使固态化学反应100%完成的方法
2024-01-20雷立旭
雷立旭
(东南大学化学化工学院,南京 211198)
According to thermodynamics, Professor Xin Xinquan of Nanjing University pointed out that solidstate reactions without solutions formed could reach 100% completion in the early 1990s[1], which has already been verified by a large number of experimental studies[2-5].However, after 30 years, solid-state reactions have not yet been widely used in industrial chemical processes except in some traditional hightemperature inorganic materials industries, such as cement, and ceramics, which is very regrettable,because both the chemical industry and the chemical laboratory researchers have been vexed by the equilibriums, low yields and a large amount of solvent waiting to be treated by the solution reactions: the yield is only 81% after two steps of reaction even if the yield of each step is as high as 90%; after 4 steps it became 66%;and only 43% in 8 steps! Therefore, it is not right to abandon the solid - state reactions of such amiable 100% completion.
The problem may come from the difficulty of diffusion involved in solid-state reactions.Due to the poor fluidity of the molecules, and atom clusters in the solid particles, no reaction may take place between two solid compounds with very tiny separation.To overcome this difficulty, people have to use instruments such as ball mills.Ball mills do not cause problems for inflammable solids but may produce uncontrollable disasters when explosive dusts are formed during the discharging of organic products.
Therefore, we must find a way to industrialize the solid-state reactions.After careful but not very comprehensive considerations, the author proposed a concept of less solvent solid-state reactions (LSRs) for the first time[6], and its core idea is to introduce a small amount of solvent into the solid-state reaction system, which is to overcome the mass transfer difficulty, as well as to use the ordinary stirred reactor to promote the reaction to 100% completion by gradually removing the solvent at the ending period of the reaction.At the same time,because all the substances are in a state of largest concentration (saturated solution) in the vast majority of the reaction time,the chemical reaction speed could be maximized, so the LSR could also significantly shorten the reaction time.Without a doubt, this is in line with the requirements of green chemistry and a sustainable economy.In addition, because the usage of solvent is reduced, the post-treatment of the solvent is consequently reduced, and the energy consumption is naturally reduced, it will also be conducive to the realization of carbon neutralization.
We know that thermodynamics determines the direction of chemical reactions.The question of direction is always the most important because we would never go the opposite of our wishes.Therefore, in the process of theoretical deduction of the above concept,the author has been thinking about how to use thermodynamics to understand the LSRs, especially how to accurately illustrate the changes in their Gibbs energy with images.In the process of forming this paper, the authors have deeply considered some possible issues of LSRs and strived to corroborate them with some experimental studies.Therefore, this article hopes to share these understandings, hoping to attract the attention of the academic community and promote relevant research.
1 Theoretical considerations of LSRs
1.1 Gibbs energy of the mixture with a chemical reaction
If a balanced equation of a chemical reaction is written as:
whereSiincludes both the reactants (Ri) and products(Pi);νiis the stoichiometric coefficient of (Si), which is negative for Ri,and positive for Pi.After some time,the products are formed, and the reactants consumed,therefore,the amount of Siin moles is:
Whereniis the amount of Siat arbitraryξ,ξis the extent of the reaction, and its value range is,is the amount of Siatξ=0.
According to the definition of chemical potential,the Gibbs energy of the mixture system under constant temperature and pressure,Gis calculated as follows:
Suppose that all the substances in the system form ideal solutions,the chemical potential of Si,μiis:
wheremiis the molality ofSiif it is all in the solution:
wheremis the mass of solvent in kg.According to thermodynamics, we know that the direction of a chemical reaction is the direction thatGdecreases; and the lowest point of theG-ξcurve is the point where chemical equilibrium stands.Therefore, if we define ∆rG=(∂G/∂ξ)p,T, called the Gibbs energy of reaction, then,the reaction proceeds along with the direction of ∆rG<0, and it reaches equilibrium when ∆rG= 0.Therefore,if the reaction is carried out in solution,we have:
If a reaction has no solutions of all kinds, the chemical potential of pure solid Si,,therefore,
1.2 Equilibrium thermodynamics of LSRs
The key difference between an LSR from a solidstate reaction is that the LSR employs a small amount of solvent, and its difference from a solution reaction is that it contains solid particles of reactants and/or products during the reaction course.In general, the amount of solvent used is as small as possible,so that (ⅰ)at the very beginning of the reaction, all the solid reactants are in equilibrium with their saturated solutions, and the concentration of product is zero; (ⅱ)as the reaction progresses, the concentrations of products gradually increase and reach their saturation concentrations at some point one by one; (ⅲ)after that, both the solid reactants and products may coexist with their saturated solution; (ⅳ)as the reaction is approaching the end of the reaction, all the reactants enter the solution one by one, their concentrations are going to be zeros, but the solid products coexist with their saturated solutions.Therefore, the concentrations in Eq.4 should be changed according to the above situations, and so are the expressions of the Gibbs energies.
To perform accurate calculations, it is necessary to clarify the relationship between the chemical potential of the saturated solution and the standard chemical potential of the pure solid.We know that the chemical potential of a solute in a solution isμi=μi⊖+RTlnai, whereμi⊖is the standard chemical potential of solute when the activity (ai)and activity coefficient (γi) of substance Si,ai=γi= 1.When the solute forms a saturated solution, its activity is,which is a value that is only related to the properties of the substance, temperature, and solvent.According to the phase equilibrium conditions,we have:
Secondly, we need to clarify the calculation method of the activity of each substance.In this article, for the convenience of calculation, it is assumed that all solutions are ideal and the solutes do not interfere with each other.Therefore, the activity is numerically equal to the molality.It should be noted that “non-interference” in the above assumptions is a very high requirement and can deviate significantly from the actual situation.The salt effect in analytical chemistry, the relationship between ionic strength and activity coefficient,etc.are direct results of this effect.
Assume the amount of substance Siiswhenξ=0, its solubility in the solution ismol·kg-1, andmkg of solvent is used, and Siis saturated at, which means:
Thus, we can determine theξvalues of each stage of the LSR.
1.2.1 The effect of the amount of solvent used on the reaction
Take an arbitrary chemical reaction A+B=C+D proceeding under 298 K, 100 kPa as an example.Suppose (ⅰ)that the reaction can proceed in the same way in either solutions,solid-state without solutions,or solidstate in the presence of a small amount of solvent;(ⅱ)atξ=0, there are 1 mol of A and B each, but no C and D.Thus, at anyξ, there are (1-ξ) mol of A and B, and ξ mol of C and D;(ⅲ)the sum of standard Gibbs energy of formation of reactants,and that of only products,thus; (ⅳ)the solubilities of A,B,C and D are 1,1.5,2 and 2.5 mol·kg-1,respectively,which are constants and not affected by the other materials coexisted, also, all their solutions are ideal solutions;(Ⅴ)the speeds of dissolution and crystallization of the substances are so fast that they are always in equilibrium with their saturated solutions if there are still solids.
When the solvent used for the LSR ismkg, and if all the substances are in solution, the concentrations of the reactants are (1-ξ)/m,and those of the products areξ/m.According to Eq.10, A and B are all in the solution ifξ≤1-mandξ≤1-1.5m, respectively; and C and D crystallize out atξ≥2mandξ≥2.5m, respectively.Thus, the LSR is divided into the following five stages if 2.5m<1-1.5m:
(ⅰ)0 <ξ< 2m, solids A and B coexist with their saturated solution, and both C and D are all in solution.
Thus,we have the following according to Eq.3 and 9:
And ∆rGcould be deduced from the partial differential ofGwith respect toξ:
It should be noticed that the concentrations of substances in saturated solutions do not appear in both 2 equations above, thus Eq.6 should be changed into the following one for LSRs:
where the subscript of “S_us” represents unsaturated substances.This is also shown in the following deductions.
(ⅱ)2m≤ξ<2.5m,solids A and B coexist with their saturated solution, C crystallizes out, but all D still remains in the solution:
(ⅲ)2.5m≤ξ<1-1.5m, all solids coexist with their saturated solution:
(ⅳ)1-1.5m≤ξ<1-m, solids A, C and D coexist with their saturated solution,but all B dissolves in solution:
(Ⅴ)1-m≤ξ<1,solids C and D coexist with their saturated solution,but all A and B dissolve in solution:
Whenm=0.01 and 0.1 kg, the author calculates the values of the Gibbs energies of the reaction A+B=C+D with respect toξbased on the above equations,and the results are charted in Fig.1.In particular,when the solvent used is 1 kg,the corresponding reaction is a solution reaction because all the substances can be dissolved in it.Thus:
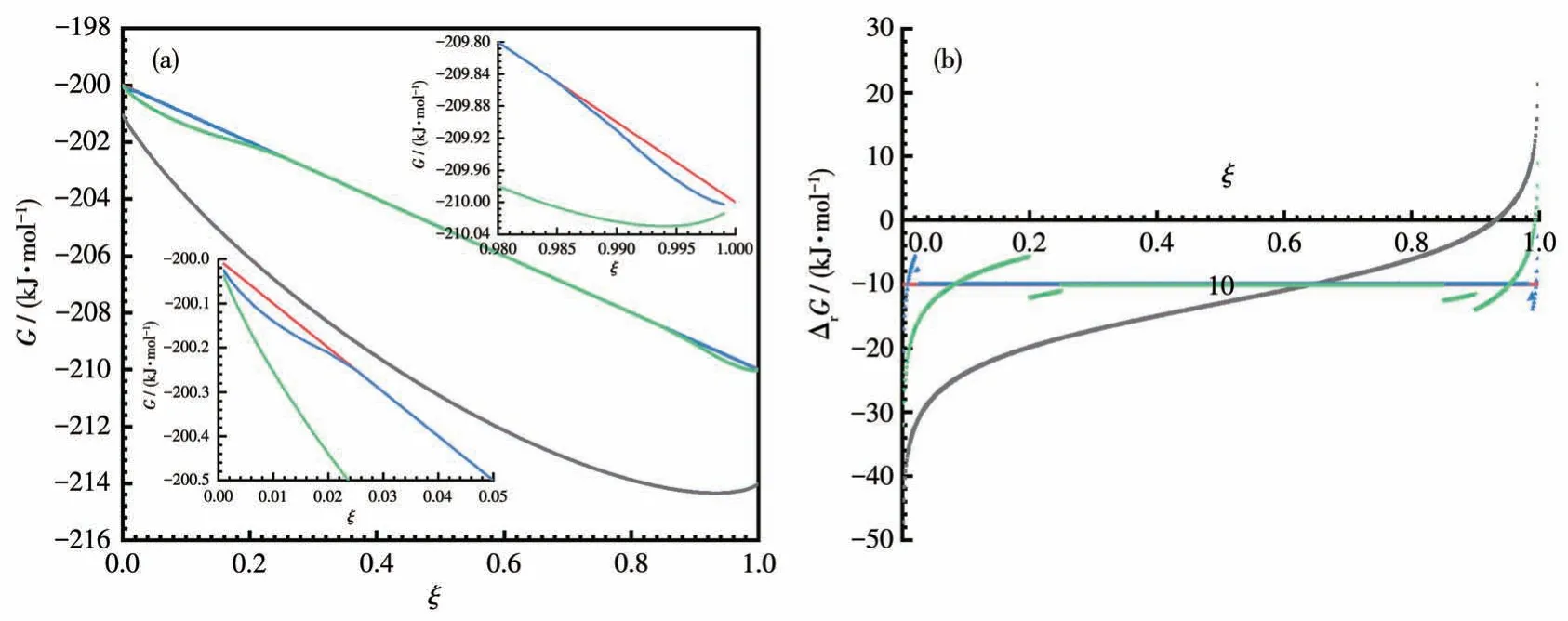
Fig.1 Curves of Gibbs energies of the system(reaction mixture)(a)and reaction(b)vs the extent of reaction
If no solvent is used, and there are no solutions among the substances,we have:
G= -200 - 10ξ
∆rG= -10
Which are the same as those of the third stage of the LSR, butξis in the full range.They are all calculated and then illustrated in Fig.1.
As can be seen from Fig.1a, whenξincreases, the G of the reaction mixture in all cases is continuously decreasing initially; However, it reaches a minimum atξ=0.93 when the solvent used is 1 kg.Similarly, ∆rG=0 atξ=0.93 in Fig.1b,which is the equilibrium point of the solution reaction.For the LSRs, the equilibrium points areξ=0.994 andξ>0.999 for the solvent used being 0.1 and 0.01 kg, respectively; The solution-free solid-state reaction with no solvent used has no equilibrium point at all and proceeds until all the reactants are exhausted.Therefore, LSRs can definitely lead to a higher degree of conversion than solution reactions,but there are still equilibriums.
Thus, we can say that an LSR is a combination of reactions affected by the full dissolution of the products or reactants at both ends and a reaction equivalent to the solid-state reaction without solutions in the middle; moreover, it is possible to override the equilibrium by removing the solvent gradually and completely at the end of the reaction, so that the middle period of reaction is extended to 100% completion.
We know that there must be a chemical equilibrium in solution reactions.This can be seen from Formula 6: ∆rGis determined by both, which is a constant for a given reaction, and, which contains the concentration quotient,is a monotonic increasing function and goes to infinity whenξincreases, so there must be aξvalue that makes ∆rG= 0, that is, the reaction is on its way to equilibrium without a doubt.
For the solid-state reaction without solution existing,, which is independent ofξ.Therefore, once the reaction starts, it continues until one of the reactants is exhausted[1].However, this is a conclusion of time-independent thermodynamics, which only gives hope, but does not care about the outcome.It is known that an ordinary reaction allowed by thermodynamics does not necessarily take place, because 3 possibilities hinder or even completely prevent the reaction from going on:(ⅰ)the activation energy of the reaction is too high to let the reaction proceed at observable speed (a good example is that hydrogen and oxygen in a clean container may never react, but a spark or a little catalyst can make the mixture explode); (ⅱ)it reaches its chemical equilibrium.After a period of reaction,the Gibbs energy of the reaction mixture cannot continue decreasing with the change ofξ, so there is no 100% completion in the solution reaction even if it has a good start; (ⅲ)there is a spatial separation between the reactants,or the diffusion of reactants is too slow so that reactants cannot contact (e.g., the oxidation process of metallic aluminum will be terminated by the dense alumina film,and the oxidation can proceed continually if the film is kept being destroyed.This is the exact reason why a mercury thermometer is not allowed on board when flying).
Thus, if a solid-state reaction without solutions existing occurred but then stopped,there must be diffusion difficulties or spatial barriers, because that it happened means that the reaction is spontaneous and the activation energy is not too high.That is to say, the diffusion difficulty of the solid-state reactant must be overcome first to make the solid-state reaction continue smoothly.This is the fundamental reason why the author proposed the concept of the LSR: by adding a small amount of solvent, liquid bridges are formed among the solid particles, which makes the reactants dissolve and react in them; Secondly, by allowing the solid partially dissolved in the solution, the solid particles are fluidized and easier to homogenize by stirring,which makes transfers of heat, momentum and mass more convenient and effective.It is also possible to avoid using ball milling that may produce harmful dust,promote solid-state reactions being used in the chemical industry,and make chemical processes greener.
1.2.2 Effectsof ∆rGm⊖
We have discussed the thermodynamics of a spontaneous LSR, which usually means ∆rGm⊖≪0.What happens if ∆rGm⊖≈0 or even bigger than 0, for which the equilibrium constant of a reaction like A+B=C+D is about or less than 1, thus only half or less than half of the reactants can be converted? By using the same conditions as above, but the ∆rGm⊖is changed, say, let it be-2 or 2 kJ·mol-1,we have Fig.2.
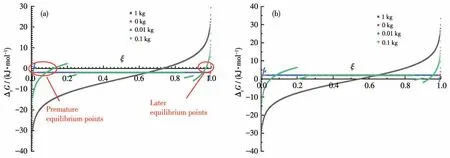
Fig.2 Curves of Gibbs energies of LSR vs the extent of reaction with ΔrGm⊖ of(a)-2 kJ·mol-1,and(b)2 kJ·mol-1
It can be seen from Fig.2 that the LSRs with small negative ∆rGm⊖could reach their equilibrium at very early stages (premature equilibrium points, where∆rG= 0), which is caused by the products fully dissolved in the solvent, if they start with no products;however, the solution reaction can proceed much further to the products.Later,the LSR could proceed even further completion than solution reaction, but there are still equilibriums (late equilibrium points) caused by the full dissolution of products.As we have shown above we can push the late equilibrium points to 100% completion by removing the solvent gradually, we have to override the premature equilibriums first.
There are two strategies, theoretically, to do that:(1) use more solvent to initiate the reaction until enough products are formed and begin to crystallize;(2) use a little solvent, as well as some solid products as the “seeds” so that there are always dissolution equilibriums for all the products.
However, the LSRs of ∆rGm⊖> 0 are basically not allowed by thermodynamics because the∆rGof the middle stage is positive, while the solution reactions can proceed and reach the equilibrium at a substantial conversion of the reactants (Fig.2b).Therefore, if the reaction system allows two reactions that one has∆rGm⊖slightly bigger than 0 and the other ∆rGm⊖slightly smaller than 0, the solution reaction could make both them proceed to produce a very substantial amount of products, while the SSR and LSR allow only the reaction of ∆rGm⊖< 0 to take place.
As we know from thermodynamics, we can couple a reaction that consumes a product of the reaction with∆rGm⊖> 0 to make the complex reaction ∆rGm⊖< 0,thus,it is possible to make it complete for LSR as well.Examples are shown to realize this later in Section 2.1.3.3.
1.3 Kinetics of LSRs
If an LSR is taking place with a little solvent, it must include the following steps: (1) The reactant dissolves in the liquid film between the solids, or is swelled by the solvent to form a fully or partially solvated substance; (2) The reactants react to form products inside the liquid film or on a solid surface;(3)The product diffuses to a nucleation site and crystallizes to form the solid product.
Undoubtedly, the above three steps occur consecutively and form a dynamic non-equilibrium process.The speed of the entire process may be determined by any one of the above three steps, or together.Concentration is no longer a good measure for the speed of LSRs,we would better use the amount of a product produced or a reactant consumed in the whole volume of the reaction mixture in a given time to measure it.Especially, when X-ray diffraction (XRD) is used to monitor the reaction, the time taken for the complete disappearance of peaks from the reactants could be used to measure the average speed of the reaction.Thus, an LSR can be divided into two periods: the dissolution of the reactants and the reaction together with the crystallization of products.If an LSR is controlled by the dissolution speed of reactants, the concentrations of the reactants will be much lower than the saturated ones, thus faster reaction is expected if more solvent is used because more reactant can be dissolved and allowed to react; if an LSR is controlled by the reaction and/or crystallization of product, its speed will not be affected too much by the amount of solvent, because the concentrations of the reactants are the saturated ones, which means the reaction will proceed in full speed.This is very different from the solution reactions because their speeds become slower definitely if more solvent is used.Researches on the LSRs of[Mg(H2O)6]Cl2and NH4Cl, Na2SO3and S8, as well asβ- PbO and PbSO4do confirm those deductions[7-9].Please find the details in the next section.
From a point of microscopic view, LSRs could take place in the liquid bridge or film between solid particles, thus the length of the liquid bridge, or the thickness of the liquid film depends on the amount of solvent, and the actual reaction site could be very close, even on a solid surface, or in the middle of the liquid bridge or film far from the solid surface.If the reaction occurs in a solution far away from the surface of the solid, it should be similar to the solution reaction; if the reaction occurs on the solid surface, it will be affected by the interaction between the solid surface and the solution.All these may greatly affect the reaction mechanism, and make the reaction quite different from that of the solution or solid-state reaction.
In addition,LSRs may involve monolayer to multilayer adsorption of solvent molecules on the surface of reactants, partial or complete solvation of the reactant species, which is very different from the solution reactions and leads to very different results.For example,it was reported that hydrated electrons on the surface of Na-K alloy if water vapor of low pressure is introduced,which conduct electricity like metals, but it is very unstable if more water is introduced, which decomposes to hydrogen and alkaline hydroxide[10], although it has been well known that electrons can be stabilized by amines/ammonia, ethers and alcohols[11-12].Therefore,partial solvation in the LSR system could bring out some new phenomena.
2 Experimental studies on the LSRs
2.1 Factors that affect the LSRs
2.1.1 Effect of solubilities and dissolution speeds of the reactants
As pointed out above, the small amount of the solvent promotes the contact of reactants by dissolving and fluidizing them, and the reaction is likely to take place in the liquid bridges connecting the solid particles of the reactants, which raises a question of whether the solubility of the reactants will have any effect.
According to Eq.9,the bigger,the smallerμi,which means it will affect the Gibbs energy of the reaction,thus, the reaction is more preferred that has bigger solubilities of products.For the speed of the reaction, we have to consider the procedures and mechanism of the reaction.As revealed in Section 1.3, if an LSR takes place in the solution film among the reactant particles,it must experience three big steps at least:(ⅰ)the dissolution of the reactants; (ⅱ)the reaction of reactants in the solution, and (ⅲ)the crystallization of products, if we monitor the reaction by monitoring the reaction mixture with XRD, the total speed could be limited by any of the three steps,or their combinations.
We have studied the reactions of (ⅰ)magnesium chloride hydrate, [Mg(H2O)6]Cl2with ammonium chloride,NH4Cl[7]; (ⅱ)le ad oxide,β-PbOand leadsulfate,PbSO4[8];(ⅲ)sulfur, S8and sodiumsulfite,Na2SO3[9]to find out the effect of solubility and dissolution speed on the reaction.These reactions are unique to each other:all the reactants and product of the first reaction is very water-soluble, but those of the second are very poor,and sulfur in the third reaction is regarded as a waterinsoluble substance.The main results are as follows:
The reaction of NH4Cl and MgCl2·6H2O, structure as[Mg(H2O)6]Cl2[7],is:
According to their crystal structures, the structure of [Mg(H2O)6]2+does not change through the reaction,hence Reaction 11 looks like actually a process that NH4+and Cl-from NH4Cl solution intercalate into the lattice of magnesium chloride hydrate, thus the Reaction 11 is not very thermodynamically favored.The speed of this reaction was found to increase with the increase in the amount of water and temperature.It is by the general chemical reaction law that the reaction speed increases with increasing temperature.The increase in the amount of solvent naturally makes more reactants (and products) enter the solution, which makes more reactants react at the same time, thus making the reaction faster.There should be no reaction in the solution and during the crystallization, and crystallization of the product could be faster because the product is more favored thermodynamically.Therefore,the control step of the reaction should be the dissolution of the reactants.
The above deduction is strongly supported by the reaction of Na2SO3and sulfur[9]:
Here, the solubility of sulfur is only 4.78×10-7mol·L-1at 80 ℃in water, and its dissolution speed is found extremely slow[13]; but the other two substances are extremely soluble in water.In particular, Na2S2O3·5H2O can be dissolved in its crystal water, and even less at higher temperatures.It has been found that Reaction 12 can be completed within 5 h under proper stirring at 115 ℃if the reactants are mixed in stoichiometric ratio.
We believe that the reason for this slow reaction should be that the dissolution speed of sulfur is too slow.As we all know, crystal sulfur has cyclic molecules of 8 S atoms, which is a non-polar soft Lewis acid, thus insoluble and very slow in dissolving in water.Experiments have also found that the reaction speed accelerates as more water is used, which strengthens the conclusion on Reaction 11[7,9].
In contrast, the reaction speed of Reaction 13 has little to do with the amount of solvent used[8]:
When the solvent used is increased by 3 times,the reaction speed changes little, both complete within 15-45 min at around 35 ℃,depending on the history of the reactants.It is known that the solubility of all the substances in Reaction 13 in water is very small[14],combined with the experimental results of Reaction 11,which indicates that the solubility of the reactants is not the main factor that determines the speed of LSRs.
It has been known that there are no water molecules in the crystal structure of 3PbO·PbSO4·H2O, but only OH and oxygen atoms[15], so a more appropriate expression for its structure is 2PbO·Pb(OH)2·PbSO4[8,16].Therefore, Reaction 13 includes the following two steps:(1)the dissolution of the reactants to form hydrated Pb2+, OH-, SO42-; (2) the formation of 2PbO·Pb(OH)2·PbSO4from hydrated Pb2+, OH-, and SO42-.Again,there should be no reaction in the solution, but the crystallization of 2PbO·Pb(OH)2·PbSO4must involve multiple steps such as ion rearrangement,condensation of OH-, which should take place at the surface of the solid particles of the products.Thus, its speed cannot be too fast.In addition, XRD can only detect crystalline substances, when we take the complete disappearance of the diffraction peaks of the reactant and the complete formation of the diffraction peaks of the product as the endpoint of the reaction, the dissolution speed of the reactants and the crystallization speed of the product become key factors that determines the speed of the reaction.Only when the crystallization speed of 2PbO·Pb(OH)2·PbSO4is the speed limiting step, is the reaction speed independent of the amount of solvent.
Therefore,we can conclude that although the solubility of the reactants could be an important factor of LSR thermodynamics, it is not the main factor determining its reaction rate, the dissolution speeds of the reactants and the crystallization speeds of the products are more important factors.
Thus,it is possible to accelerate an LSR by changing the solvent, the key is to find a good solvent for all the reactants.For example, the LSR ofβ-naphthol with FeCl3·6H2O in a little water is limited by the dissolution speed ofβ-naphthol in water, however, if the solvent is changed to a volume ratio of 50% mixture of ethanol and water, the reaction can complete much faster.This is becauseβ-naphthol dissolves much more quickly in the mixture of ethanol and water[17].
2.1.2 Reactions with the solvent as a product
Unlike all the preceding reactions, Reaction 14 has the following two characteristics: (1) there is an equilibrium in the solution reaction.Also, in the literature, the product (NH4)2[CoCl4(H2O)2] was prepared by evaporating the solvent[18]; (2) this reaction produces water that can be used as the solvent.
Due to the lack of thermodynamic data, we use a model reaction of A+2B=C+4W to represent Reaction 14, where W is the solvent water.Based on its characteristics, we assume that its ΔrGm⊖is -10 kJ·mol-1,and the solubilities of A, B, and C are 5, 8, and 6 mol·kg-1H2O, respectively; and the mass of water used are 0,0.075,1.0 kg,respectively atξ=0.It must be noticed that the molality of A, B, and C are (1-ξ)/(m+0.072ξ), 2(1-ξ)/(m+ 0.072ξ), andξ/(m+ 0.072ξ),respectively, if substance Siis all dissolved in water,because more water is produced during the reaction and should add to the original dosage of solvent water,then Fig.3 is produced.
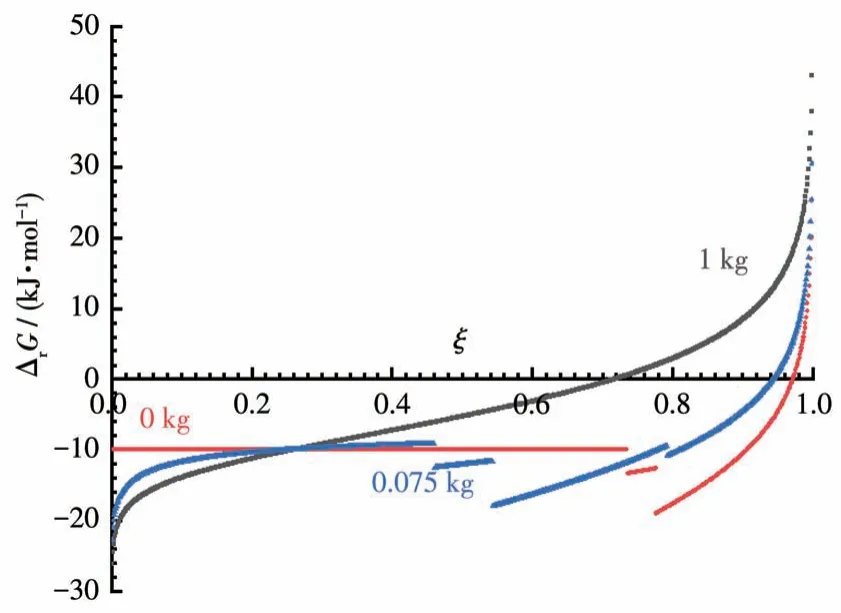
Fig.3 Gibbs energy of the LSR with the solvent released
As can be seen from Fig.3, the solution reaction reaches its equilibrium atξ=0.72.For the LSRs, when the solvent used is increased from 0 to 0.075 kg, theξvalues of the reaction reaching equilibrium decrease from 0.97 to 0.94.Therefore, even in the case of the reaction itself producing solvents, the equilibrium of the LSR is pushed towards 100% completion as the amount of solvent used decreases.Experiments do prove that Reaction 14 can be completed if the solvent is gradually removed at the end of the reaction.The details of this research will be published soon.
2.1.3 Consecutive reactions
The author and his schoolmates under the supervision of Professor Xin Xinquan carried out a series of studies on solid-state coordination chemistry in the early 1990s, they found the so-called stepwise reaction phenomenon that the products of some consecutive reactions are determined by the initial ratio of the reactants[19-23].For example, the reaction of solid CuCl2·2H2O and 2, 2′ - bipyridyl (short as bipy) produces Cu(bipy)Cl2if their molar ratio is 1∶1, or Cu(bipy)2Cl2·H2O if the molar ratio is 1∶2[22].Monitoring the reaction with a differential scan calorimetry (DSC) shows that the reaction temperature of the first step of the reaction is about 20 ℃, but that of the second step is about 70 ℃.Consequently, the second step of the reaction cannot take place at room temperature if the reactants are mixed in a 1∶1 molar ratio, although the reactant/reactants has/have to pass over Cu(bipy)Cl2[22].
Similarly, the solid-state reaction of CuCl2·2H2O and 8-hydroxyquinoline (HQ) produces Cu(HQ)Cl2if the reactants are mixed in a 1∶1 molar ratio,where HQ replaces two water molecules in CuCl2·2H2O,while the reaction produces Cu(Q)Cl and (H2Q)Cl·H2O if they are mixed in molar ratio of 1∶2, where the second HQ molecules takes a proton and a Cl-together from Cu(HQ)Cl2.DSC measurements show that the reaction temperatures of the reactions are 47 and 75 ℃, respectively[21], which reveals that the phenomenon is also the result of kinetic controls.
However, the observations with energy dispersive X-ray diffraction (EDXRD) on the solid-state reaction of FeSO4·7H2O ando-phenanthroline (phen)in a molar ratio of 1∶1 show that the reaction produces [Fe(phen)3]SO4·5H2O at 54 ℃firstly,as indicated by DSC studies,then [Fe(phen)3]SO4·5H2O reacts with unreacted FeSO4·7H2O to produce [Fe(phen)(H2O)3SO4] at 60 ℃;EDXRD also reveals the second step of the reaction does not take place at 50 ℃[23].This research indicates that solid-state reaction is very sensitive to reaction temperature, which should be caused by the similar reason that crystals have a very narrow range of melting points.It also supporting the conclusions on the above two reactions of CuCl2·2H2O why the second step of the reaction will not take place even if their reactants encounter.
However, a question arises: we know that the first step of the reaction, in which FeSO4·7H2O react with phen to produce [Fe(phen)3]SO4·5H2O, is spontaneous,thus the reversed one is definitely not spontaneous,how could the second step, which is the reaction of[Fe(phen)3]SO4·5H2O and FeSO4·7H2O to produce Fe(phen)(H2O)3SO4, take place? Is it not the reversed one? More importantly, can we expect a stepwise reaction in LSRs?
Recently, we have studied the LSR of CuCl2·2H2O and bipy in molar ratio 1∶1 with a little water as the solvent.We have found that Cu(bipy)Cl2and[Cu(bipy)2Cl]Cl·H2O coexist initially, but in the end,only Cu(bipy)Cl2is the product[24].This is certainly a very different result from the solid-state reaction of CuCl2·2H2O and bipy.But why?
Here, we use the consecutive reactions of Reactions 15 and 16 as the example to illustrate the related problem.
If the initial amount of substanceiis, and the extent of Reaction 15 is represented withx, and that of Reaction 16 withy, thusx≥y≥0; the mass of solvent used is m kg, then the amountniand concentrationmi(in mol·kg-1)of substance Siare:
Still, the solutions are all ideal, the saturated concentration of substance Siis,the chemical potential of the solid Siis,then,the chemical potential of substanceiat arbitrary concentration/activity is defined as Eq.9, and the Gibbs energy of the mixture as Eq.3.To find the equilibrium point, we need to derivate it with respect toxandy,respectively.Therefore,
Now, we can discuss the issues in different cases in solution,solid-state,and LSRs.
2.1.3.1 The solution reactions
For the solution reactions,assume they are reversible,and.According to the mass conservation, we have.Deform the Eq.23-24 and substitute in them:
IfK15=2.0×104,K16=4.2×103;;m=1 kg.Fig.4 is obtained, which shows there is no way definitely to obtain pure C or D by adjusting the initial molar ratio of A and B in a solution equilibrium system.
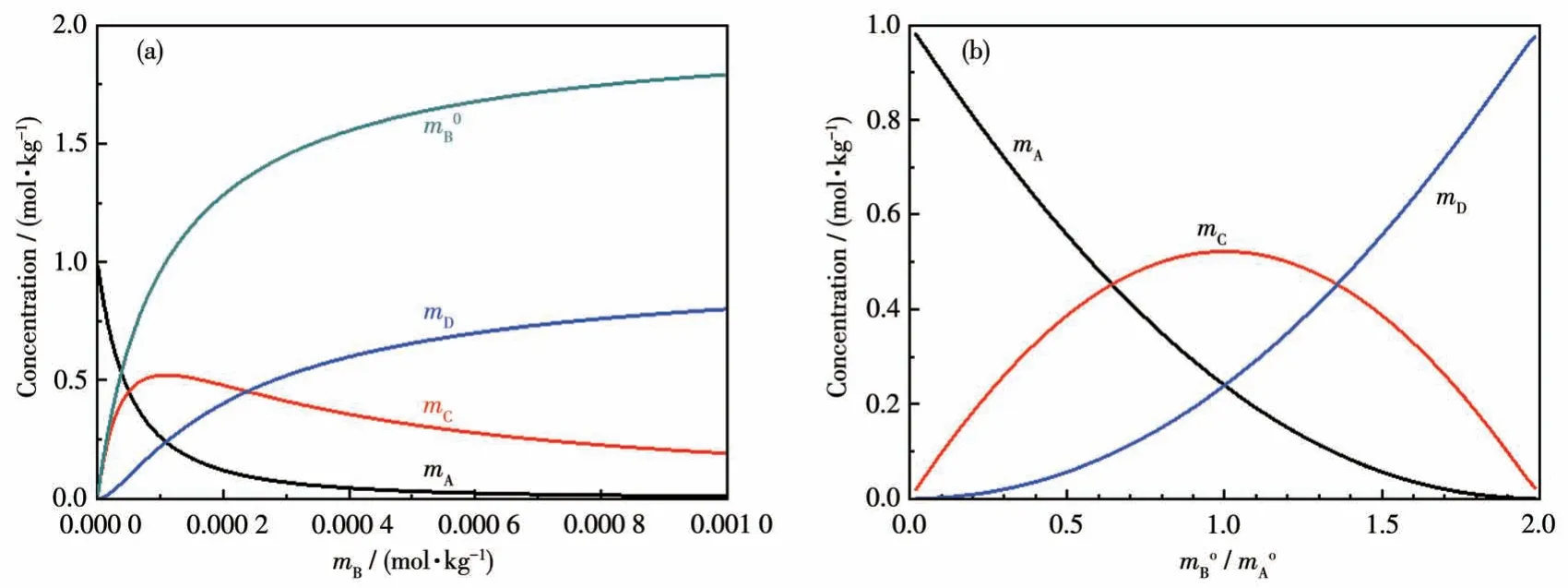
Fig.4 Concentrations of various species determined by equilibrium thermodynamics:(a)with respect to the concentration of B in solution;(b)with respect to the initial molar ratio of B to A
2.1.3.2 Solid-state reaction without solutions and LSRs
For the solid-state reaction without solutions, the chemical potentials of all substances are constants that are not related to the extent of the reaction.Therefore,it goes until one of the reactants is exhausted.However, if the solid-state reaction is not agitated by any means, at least one of the reactants must penetrate the product layers accumulated between the reactants,then, if the activation energies of subsequent reactions are not too high, the reactant may react with the product first to produce the products of the post steps successively.This is what we have found in the solid-state reaction of FeSO4·7H2O and phen[23].It is known that FeSO4·7H2O has a structure of [Fe(H2O)6]SO4·H2O,thus all the waters coordinated to Fe2+are very easy to be replaced subsequently by phen because the activation energy for the substitutions is similar:
However, the second step of the reaction is much different:
The activation energy of reaction 29 must be higher than the previous substitution Reaction 28,because Fe—N bonds between Fe2+and phen are stronger than Fe—OH2, and it has to break two of them at almost the same time.
But why can Reaction 29 take place? Does it violate thermodynamics? To simplify the reasoning, we use A to present FeSO4·7H2O, and B to present phen,their coordination compounds are AB, AB2, and AB3.Thus,the reactions take place sequentially:
Thus,the Reaction 33 could be spontaneous if.Generally, it is known that, for which the examples can be found in Lange′s Handbook of Chemistry[25], therefore, the Reaction 29 is very possibly spontaneous.However, we have to point out that the reaction of Fe2+and phen in aqueous solution could produce [Fe(H2O)4(phen)]2+, [Fe(H2O)2(phen)2]2+, [Fe(phen)3]2+, and their formation constants, lgKiare 5.85, 5.6, and 9.85[25],thus the values of ∆rGm⊖are -38.4,-36.7,and-64.6 kJ·mol-1, respectively, which means that the solution reaction[Fe(phen)3]2++2[Fe(H2O)6]2+=3[Fe(H2O)4(phen)]2+could not be spontaneous, and the most abundant species in Fe2+and phen mixture is [Fe(phen)3]2+.In the solid state, Fe(H2O)3(phen)(SO4) should be a molecular solid, since SO42-is a stronger ligand than H2O, thus replaces water to enter into the coordination sphere[23],therefore, it is very different from the solution reaction,which makes Reaction 29 proceed to 100% completion.
Similarly, it is possible to get AB2in LSR by mixing A and B in 1∶2 molar ratio,since ∆rGm⊖of 2AB3+A=3AB2equals, therefore, if,the reaction is spontaneous.As it is stated above,is very possible for consecutive reactions.For the reaction of FeSO4·7H2O, and phen in 1∶2 molar ratio,it is more probable that [Fe(H2O)(phen)2(SO4)] is the product in the solid state.
Thus, the LSR of CuCl2·2H2O and 2,2′-bipyridyl in a 1∶1 molar ratio with a little water produces Cu(bipy)Cl2as well as Cu(bipy)2Cl2·2H2O at first is understandable[24].We believe that it is caused by the formation of CuCl2(H2O)4when CuCl2·2H2O partially dissolves in water, as we know that CuCl2·2H2O is a polymer oftrans-[CuCl2(H2O)2] connected by intermolecular Cu—Cl bonds.Just like what has been found in the reaction of FeSO4·7H2O and phen[23], the substitution of four water molecules by two 2,2′-bipyridyl molecules would be easy and fast in aqueous solution,therefore both Cu(bipy)Cl2and Cu(bipy)2Cl2·2H2O are found in the early stage of the reaction.However,the latter reacts with unreacted CuCl2·2H2O and produces Cu(bipy)Cl2because the standard Gibbs energy of the second step reaction is bigger than that of the first one.Therefore, for LSRs, it is still possible to obtain the stepwise product when a bit solvent is used.
The solid - state reaction of CuCl2·2H2O and NH4Cl in a little water produces (NH4)2[CuCl4(H2O)2]only because NH4CuCl3is not stable in water.However,the reaction of anhydrous CuCl2and NH4Cl in a 1∶1 molar ratio with a bit of absolute alcohol can produce NH4CuCl3and completes in 3 h, but it takes a much longer time to produce (NH4)2[CuCl4] completely.This should be related to the slow and difficult dissolution of NH4Cl and NH4CuCl3in absolute alcohol[24].
2.1.3.3 A deduction for the competitive reactions
For a reaction system that contains competitive reactions:
Therefore,no F and G would be formed in LSRs.
Thus, we have a new reaction B+F+G+H=D+E+K+L, which is spontaneous since
Consequently, LSR can do very much to make chemical processes greener and more selective.
2.2 LSR systems other than the above
Some LSR systems have been industrialized.Among them, commercial electrochemical cells and solid - state fermentation processes are starring.For example, to increase the energy density of batteries,the amounts of electrochemically non-active substances are always reduced as much as possible in all commercial cells, so the solvent used in the electrolyte is always minimal.Especially, we know that the electrochemically active substances in all batteries are solids,except the flow cells and fuel cells, they are the most proper industrial examples of LSRs.Table 1 lists the chemical reactions involved in common battery systems.
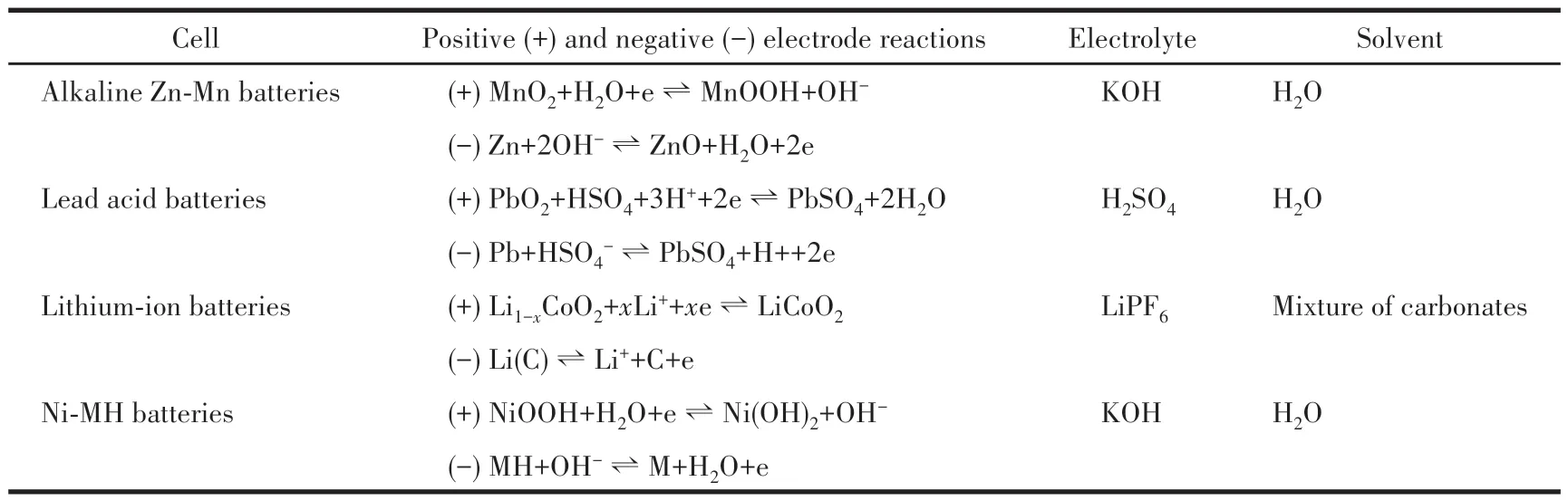
Table 1 LSRs in most popular electrochemical cells
It is well known that the positive and negative electrodes are separated by separators in the electrochemical cells, so the reaction must include the exchange of substances between the two electrodes.For example, in a lead-acid cell, the protons produced by the negative electrode must pass the separator to the positive electrode, and as a result, water is generated on the positive electrode, which reduces the concentration of sulfuric acid in the positive electrode region more than the negative one, thus leads to the diffusion of water molecules to the negative electrode region.Similarly, when a lithium-ion battery is discharged, the positive electrode material receives the Li+released by the negative electrode.Those exchanges between the electrodes must be done by ions and/or molecules flowing across the electrolyte solution or fast ion conductor.As we know ions have to be solvated or cling to the lattice of fast ion conductors, which means that the binding force between the medium and the ions must not be too strong so that the ions can move faster.The author believes that the research that pursues “all-solid” electrolytes should consider the theoretical thinking on LSRs in this paper, and add a little liquid substance(so that no harmful flames will be produced when thermorunaway takes place) to promote the movement of ions properly.
Solid-state fermentation refers to the process that uses an insoluble solid matrix to cultivate microorganisms, including both deep fermentation that solid-state materials that are suspended in liquids, and processes that cultivate microorganisms on wet solid materials with no(or almost nothing)free water[26].
In addition,in the biochemical process in the cell,the amount of solvent is the least, and there are semisolid substances such as solvated enzymes, DNA, and RNA,which can also be regarded as LSRs.
Liquid-assisted mechanochemistry is a technique to speed up the solid-state reaction by adding a tiny amount of liquid.Generally, the liquid additives mainly play the role of lubrication and solvent,and they can improve the uniformity and speed of reactant mixing[27].Unlike the LSRs described above, liquid-assisted mechanochemical reactions use ball mills,so the spacetime productivity of the reactor will be much reduced because grinding balls take up a lot of reactor space.
3 Conclusions
In summary, the following conclusions can be drawn:
(1) The LSR technique employs a little solvent to promote the reaction.The small amount of solvent, just like the magpie bridge of the 7th of Chinese lunar July,connects the immobile solid components and makes them meet and react, also giving the fluidity of solid particles in a stirred reactor.Thermodynamically, it results in an intermediate stage equivalent to the solidstate reaction without solutions, of which Gibbs energy of reaction does not change; and the two ends like a solution reaction, of which Gibbs energy of reaction changes sharply, which brings out an equilibrium point.However, by gradually removing the solvent at the end of the reaction, the intermediate stage can be extended to the far end of the reaction, so that the LSRs can eventually reach 100% completion.Because the solvent must be removed anyway, it is not superfluous, and just kills three birds with one stone: making chemical processes greener by reducing solvent usage,pushing the chemical reaction 100% completed, and making the solid-state reaction easily carried out in an ordinary stirred reactor;
(2) It is possible for consecutive reactions to get the intermediate product by using LSRs if the ∆rG⊖mof the subsequent step is bigger than that of the previous step.Very luckily, that is the general case of consecutive reactions;
(3) It is possible to make a solid-state reaction with near-zero negativeΔrG⊖mcomplete by using more solvent or product “seeds”,otherwise,it may stop at its very beginning because of full dissolution of products.Of course, removing the solvent is still needed to push the reaction to 100% completion;
(4) For reversible parallel reactions, if the ∆rG⊖mof the unwanted reaction is bigger than that of the wanted one, or if a reaction of very negative ∆rG⊖mis coupled with the wanted reaction by consuming one of the products, it is also possible to get the sole products of the wanted reaction.Thus, LSRs supply effective means of selectivity;
(5) The speed of an LSR is determined by the dissolution speeds of reactants,the chemical reaction,and the crystallization speeds of products, it has little to do with the solubilities of the substances in the reaction.
Acknowledgment:Thanks must be first given to Professor Xin Xinquan, who started the room temperature solid state reaction and made me think about the issues discussed in this paper.Also,thanks to my financial supporters from the industry,who support my other projects.Thirdly,in the process of composing this article, I have received constructive suggestions and kind encouragement from Professor Hou Wenhua of Nanjing University, Professor Sun Yueming of Southeast University, and Professor Zhou Yiming of Nanjing Normal University.Also, I received some tough and even wild criticism from unknown reviewers for the previous unpublished versions of this paper,which helped me remove the flaws.Criticism is definitely and always welcome, although friendly and constructive ones are more favored.Big thanks to all of them!
