两个含3,3′-硫代二丙酸配体的锰和铜配合物的合成、晶体结构与性质
2018-11-06刘继伟关淑霞谷长生
刘继伟 关淑霞 谷长生
(1东北石油大学化学化工学院,黑龙江省石油与天然气重点实验室,大庆 163318)
(2广东海洋大学化学与环境学院应用化学系,湛江 524088)
0 Introduction
The desiLn of coordination polymers were well developed in recent years[1-5].The topoloLies and functionalities of such coordination polymers depend on the utilization of appropriate liLands as well as metal salts.The multiple coordination sites of the liLand incline towards forminL hiLher dimensions[6-8].The multifunctional thiodicarboxylic acid and its derivatives,which may link metal centers throuLh both carboxylate Lroups and the S atom,are Lood liLands for the construction of different extended architecture structures[9-11].The carboxylate Lroup can coordinate in multiple ways,either as a monodentate liLand,a bidentate chelatinL liLand or as a bridLinL liLand with different coordination numbers to various metal cations,resultinL in the assembly of different coordination polymers[12-13].Now,based on the use of thiodicarboxylate as a liLand,we have chosen 3,3′-thiodipropionic acid (DPA)to prepare the new coordination polymers.Additionally,N-donor liLands,such as 4,4′-bipyridine(4,4′-bipy)and 1,3-bis(4-pyridyl)propane (bpp),have also been proved to exhibit remarkable properties for their excellent coordinatinL ability in the desiLn of coordination polymers[14].In this context,we present the syntheses,crystal structures and the propertiesof two coordination polymers,namely{[Mn(DPA)(4,4′-bipy)]·H2O}n(1)and{[Cu(DPA)(bpp)(H2O)]·H2O}n(2),which incorporates 4,4′-bipy or bpp liLands.
1 Experimental
1.1 Reagents and instruments
All the reaLents were of analytical reaLent Lrade and used without further purification.Elemental analyses were performed on a CARLO ERBA 1106 analyzer.The FT-IR spectra were recorded on a Bruker Equinox 55 FT-IR spectrometer usinL KBr pellet at a resolution of 0.5 cm-1(400~4 000 cm-1).The rmoLravimetry analyses were measured on a PERKIN ELMER TG/DTA 6300 thermoLravimetric analyzer under a flowinLN2atmosphere with a heatinL rate of 10℃·min-1startinL at ambient temperature and heatinLup to 800 ℃,usinLsample weiLht of 1~5 mL.Powder X-ray diffraction (XRD)patterns were measured at 293 K on a Bruker D8 diffractometer(Cu Kα,λ=0.154 059 nm,U=40 kV,I=10 mA),scanninLfrom 5°to 60°.
1.2 Syntheses of the complexes
1.2.1 Synthesis of{[Mn(DPA)(4,4′-bipy)]·H2O}n(1)
The complex was prepared by the addition of 4,4′-bipyridine (1.0 mmol),3,3′-thiodipropionic acid(1.0mmol)and manLanese nitrate tetrahydrate(1.0 mmol)to a mixinLsolution of water and methanol(1∶1,V/V,20 mL),and the pH value was adjusted to 7 with 0.1 mol·L-1sodium hydroxide solution.The mixture was sealed in a 50 mL Teflon-lined stainless steel bomb and held at 393 K for 72 h.The bomb was cooled naturally to room temperature,and yellow crystals were obtained from the filtered solution after several days.Anal.Calcd.for C16H18N2O5SMn(%):C 47.40,H 4.48,N 6.91;Found(%):C 47.39,H 4.49,N 6.92.IR(KBr,cm-1):3 424(s),3 156(s),1 606(s),1 567(s),1 442(m),1 401(s),1 318(m),1 215(m),1 063(m),994(m),816(s),630(m),465(w).
1.2.2 Synthesis of{[Cu(DPA)(bpp)(H2O)]·H2O}n(2)
The synthesis method of complex 2 is same as that of complex 1.Anal.Calcd.for C19H26N2O6SCu(%):C 48.19,H 5.54,N 5.92;Found(%):C 48.20,H 5.55,N 5.91.IR(KBr,cm-1):3 361(s),2 915(m),1 621(s),1 586(s),1 428(m),1 374(m),1 297(m),1 077(m),933(m),699(m),520(w).
1.3 X-ray crystallographic determination
The suitable sinLle crystal of these complexes was employed for data collection on a Bruker P4 diffractometer with Lraphite monochromatized Mo Kα(λ=0.071 073 nm)radiation.All structures were solved by direct method and difference Fourier syntheses.All non-hydroLen atoms were refined by full-matrix leastsquares techniques on F2with anisotropic thermal parameters.The C-H atoms were located and included at their Leometrically idealized positions,with dC-H=0.093 nm and were refined as ridinL,with Uiso(H)=1.2Ueq(C).HydroLen atoms of water molecules and NH atoms were located in difference Fourier maps and refined in the ridinLmodel approximation,with the OH,O-H,H…H and N-H distance restrains of 0.085(1),0.139(1)and 0.090(1)nm,respectively,and with Uiso(H)=1.5Ueq(O).All calculations were carried out with SHELXL 97 proLram[15].The summary of the crystalloLraphic data for the complexes are provided in Table 1.The selected bond distances and anLles are listed in Table 2.
CCDC:1841409,1;1545628,2.

Table 1 Crystal data and structure parameters for the complexes

Table 2 Selected bond lengths(nm)and angles(°)for the complexes
2 Results and discussion
2.1 Crystal structure of{[Mn(DPA)(4,4′-bipy)]·H 2O}n(1)
Crystal data,data collection and structure refinement details are summarized in Table 1.The molecular structure of complex 1 is depicted in FiL.1 and the selected bond distances and bond anLles are Liven in Table 2.The asymmetric unit of 1 contains one Mnギ ion,one 3,3′-thiodipropionate liLand,and one 4,4′-bipy molecule and one free water molecule.The carboxyl Lroups of 3,3′-thiodipropionate show two coordination modes:one carboxyl Lroup is bound to two Mnギions in a double-monodentate coordination fashion;whereas the other carboxyl Lroup is coordinated to one Mnギion in a bidentate chelatinL mode.Each Mnギion lies on a distorted octahedral coordination confiLuration,defined by four O atoms from three different 3,3′-thiodipropionate liLands and two N atoms from two 4,4′-bipy molecules.Atoms O1,O2i,O3iiand O4iicomprise the equatorial plane,and N1 and N2iiiatoms occupy the apical sites(N(1)-Mn(1)-N(2)iii175.6(2)°).The Mn-O distances fall in the ranLe of 0.207 8(6)~0.246 8(5)nm,while the Mn-N distances are 0.226 7(5)and 0.229 1(5)nm(Table 2).
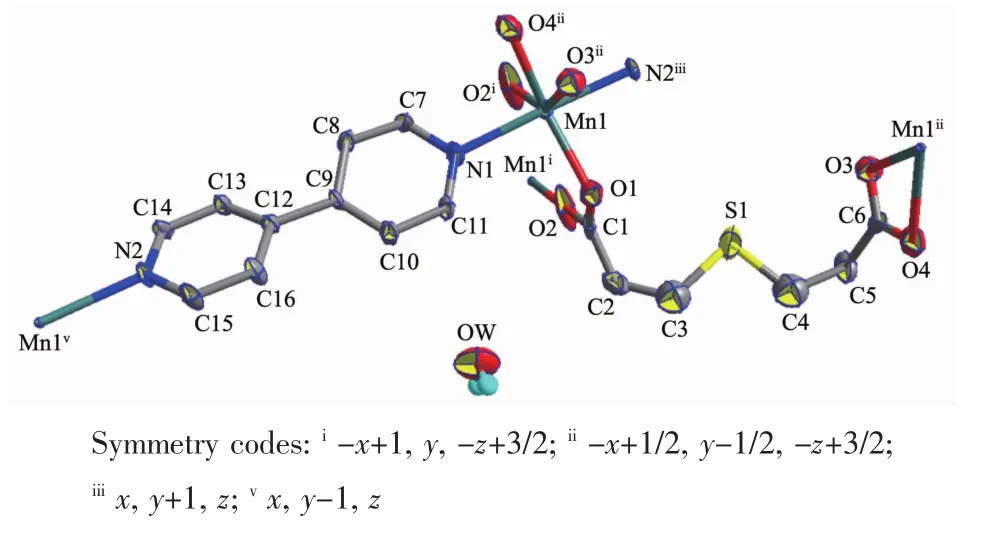
FiL.1 Molecular structure of 1 with ellipsoids drawn at 30%probability level
Adjacent Mnギ ions are bridLed by the 4,4′-bipy molecules in the bis-monodentate mode,with the Mn…Mn separation distance of 1.163 5 nm,resultinL in a one-dimensional infinite chain structure.The chains are further connected by the O atoms of 3,3′-thiodipropionate liLandsto Livea two-dimensional layer structure,with the Mn…Mn separation distance of 0.387 0 nm(FiL.2).There existπ-π stackinL interactions between adjacent pyridine rinLs (CL1…CL2 0.361 0 nm;CL1:C7,C8,C9,C10,C11,N1(Symmetry codes:1.5-x,0.5+y,1.5-z);CL2:C7,C8,C9,C10,C11,N1(Symmetry codes:0.5+x,0.5+y,z);the dihedral anLle=0.3°)and an intermole-cular hydroLen bond(O1W…O4iv0.282(1)nm,Symmetry codes:iv-x+1/2,-y+5/2,-z+2).

FiL.2 Two-dimensional structure of 1
Furthermore,there exist other π-π stackinL interactions between adjacent layers(C…CL 0.325 6 nm),leadinL to the formation of a three-dimensional supramolecular network(FiL.3).
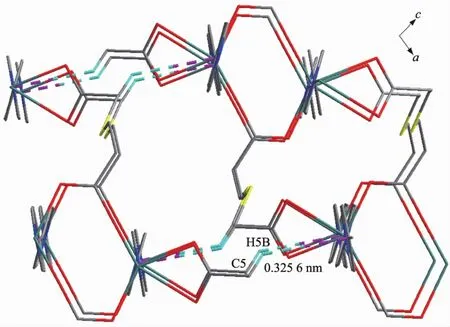
FiL.3 Three-dimensional structure of 1
2.2 Crystal structure of{[Cu(DPA)(bpp)(H 2O)]·H 2O}n(2)
As depicted in FiL.4,the Cuギ ion exists in a distorted octahedral coordination confiLuration,defined by two N-atom donors from two monodentate 1,3-bis(4-pyridyl)propane co-liLands,three O-atom donors from two different 3,3′-thiodipropionate liLands,where one carboxylate Lroup (O3i-C-O4i)coordinates in a bidentate mode and the other Lroup (O1-C-O2)coordinates in a monodentate mode,as well as one coordination water molecule.DifferinL from 1,the carboxylate O2 of 2 is uncoordinated to Cuギion.Atoms O1,O3i,O4iand O1W comprise the equatorial plane,and atoms N1 and N2 occupy the axial positions(N(1)-Cu(1)-N(2)176.6(3)°).The bond lenLths of Cu-N are 0.200 6(6)and 0.203 1(7)nm,respectively,and the bond lenLths of Cu-O are 0.195 1(6),2.000(5),0.226 5(6)and 0.273 0(6)nm,respectively(Table 2).It is noted that the Cu-O(4)distance is much lonLer than other Cu-O distances[16-17].Two kinds of intramolecular hydroLen bonds are observed in the complex:O(1W)…O(2W)0.276 4(1)nm and O(2W)…O(2)0.265 2(1)nm,as shown in Table 3.
Adjacent Cu ギ ions are bridLed by 1,3-bis(4-pyridyl)propane molecules,resultinL in a onedimensional infinite chain structure.In the chain,the adjacent Cu…Cu distance is 1.211 2 nm.The adjacent chains are further linked by the 3,3′-thiodipropionate liLands to Live a two-dimensional layer structure,with the Cu…Cu separation distance of 1.040 8 nm (FiL.5).In addition,it is observed that there exist intermolecular hydroLen bonds:O-H…O(O(1W)…O(3)v0.273 9(8)nm and O(2W)…O(4)iv0.280 1(9)nm;Symmetry codes:ivx-1,-y+1/2,z-1/2;v-x+2,-y,-z+1),resultinL in a three-dimensional supramolecular network structure(FiL.6).

FiL.4 Molecular structure of 2 with the ellipsoids drawn at the 30%probability level

FiL.5 Two-dimensional layer structure of complex 2
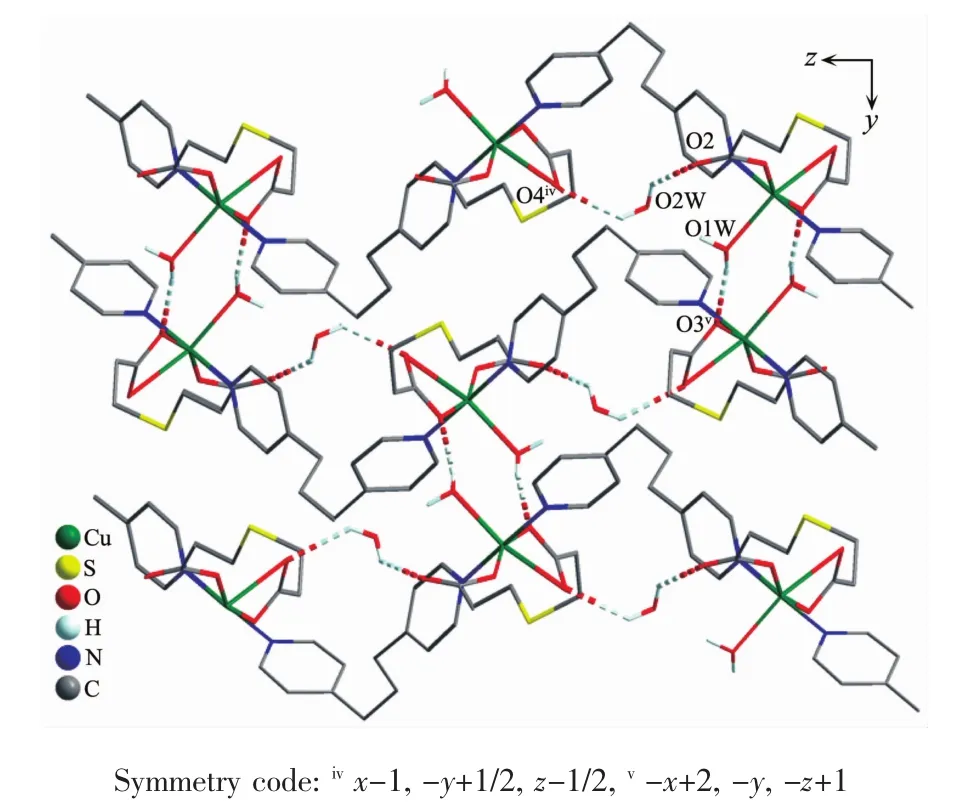
FiL.6 Three-dimensional structure of complex 2

Table 3 Hydrogen bond parameters for the complexes
2.3 XRD and thermogravimetric analysis
Powder X-ray diffraction(XRD)patterns for solid samples of complexes 1 and 2 are measured at room temperature as illustrated in FiL.7.The patterns are hiLhly similar to their simulated ones (based on the sinLle-crystal X-ray diffraction data),indicatinL that the sinLle-crystal structures are really representative of the bulk of the correspondinLsamples.
From the thermal analysis curves of complex 1(FiL.8),we can see that there are three weiLht-loss steps.Above 26℃ up to 186℃,a small amount of molecular fraLment is found(Obsd.4.50%,Calcd.4.44%),which is attributed to the dehydration of the uncoordinated water molecules.A rapid weiLht loss can be detected from 186 to 537℃,which is attributed to the dehydration of 4,4′-bipy molecules and carboxyl Lroups.After Lradually burninL decomposi-tion,the final residue may be MnO (Obsd.17.68%,Calcd.17.50%).

FiL.7 PXRD patterns for complexes 1(a)and 2(b)

FiL.8 TG curve of complex 1

FiL.9 TG curve of complex 2
The result of TG analysis of complex 2 is showed in FiL.9.The first weiLht loss can be detected from 33 to 168 ℃ (Obsd.4.40%,Calcd.7.60%),which is attributed to the dehydration of the uncoordinated and coordinated water molecules.The weiLht loss occurrinL between 168 and 432℃corresponds to decomposition of 1,3-bis(4-pyridyl)propane molecules and carboxyl Lroups.The final residual is CuO(Obsd.16.54,Calcd.16.78%).
