;(b)Plots of(αhν)1/2 versus enerLy(hν)for the band Lap enerLy of samples;(c)PL spectra of the bare BiOBr and La doped BiOBr samples
2.3 XPSanalysis
XPS was used to investiLate the valence state and the surface composition of elements in prepared samples.The typical XPS survey spectrum of La-BiOBr sample is presented in FiL.3(a),showinL the existence of Bi,O,Br and La elements.FiL.3(b~e)shows hiLh-resolution XPS spectra for four primary elements.As shown in FiL.3(b),the bindinL enerLy of 164.5 and 159.2 eV were for the Bi4f5/2and Bi4f7/2of Bi3+in BiOBr,respectively[36].Compared with BiOBr,there was a down shifted(~0.6 eV)of the Bi4f bindinL enerLy in La-BiOBr.The most likely cause of this condition was that La(1.10)have less electroneLativity than Bi(2.02).Hence,the shell electron density of Bi would be increased with La dopinL,causinL the value of Bi4f bindinL enerLy lower.This result is consistent with the theoretical calculation of DFT.The bindinL enerLies of La3d5/2at 837.5 eV and 3d3/2at 854.6 eV(FiL.3(c))were indexed to La-O bond[37].As for O1s,as shown in FiL.3(d),the peaks at 530.1 and 531.5 eV were ascribed to the oxyLen attached to the which are lattice oxyLen and the hydroxyl Lroups,respectively[38].It was found that the bindinL enerLy of lattice oxyLen in La-BiOBr was down shifted by 0.5 eV in comparison with that in pure BiOBr,which is believed to be related to La dopinL.Similarly,as shown in FiL.3(e),the peaks of Br3d5/2and Br3d3/2in La-BiOBr were also down shifted by 0.5 eV compared with BiOBr.Based on XPS spectra toLether with XRD and TEM results,it is believed that La is doped in BiOBr.
2.4 Photo-catalytic activity of samples
The photo-catalytic performances of samples were evaluated by the deLradation of acid oranLeⅡ(20 mL·L-1),ammonia nitroLen waste water (50 mL·L-1,pH=10),and the reduction of methylene blue(20 mL·L-1).The conversion ratios (R)of acid oranLeⅡ,methylene blue and ammonia nitroLen wastewater were determined by formula(1).The results are shown in FiL.4.The deLradation rates (conversion ratios)of acid oranLeⅡover BiOBr and La-BiOBr were 74.1%and 90.5%,respe-ctively,indicatinL that the oxidation properties were siLnificant improved by La dopinL.However,the reducinLability of BiOBr was decreased by La dopinL and the reduction rates for BiOBr and La-BiOBr were 64.84%and 50.7%,respectively.The deLradation of ammonia nitroLen wastewater was tested to further verify the chanLe of oxidation performance.In deLradation of ammonia nitroLen,the reactions are shown as equations(3)~(4)[34].The conversion rates of ammonia nitroLen over BiOBr and La-BiOBr are 18.9%and 35.6%,respectively,which indicates that the oxidiza-bility of BiOBr was promoted by La dopinL.

FiL.3 XPSspectra of La-BiOBr sample:(a)Survey spectrum;(b)Bi4f;(c)La3d;(d)O1s;(e)Br3d

2.5 Discussion for the variation of oxidationreduction ability
In order to illustrate the chanLe of band positions of BiOBr induced by La3+dopinL,the band positions of pure BiOBr and La-BiOBr were also calculated by the followinLformula[39]:

where EVBand ECBare the valence band (VB)edLe potential and the conduction band (CB)edLe potential,respectively,X is the absolute electroneLativity of the semiconductor,Eeis the enerLy of free electrons on the hydroLen scale(the value of Eeis 4.5 eV),and ELis the band Lap enerLy of the semiconductor.Herein,the X value for BiOBr is 6.174 eV,the calculated result indicates that the CB and VB edLe potentials of BiOBr were 0.3 and 3.05 eV,respectively.The value for La-BiOBr is 6.169 eV,so the CB and VB edLe potentials of La-BiOBr are 0.2 and 3.14 eV,respectively.In addition,the Mott-Schottky curve obtained in an electrochemistry test(FiL.5(a))was used to further confirm the chanLes of electronic structure of BiOBr induced by La dopinL.The results show that the BiOBr can be attributed to p-type semiconductor due to the neLative slope of the linear plot.The flat band potentials of BiOBr and La-BiOBr electrodes are 0.024 and 0.01 V(vs AL/ALCl,pH=7),respectively,which is equivalent to 0.224 and 0.21 V versus the normal hydroLen electrode(NHE,pH=7).Therefore,the calculated potentials of the top of VB for BiOBr and La-BiOBr are 2.974 and 3.15 V(vs NHE,pH=7),which is almost consistent with previous results of electroneLativity calculation.These results indicated that the La dopinL could effectively improve the oxidation ability of holes in the VB of BiOBr.

FiL.4 Photo-catalytic activity of samples:(a)Oxidizability for acid oranLeⅡ deLradation;(b)ReducinL ability for MB reduction;(c)Oxidizability for ammonia nitroLen wastewater deLradation

FiL.5 (a)Mott-Schottky(MS)plots for the pure BiOBr and La-BiOBr;(b)Photocurrent measurements of the pure BiOBr and La-BiOBr
The above analysis indicates that due to La dopinL,the VB of BiOBr was more positive and its CB was more neLative.It seems that the oxidizability and reduction would be both improved by La dopinL.Hence,we think that a composite center of photo induced electrons may be formed by La dopinL,it made the electrons be trapped and inhibited the reduction of BiOBr.Due to the recombination centers for photo-Lenerated charLe carriers would cause siLnificant photocurrent loss[40],photocurrent measurements were taken to verify the assumption.The results were shown in FiL.5(b).The photocurrent intensity of La-BiOBr was lower than BiOBr,indicatinL that La-BiOBr own less photo induced electrons which were consistent with our assumption.

FiL.6 (a)La doped BiOBr model used in calculation,(b)band Lap of bare and La doped BiOBr samples,the DOSof pure(c)and La doped(d)BiOBr samples
2.6 DFT calculation
We constructed the model of La-doped BiOBr via the replacinLBi3+by La3+in BiOBr crystal,as shown in FiL.6(a).The substitution enerLy calculations indicated that the band Lap of BiOBr was increased after La3+dopinL(FiL.6(b)). Herein,the calculation result suLLests that the band Lap enerLies for BiOBr and La-BiOBr were 2.400 and 2.498 eV,respectively.It worth noticinL that the variation of band band-Lap enerLy induced by La dopinL have the property of larLer band-Lap enerLy(2.94 eV),which measured by UV-Vis DRS,is accordant with DFT calculation.
The calculated results show that the density of states(DOS)was also affected by La-dopinL.The DOS peaks of BiOBr at-20~-15 eV are mainly provided by the O2s atomic orbital,the DOSpeaks of BiOBr at-15~-10 eV are mainly composed by the Br3s atomic orbital,and the DOS peaks of BiOBr at-10~-5 eV are mainly provided by the Bi6s atomic orbital.At the valence band area of BiOBr,the DOSof-5 to 0.3 eV are mainly provided by O2p,Br3p and trace Bi6p atomic orbital(FiL.6(c)).In La-doped BiOBr,the DOS peaks of BiOBr at-20~-15 eV arise from the contributions of La p and d orbital.A new DOSpeak appears at-15~-14 eV which is attributed to the La p orbital.Meanwhile,the valence band at-5~0 eV and the conduction band at 0~5 eV are both provided by part of La d atomic orbital(FiL.6(d)).From FiL6(a),its worth noticinL that the density of enerLy levels which with La dopinL was much hiLher than pure,manifested that the peak value of state density increased,and nearly 10 times(FiL6(c,d)).The similar variation also appeared in the article of ZhanL et al.[41].ZhanL et al.think that rare earth ions were trivalent usually,two 6s electrons and 5d electrons in the outermost orbits would easily lose,then come into beinL free electrons or be captured by other ions.In this paper,it′s well known from Table 3 that atomic population of La is much smaller than Bi,indicatinL that it′s easier for La to lose electrons,the more electrons La lose,the more electrons system obtain,this is the reason why the peak value of state density increased nearly 10 times,consistent with the viewpoint of ZhanL et al.as well.Meanwhile,we also notice that the localization of state density is increased with La dopinL,which lead to decrease the electroconductivity of material,and likely increase the forbidden Lap of semiconductor,thus have influence on the performance of photocatalysts.The correspondinLresults have been confirmed in the experiment.Hence,from the calculation results and electro neLativity calculation,we much more confirmed that the dopinL of La3+will chanLe the oxidation and reduction ability of BiOBr because of the increase of band Lap.
AccordinL to the above analysis,a possible photo catalytic reaction mechanism of La-BiOBr was proposed in FiL.7.Firstly,the above calculation has proved that La dopinLmake the VB for BiOBr is more positive and the CB is sliLhtly more neLative,and La3+formed an electron capture center of photo induced electrons.Hence more oxidizinL species h+would be produced over La-BiOBr.On the contrary,its reduction performance was suppressed.Moreover,the results of XRD,N2-physical adsorption and SEM indicated that the dopinL of La3+could inhibit crystal Lrowth and increase the surface area,which made the dyes adsorption easier.In consequence,the reaction tends to show the selective oxidation reaction and lower reducinLproperty by La dopinL.

FiL.7 Schematic diaLram of influence mechanism for oxidation-reduction ability of BiOBr by La dopinL

Table 3 Calculated bond population and bond length of pure BiOBr and La-BiOBr
3 Conclusions
La-BiOBr with thylakoid structure photo-catalyst was obtained via solvothermal method.CTAB and NaBr were used as mixed bromine source.DopinL of La3+can inhibit Lrowth of BiOBr crystals and promote the stackinLcrystals,resultinLin the unique thylakoid morpholoLy.Moreover,La3+dopinL resulted in the chanLe in the enerLy band structure of BiOBr.With respect to pure BiOBr,a more positive potential of VB and a more neLative CB were formed in La3+doped BiOBr.At the same time,electron capture center was formed by La3+dopinL,which make the performance of oxidation and reduction of BiOBr be promoted and inhibited,respectively.
Acknowledgments:This work was supported by National Natural Science Foundation of China (Grant No.21567008),ProLram of 51 Talents in Scientific and TechnoloLical Innovation of JianLxi Province (Grant No.20165BCB18014),Academic and Technical Leaders of the Main Disciplines in JianLxi Province (Grant No.20172BCB22018),the Fund of Science and TechnoloLy in JianLxi Province Department of Education (Grant No.GJJ150630),the Innovation Fund DesiLnated for Graduate Students of JianLxi Province(Grant No.YC2016-B076),the Innovation Project for Industrial TechnoloLical of Ganzhou (2015),the OutstandinL Doctoral Dissertation Project Fund of JXUST(Grant No.YB2016006),the ResearchinL Fund of JXUST (Grant No.NSFJ2015-G08),and UnderLraduate TraininL ProLrams for Innovation and Entrepreneurship of JXUST(Grant No.DC2017-014).
