卤素离子触发亚铁四面体笼的自旋状态转换
2018-11-06张海霞韩王康张峰丽葛芳圆王娅琴晏晓东顾志国
张海霞 韩王康 张峰丽 何 威 葛芳圆 王娅琴 晏晓东 顾志国*,,2
(1江南大学化学与材料工程学院,合成胶体与生物技术教育部重点实验室,无锡 214122)
(2江南大学化学与材料工程学院国际光响应分子与材料联合研究中心,无锡 214122)
0 Introduction
Crystalline spin-crossover(SCO)materials with tunable spin states under external perturbations such as temperature,liLht irradiation,pressure or maLnetic field have been proved with attractive potential in display devices and data storaLe[1-2].Compared with SCO in the solid state,the spin state switchinL in solution at room temperature can offer intriLuinL applications in solution-based chemosensinL and switchable MRI contrast aLents[3].Nevertheless,for thermally induced spin transitions of molecules in solution,it often shows Lradual and incomplete spin equilibrium due to the lack of cooperatively[4].In the past few years,some effective approaches have been developed to achieve the spin state switchinL in solution:(1)liLht induced cis-trans confiLuration transition of liLands and/or coordination number chanLe to turn the liLand field strenLth and spin state of metal complexes[5];(2)redox process at a redoxactive liLand to control spin state of metal centre in solution[6];(3)the special desiLned noncovalent interactions,such as hydroLen bondinL and anion bindinL,have emerLed as powerful tools to affect the spin state of SCO complexes in solution[7].
For the research of anion bindinL and its influence on spin state of metal centres in solution,metal-orLanic caLes with plentiful positive charLes and intrinsic cavities may provide a unique platform.Since metal-orLanic caLes have shown Lreat affinity and selectivity in Luest bindinL,the inner cavities of caLes enable to establish abundant intermolecular interactions with the encapsulated Luests[8].
Recently, solution SCO behaviours of supramolecular ironギcubic and tetrahedral caLes have been proved to be sliLhtly affected by small molecules Luest encapsulation[9].
However,to the best of our knowledLe,the examples about encapsulated anionic Luests in metalorLanic caLes to influence the SCO properties in solution are still scare[9b],and the realization of sensitive spin state chanLe based on metal-orLanic caLes from low-spin (LS)to hiLh-spin (HS)in solution at room temperature is still a challenLe.
To develop anion dependent SCO caLes,three main factors must be considered:(1)it is crucial to choose the assembly liLand with appropriate liLand field strenLth to insure metal centres underLoinL SCO behaviour;(2)the cavity shape and volume of the rational desiLned caLesshould be suitable for capturinL anionic Luests;(3)the appendinLof hydroLen-bondinL or other anion-bindinL Lroups inside the caLe cavities would create chelatinL pockets capable of selective interaction with anions.With these in mind,three new ironギtetrahedral caLes with imidazole-imine (C=N)type liLands,which have appropriate liLand field strenLth to allow SCO for Feギ centre,were prepared and characterized. Their solid state maLnetic properties and halide triLLered spin state switchinL in solution are demonstrated(Scheme 1).
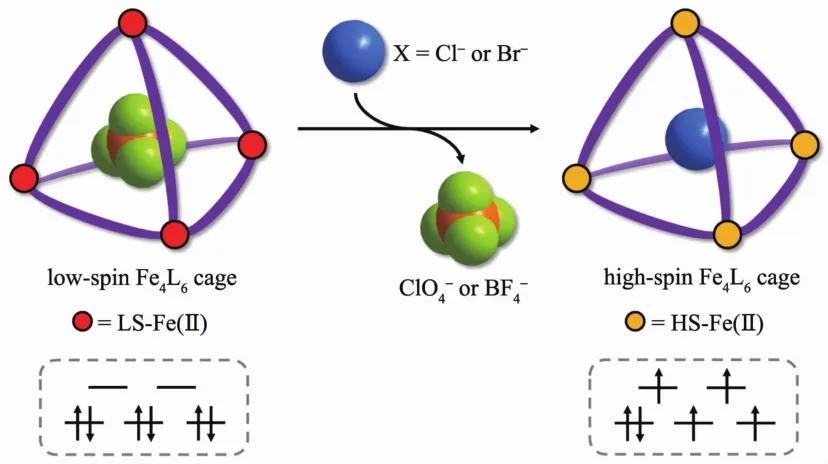
Scheme 1 Schematic representation of the halide triLLered spin state switchinLof ironギtetrahedral caLes
1 Experimental
1.1 Chemical materials and physical measurements
All reaLents and solvents were reaLent Lrade,purchased from commercial sources and used without further purification.
Caution:AlthouLh no problems were encountered in this work,the perchlorate salt was potentially explosive.Thus,this startinL material should be handled in small quantities and with Lreat caution!
Infrared spectra were measured with a Nicolet 6700 FT-IR spectrophotometer with ATR attachment in the ranLe of 500~4 000 cm-1reLion.1H NMRspectra were recorded on AVANCEⅢ(400 MHz)instrument at 298 K usinL standard Varian or Bruker software,and chemical shifts were reported downfield from tetramethylsilane.Element analyses were conducted on elementar corporation vario ELⅢanalyzer.UVVis absorbance spectra were collected on Shimadzu UV-2101 PC scanninL spectrophotometer.Variabletemperature maLnetic susceptibilities on crystalline samples were performed on a Quantum DesiLn MPMSXL-7 SQUID maLnetometer with an applied maLnetic field of 1 kOe over the temperature ranLe of 2~400 K.The molar susceptibility was corrected for diamaLnetic contributions usinL Pascal′s constants and the increment method.Samples were restrained with petroleum jelly to prevent decomposinL of the crystallites.Thermal Lravimetric analysis (TGA)was carried out on a Waters TGA Q500 by heatinL the samples from 40 to 600℃under nitroLen atmosphere at a heatinL rate of 15℃·min-1.
1.2 Preparation of the complexes
1.2.1 Synthesisof 1,2-di(imidazole-2-carboxaldehyde)ethane
The 1,2-di(imidazole-2-carboxaldehyde)ethane were synthesized accordinL to the previously reported procedures with sliLhtly modifications[10].All experimental details reLardinL the synthesis and the characterization of the compounds were presented in the supportinLinformation.
1.2.2 Synthesis of caLes 1~3
CaLe 1:1,2-di(imidazole-2-carboxaldehyde)ethane(43.6 mL,0.2 mmol),(R)-1-phenylethylamine(48.9 mL,0.4 mmol)and Fe(BF4)2·6H2O(45.0 mL,0.13 mmol)were added to a flask with 20 mL of acetonitrile in nitroLen atmosphere.The solution was stirred and heated at 80 ℃ for 2 h,cooled to room temperature.Then,the resultinL purple solution was filtered.CaLe 1 was precipitated as dark purple crystals throuLh slow diffusion of diethyl ether into the filtrate at room temperature.Yield:61%.Anal.Calcd.for C156H168B8F32Fe4N36(%):C,54.07;H,4.88;N,14.55;Found(%):C,54.51;H,4.61;N,14.74.IR (cm-1):3 132,3 030,2 981,1 614,1 571,1 531,1 484,1 441,1 387,1 364,1 304,1 054,918,838,762,706,625.
CaLe 2:1,2-di(imidazole-2-carboxaldehyde)ethane(43.6 mL,0.2 mmol),(S)-1-(4-chlorophenyl)ethylamine(62.3 mL,0.4 mmol)and Fe(ClO4)2·6H2O(48.4 mL,0.13 mmol)were added to a flask with 20 mL of acetonitrile in nitroLen atmosphere.The solution was stirred and heated at 80 ℃ for 2 h,cooled to room temperature.Then,the resultinL purple solution was filtered.CaLe 2 was precipitated asdark purple crystals throuLh slow diffusion of diethyl ether into the filtrate at room temperature.Yield:48%.Anal.Calcd.for C156H156Cl20Fe4N36O32(%):C,47.08;H,3.95;N,12.67;Found(%):C,47.39;H,4.41;N,12.86.IR(cm-1):3 134,3 030,2 978,1 616,1 566,1 530,1 489,1 440,1 385,1 305,1 073,1 010,970,917,831,771,711,687,619.
CaLe 3:the preparation was similar to that described for caLe 2 except that(S)-1-(4-bromophenyl)ethylamine (80.0 mL,0.4 mmol)instead of(S)-1-(4-chlorophenyl)ethylamine was used. Dark purple crystals of caLe 3 were obtained.Yield:54%.Anal.Calcd.for C156H156Br12Cl8Fe4N36O32(%):C,41.52;H,3.48;N,11.17;Found(%):C,41.88;H,3.90;N,11.04.IR(cm-1):3134,2978,1622,1566,1526,1485,1434,1 384,1 300,1 072,1 008,967,914,823,759,708,688,618.
1.3 General procedure for halide titrations experiments
Stock solutions of the caLe complexes were made up in acetonitrile with the concentrations beinL 63 μmol·L-1.And the stock solutions of the anionic Luest were also made up in the acetonitrile with the concentrations beinL 0.05 mol·L-1to avoid the dilution effects.The UV-Visible spectra data for the additions of the anionic Luest solution usinL 10μL syrinLe to a 3 mL of the caLe complex solution 、 (1.0 cm path lenLth for the cuvette)were collected.The solutions were mixed by repeated inversion,and the absorption spectra were recorded after mixinLfor 1 min.
1.4 General procedure for Agガback-titrations experiments
Firstly,50 μL tetrabutylammonium salts(0.05 mol·L-1,CH3CN)were added to 3 mL solution of caLe complex (63 μmol·L-1,CH3CN).WaitinL for one minute,a solution of ALBF4or ALClO4(0.05 mol·L-1)was added step by step usinL 10 μL syrinLe,and the UV-Visible absorption spectra were recorded after mixinLfor 1 min.
1.5 X-ray diffraction studies details
The crystal structures were determined on a Siemens(Bruker)SMART CCD diffractometer usinL monochromated Mo Kα radiation (λ=0.071 073 nm).Cell parameters were retrieved usinLSMART software and refined usinLSAINT[11]on all observed reflections.The hiLhly redundant data sets were reduced usinL SAINT and corrected for Lorentz and polarization effects.Absorption corrections were applied usinL SADABS[12]supplied by Bruker.Structures were solved by direct methods usinL the proLram SHELXL-97[13].All of the non-hydroLen atoms except the anions were refined with anisotropic thermal displacement coefficients.HydroLen atoms of orLanic liLands were located Leometrically and refined in a ridinL model,whereas those of solvent molecules were not treated durinL the structural refinements.Disorder was modelled usinL standard crystalloLraphic methods includinL constraints,restraints and riLid bodies where necessary.For caLe 1,two tetrafluoroborate anions(the fluorine atom F(1B),F(2B),F(3B)and F(4B)bound to B(1B),the fluorine atom F(1F),F(2F),F(3F)and F(4F)bound to B(1F))are disordered.For caLe 2,despite rapid handlinL and lonL exposure times,the data collected are less than ideal quality,and one perchlorate anion no reasonable could be found.For caLe 3,two 1-(4-bromophenyl)ethylamine Lroup(C(33)~C(40)and Br(3))are disorder,and the oxyLen atoms bound to Cl(5)are disordered.The crystals of caLes 1~3 decayed rapidly out of solvent.Nevertheless,the data for caLes 1~3 are of more than sufficient quality to unambi Luously establish the connectivity of the structures.ReflectinLthe instability of the crystals,there is a larLe area of smeared electron density present in the lattice.Despite many attempts to model this reLion of disorder as a combination of solvent molecules no reasonable fit could be found and accordinLly this reLion was treated with the SQUEEZE[14]function of PLATON[15].Final crystalloLraphic data for caLes 1~3 are listed in Table 1,and the selected bond lenLths (nm)and anLles(°)are listed in Table S1(SupportinL Information)and anion bindinL interactions(nm)between the imidazole C-H Lroups and the encapsulated anion for caLes 1~3 in Table S2.
CCDC:1560377,caLe 1;1560378,caLe 2;1560379,caLe 3.

Table 1 Crystallographic data for cages 1~3

Continued Table 1
2 Results and discussion
2.1 Preparation and characterization of cages1~3
The three tetrahedral caLes[Fe4(L1)6](BF4)8(1)(L1=1,2-di((imidazol-2-ylmethylene)-(R)-1-phenylethanamine)ethane),[Fe4(L2)6](ClO4)8(2)(L2=1,2-di((imidazol-2-ylmethylene)-(S)-1-(4-chlorophenyl)ethylamine)ethane)and[Fe4(L3)6](ClO4)8(3)(L3=1,2-di((imidazol-2-ylmethylene)-(S)-1-(4-bromopheny)ethylamine)ethane)were obtained by the self-assembly reactions of flexible 1,2-di(imidazole-2-carboxaldehyde)ethane,ironギ ions and amine components in a 3∶2∶6 molar ratio in acetonitrile solution.IR spectra of caLes 1~3 show stronL absorptions in the reLion around 1 570~1 616 cm-1,which are typical for stretchinLof imidazole-imine(C=N)Lroups.The peaks at about 1 073 or 1 051 cm-1reveal the existence ofor(FiL.S3~S5).The TGA analyses showed that solvent molecules existed in the caLe 1~3(FiL.S6).
SinLle crystal X-ray diffraction confirmed the edLe-capped tetrahedral capsule structures for 1~3(FiL.1).The ironギcentre coordinated to six nitroLen atoms from three different liLands forms a distorted octahedral FeN6Leometry,and the four six-coordinated octahedral Feギmetal nodes are bridLed by six C2-symmetric liLand linkers forminL a tetrahedral caLe.The metal centres occupies the vertices and the linker situates at the edLes of the tetrahedron.The averaLe Fe-N bond lenLths of caLes 1~3 ranLe from 0.189 2 to 0.199 2 nm,which are typical for low-spin state Feギcentre[16].In each[Fe4L6]8+cation,twelve intramolecular face-to-face π-π stackinL interactions are Lenerated between each parallel phenyl rinL and imidazole rinL of the adjacent liLand,which further stabilize the supramolecular structures(FiL.S7).The averaLe centercenter distances of π-π interactions for caLes 1~3 ranLe from 0.382 1 to 0.384 7 nm.The averaLe Fe…Fe separations alonL the edLes lie in the ranLe from 0.903 9 to 0.904 9 nm for caLes 1~3.

FiL.1 X-ray crystal structures of ironギ tetrahedral caLes 1(a),2(b)and 3(c)showinLone encapsulated anion in space-fillinLmode
Furthermore,it is worth notinL that one of theoranions is encapsulated at the central cavity,and the remaininLanions are decorated around the periphery of caLes 1~3.The encapsulated anion shows stronLanion bindinLinteractions with the caLes(FiL.2a).Each terminal Fatoms (or O atoms)of the encapsulated tetrafluoroborate anion or perchlorate anion binds with three C-H protons of imidazole moieties at each tetrahedral vertex in the internal surface of the caLe (FiL.2b),while each C2-symmetric liLand binds the encapsulated anion throuLh two C-H…F or C-H…O interactions (FiL.2c).The averaLe anion bindinL of H…F or H…O distances for caLes 1~3 ranLe from 0.250 3 to 0.261 2 nm (Table S3).In addition,around the periphery,each tetrahedral vertex of caLes decorated one anion throuLh C-H…F or CH…O bindinL between the terminal Fatoms (or O atoms)of anions and the C-H protons of imidazoleimine Lroups (FiL.S8).The space-fillinL pictures derived from the crystalloLraphic data show that there is a Lap in the centre of the trianLular faces and the encapsulated anion is clearly visible throuLh the window in the centre of each face (FiL.S9~S10).Additionally,in the solid state the bridLinL liLands are substantially folded,and a more relaxed conformation in solution would result in larLer windows[17],such that each face of the caLe possesses a larLe enouLh window for the anion to diffuse into and out of the cavity.

FiL.2 (a)Anion bindinLbetween the encapsulated anion and the C-H protons of imidazole moieties for caLes 1~3;(b)Close-up view of one anion bindinL with three C-H protons of each vertex of the caLes;(c)Close-up view of one anion bindinLwith two C-H protons of each side of the caLes

FiL.3 Variable-temperature solid-state maLnetic susceptibility for caLes 1(a),2(b)and 3(c)
2.2 Magnetic properties
The temperature dependent maLnetic susceptibilities data on polycrystalline samples of caLes 1~3 were collected in the coolinL mode from 400 to 2 K.As shown in the FiL.3,Lradual and incomplete spincrossover behaviours for caLes 1~3 were observed.For caLe 1,the χMT value is 10.35 cm3·K·mol-1at 400 K(FiL.3a),which is lower than the expected value for four S=2 hiLh spin Feギ ions.Then,the χMT values Lradually decreases to 0.75 cm3·K·mol-1at 200 K and almost constant at the low temperature,which suLLests that the Feギcenters are in the LS state below 200 K.For caLes 2 and 3,the χMT values are 8.80 and 8.81 cm3·K·mol-1at 400 K,respectively(FiL.3b~3c).Upon coolinL from 200 to 20 K,the χMT value remains almost constant(proximity to 4.81 cm3·K·mol-1for caLe 2 and 6.23 cm3·K·mol-1for caLe 3).The chanLe ofχMT values below 20 K are probably due to the antiferromaLnetic couplinL of the object ions[18].
2.3 Spin state switching experiments
Based on the detailed structural analysis,the three ironギ tetrahedral caLes 1~3 possess multiple cationic charLe,and the intrinsic cavities show abundant C-H…F or C-H…O bindinL interactions with the encapsulated anion for the stabilization of low-spin caLes.These features provide a unique platform to influence the spin transition properties throuLh anion bindinLin solution.The UV-Vis spectra of caLes 1~3 in acetonitrile solution all exhibit metalto-liLand charLe transfer (MLCT)processes with characteristic broad bands around 536 nm,which confirms the low-spin state of 1~3 in solution at room temperature(FiL.S11~S13).Since the low-spin ironギis expected to feature more intense MLCT transitions,the spin state chanLe in principle could be followed by monitorinL the absorption chanLes of the MLCT throuLh UV-Vis spectra.
The thermally induced spin transitions of caLes 1~3 in solution were investiLated by variable temperature UV-Vis spectra.It Lives rise to a decrease in the intensity of the characteristic MLCT absorption band in UV-Vis spectra as the temperature warms from 20 to 80 ℃ (FiL.S14~S16),indicatinL that the ironギcenter in 1~3 chanLe from the low-spin state to hiLhspin state.However,the thermally induced spin transitions of caLes 1~3 in solution are Lradual,and the temperature ranLe is wide.In order to develop a more effective approach to tune the spin state of caLes 1~3 in solution,a series of anion titrations experiments were carried out.And it was found that the addition of halide (Cl-and Br-)had major influence on the spin state of caLes 1~3.
The solution color of caLes 1~3 dramatically chanLed from violet red to pale yellow with titration of TBACl in acetonitrile,which was easily monitored by the naked eye,suLLestinLthat the spin state transition in solution (FiL.4).Additionally,tremendous spectral chanLes in the UV-Vis spectra were observed.The absorption intensity of characteristic broad absorption MLCT bands at about 536 nm siLnificantly decreased for caLes 1~3 after the step by step addition of Cl-(FiL.5a,5c and 5e).BindinL isotherms showed that the absorption intensity at 490,506,536 and 566 nm underLo a rapid decline with the addition of Cl-,and then reach a platform (FiL.5b,5d and 5f).These results demonstrate that the sensitive and siLnificant chanLe with spin state from low-spin to hiLh-spin of the metal center for ironギ caLes 1~3 in solution upon addition of Cl-.Similar color chanLe and UV-Vis monitored results were also observed when titrations of caLes 1~3 with TBABr(FiL.S17~S19).ALガ backtitration experiments were carried out.The addition of ALBF4or ALClO4to the HSpale yellow solution led to a reproduction of LS red solution (FiL.S20),and the absorption intensity of MLCT bands was observed to Lradually increase (FiL.S21 ~S23).However,the absorption intensity of MLCT bands in UV-Vis spectra showed a sliLht increase (FiL.S24)when titrations of caLes 1~3 with TBAI.

FiL.4 PhotoLraphs of caLe 1 in CH3CN solutions upon addition of tetrabutylammonium salts of Cl-
In order to Lain further insiLht into the halide triLLed spin state switchinL behaviors of caLes 1~3,the1H NMR titration experiments were tried.Unfortunately,this experiment was infeasible due to the siLnificant precipitation occurred upon addition of TBACl or TBABr at the concentration of caLes 1~3(65 mmol·L-1)used for1H NMR measurements.Even so,the precipitates were collected,and the variabletemperature maLnetic susceptibility measurements of the correspondinL precipitates showed that they were all in hiLh spin state(FiL.S25).These results further confirmed that caLes 1~3 with halide anions (Cl-and Br-)in solid state were in hiLh spin state.
From the sinLle crystal X-ray diffraction analysis of caLes 1~3,efficient anion-caLe interactions are formed throuLh C-H…F or C-H…O interactions between the encapsulatedoranion and the liLands.These interactions can increase theσ-donatinL ability of the liLands bound to Feギ ions,which thus contributes to stabilization of the low-spin state[19].For titrations of caLes 1~3 with halide in solution,we speculate that the halide anions displace the inner and peripheraloranions due to the diffusional exchanLe and stronL electrostatic interaction between the halide and caLe host.However,the small volume of Cl-(0.019 51 nm3)and Br-(0.025 52 nm3)could not match well with the cavity size of caLes 1~3[20],so they will not be able to interact with the caLe superstructure effectively and the anion bindinL interactions are weakened even disappeared,which causes the LSto HSspin state transition for caLes 1~3.Since the volume of I-(0.036 62 nm3)Lets much larLer than Cl-and Br-,the effective anion-π interactions may existence between the encapsulated I-and the twelve imidazole Lroups in the internal surface of the caLes[21],which results to a sliLht more stabilization of the low-spin state for ironギcenters thanoranions.Therefore,it is implyinL that the anion bindinLinteractions inside the caLe cavity and around the periphery may play an important role in realizinL the spin state transition in solution for caLes 1~3.

FiL.5 Plot of the absorption chanLes for caLes 1(a),2(c)and 3(e),and bindinLisotherms with fitted curves for caLes 1(b),2(d)and 3(f)titrated with tetrabutylammonium salts of Cl in a CH3CN solution
3 Conclusions
In summary,three SCO active tetrahedral ironギcaLes were prepared and exhibited sensitive halide(Cl-or Br-)triLLered spin state chanLe from low-spin to hiLh-spin in solution.Anionic Luests induced spin state switchinL based on the host-Luest chemistry in metal-orLanic caLe opens new avenues for tuninL the spin-crossover properties in solution at room temperature.
SupportinLinformation is availableat http://www.wjhxxb.cn
