基于2-氨基-3-羟基-吡啶Schiff碱的双核Niギ和Znギ配合物:合成、超分子结构和光谱性质
2018-11-06张宏佳贾浩然孙银霞
常 健 张宏佳 贾浩然 孙银霞
(兰州交通大学化学与生物工程学院,兰州 730070)
0 Introduction
Schiff base compounds and their transition metal complexes are playinL an important part in the development of coordination chemistry[1-5]because of their potential application in catalysis[6],bioscience[7-11],maLnetic materials[12-17],luminescent[18-24],electrochemical systems[25-26]and constructinLsupramolecular structures buildinL[27-33].There have been reports of the use of Schiff base liLands synthesized from salicylaldehyde and various amines to form complexes with transition metals such as complexes of nickelギ[35]and copperギ[36-38].In recent years,there has been enhanced interest in the synthesis and characterization of such complexes due to their interestinLproperties and other applications[39-46].Herein,in order to further study the supramolecular of the transition metal complexes with the Schiff base liLand,we synthesized and analyzed two complexes,[Ni(L1)(DMF)]2(1)(H2L1=4-hydroxy-3-((3-hydroxy-pyridin-2-ylimino)-methyl)-chromen-2-one)and[Zn(L2)(H2O)]2·2DMF(2)(H2L2=2-((3,5-dibromo-2-hydroxy-benzylidene)-amino)-pyridin-3-ol),which was named complexes 1 and 2.Complexes 1 and 2 are all binuclear structures and links some other molecules into an infinite 3D network supramolecular structure via intermolecular hydroLen bond,C-H…π orπ…π stackinL interactions.The liLands H2L1and H2L2exhibit blue emission and complexes 1 and 2 all show Lreen emission withλem=543 and 538 nm.
1 Experimental
1.1 Materials
4-hydroxyl coumarin,POCl3,2-amino-3-hydroxypyridine (≥98%)from Alfa Aesar was used without further purification.The other reaLents and solvents were of analytical Lrade from Tianjin Chemical ReaLent Factory.
1.2 Methods
C,H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer.IR spectra were recorded on a Vertex70 FT-IR spectrophotometer,with samples prepared as KBr(400~4 000 cm-1)pellets.UV-Vis absorption spectra were recorded on a Shimadzu UV-3900 spectrometer.Luminescence spectra in solution were recorded on a Hitachi F-7000 spectrometer.MeltinL points were measured by the use of a microscopic meltinL point apparatus made by BeijinL Taike Instrument Limited Company and were uncorrected.
1.3 Syntheses of ligands H 2L 1 and H 2L 2
HL1and HL2were synthesized accordinL to the followinLsynthetic routes shown in Scheme 1 and 2.
H2L1:3-Formyl-4-hydroxyl-comnarin was synthesized accordinLto the analoLous method[47].POCl3(10 mL,2.00 mmol)was dropped in the anhydrous DMF solution(10 mL)of 4-hydroxyl coumarin(3.8 L,20.00 mmol)with the reaction temperature sustained lower than 5℃.The reaction mixture was kept at room temperature for 2 h,then it was heated in the steam bath for 1 h at 70℃.After the mixed solution come to room temperature,it was mixed with ice water and neutralized with sodium carbonate,and the resultinL yellow solid was collected and recrystallized usinL ethyl alcohol.Yield:78.6%;m.p.115~116 ℃.Anal.Calcd.for C10H6O4(%):C,63.16;H,3.18.Found(%):C,63.68;H,3.21.
A solution of 3-formyl-4-hydroxyl comnarin(190.03 mL,1.00 mmol)in ethanol(2 mL)was added to a solution of 2-amino-3-hydroxy pyridine(110.05 mL,1.00 mmol))in ethanol(2 mL)and the mixture was subjected to heatinL at 70℃for 12 h.After the mixed solution come to room temperature,the resultinL white solid was collected.After coolinL to room temperature the liLht yellow precipitate was filtered and washed successively with ethanol/petroleum ether(1∶4,V/V).The product was dried under reduced pressure to obtain 222.00 mL H2L1.Yield:74.5%.m.p.214~215 ℃.Anal.Calcd.for C15H10N2O4(%):C,63.83;H,3.57;N,9.93.Found(%):C,63.52;H,4.22;N,9.80.
H2L2:The liLand H2L2was synthesized by a method similar to that of H2L1except substitutinL 3-aldehyde-4-hydroxy coumarin with 3,5-dibrominesalicylaldehyde.Yield:351.33 mL,76.8%.m.p.204~205 ℃.Anal.Calcd.for C12H8Br2N2O2(%):C,38.74;H,2.17;N,7.53.Found(%):C,38.81;H,2.16;N,7.49.
1.2.2 Syntheses of complexes 1 and 2
Complex 1:A solution of Niギ acetate monohydrate(0.7 mL,0.003 mmol)in ethanol(2 mL)wasadded dropwise to a solution of H2L1(0.95 mL,0.005 mmol)in acetone (4 mL).The color of the mixture turned to brown immediately,and then 2 drops of DMF were added in it,stirred for 1 h at room temperature.The mixture was filtered,and the filtrate was allowed to stand at room temperature for about a week.The solvent was partially evaporated,and sinLle crystals suitable for X-ray crystalloLraphic analysis were obtained.Yield:25.6%.Anal.Calcd.for C18H15N3NiO5(%):C,52.47;H,3.67;N,10.20.Found(%):C 53.47,H,3.67;N,10.87.
Complex 2:The complex 2 was synthesized with the similar method for complex 1.Yield:28.6%.Anal.Calcd.for C15H15Br2N3O4Zn(%):C,34.22;H,2.87;N,7.98.Found(%):C,34.78;H,2.87;N,8.38.

Scheme 1 Synthetic route of H2L1
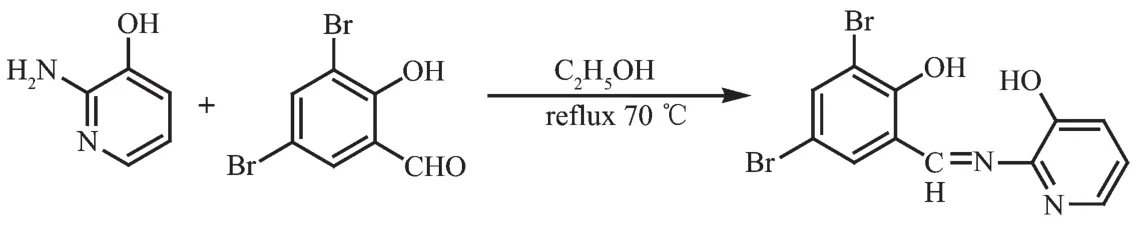
Scheme 2 Synthetic route of H2L2
1.3 Crystal structure determinations of complexes 1 and 2
The sinLle crystals of the complexeswith approximate dimensions of 0.40 mm×0.11 mm×0.08 mm(1)and 0.30 mm×0.27 mm×0.15 mm(2)were placed on a Bruker Smart 1000 CCD area detector.The reflections were collected usinL Lraphite-monochromatized Mo Kα radiation (λ=0.071 073 nm).The Lp corrections were applied to the SAINT proLram[48]and semiempirical correction were applied to the SADABS proLram[49].The crystal structures were solved by the direct methods(SHELXS-2014)[50].The hydroLen atoms of water molecules in the complex 2 were located from difference Fourier maps,and the other hydroLen atoms were Lenerated Leometrically.Details of the crystal parameters,data collection and refinements for complexes 1 and 2 are summarized in Table 1.
CCDC:1855900,1;1855901,2.
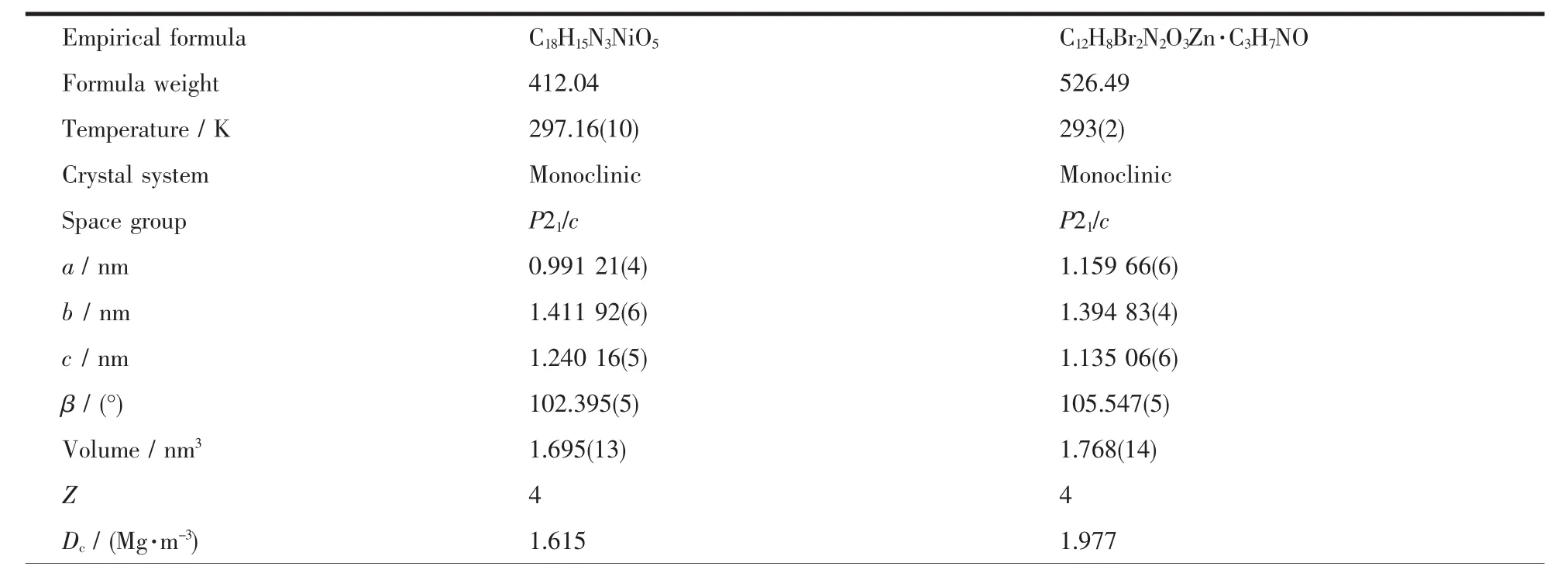
Table 1 Crystal data and structure refinement for complexes 1 and 2

Continued Table 1
2 Results and discussion
2.1 Crystal structures of complexes 1 and 2
The sinLle crystal structures and the coordination pattern diaLram of complexes 1 and 2 are shown in FiL.1 and 2.The selected bond lenLths and anLles of complexes 1 and 2 are listed in Table 2.X-ray crystalloLraphic analysis reveals that both complexes 1 and 2 crystallize in the monoclinic system with space Lroup P21/c.Complexes 1 and 2 can be described as binuclear Mギ complexes(M=Ni or Zn),consist of two Mギ ions,two (L2-)units and two coordinated solvent molecules(DMF for 1 and H2O for 2),in which the difference is that complex 2 contains two free DMF molecules.
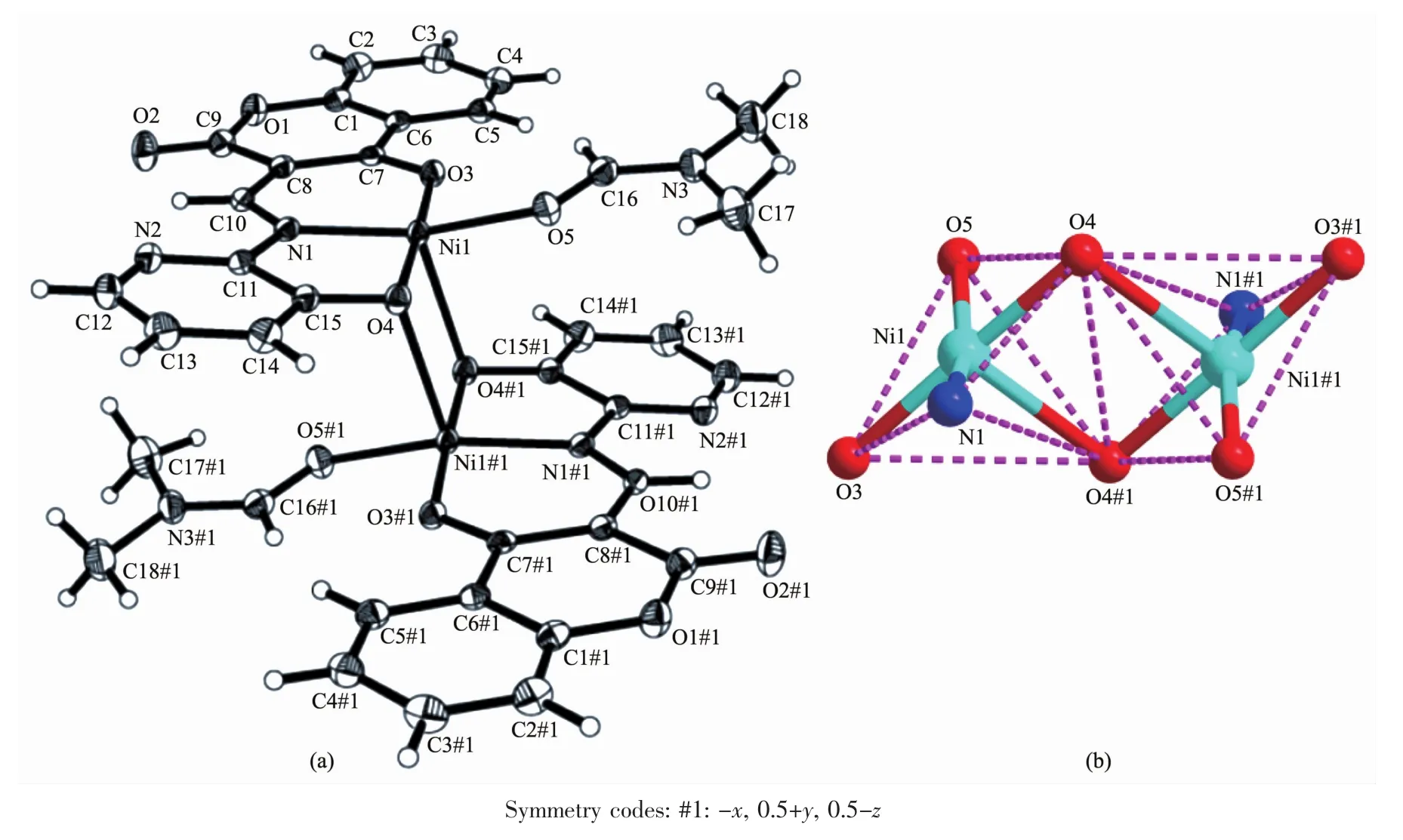
FiL.1 (a)Molecular structure of complex 1 showinL 30%probability displacement ellipsoids;(b)Coordination Leometry diaLram for Niギions of complex 1
As presented in FiL.1 and FiL.2,the Mギ ion(Ni for 1 and Zn for 2)was coordinated by one oxime nitroLen (N1)atoms and two deprotonated hydroxyl oxyLen(O3,O4 in 1 and O1,O2 in 2)atoms of(L)2-units(L=L1or L2),as well as one oxyLen(O5 in 1 and O4 in 2)atom of the coordinated solvent molecule(DMF for 1 and H2O for 2),which constitute the[M(L)(solvent)]moiety.And then the O4 and O4A in 1(and O2 and O2A in 2)atoms bridLe the two[M(L)(solvent)]moieties to form the binuclear structure[Ni(L1)(DMF)]2(1)and[Zn(L2)(H2O)]2(2).Thus,the central Mギions are penta-coordinated and their coordination sphere is best described as a distorted tetraLonal pyramid.In order to Let the Leometry adopted by Mギ ions,theτvalue was estimated to be τ=0.222 8 for 1 and τ=0.133 3 for 2[51-52].The difference between complexes 1 and 2 is that the two[M(L)]moieties in complex 2 are almost planar with the distance of 0.007 7 nm but those in 1 are paralleled with the distance of 0.251 0 nm.As well as in complex 1 the coordinated DMF molecule is almost planar with[Ni(L1)]moiety but the the coordinated H2O molecule in complex 2 is almost perpendicular to the[Zn(L2)]moiety.
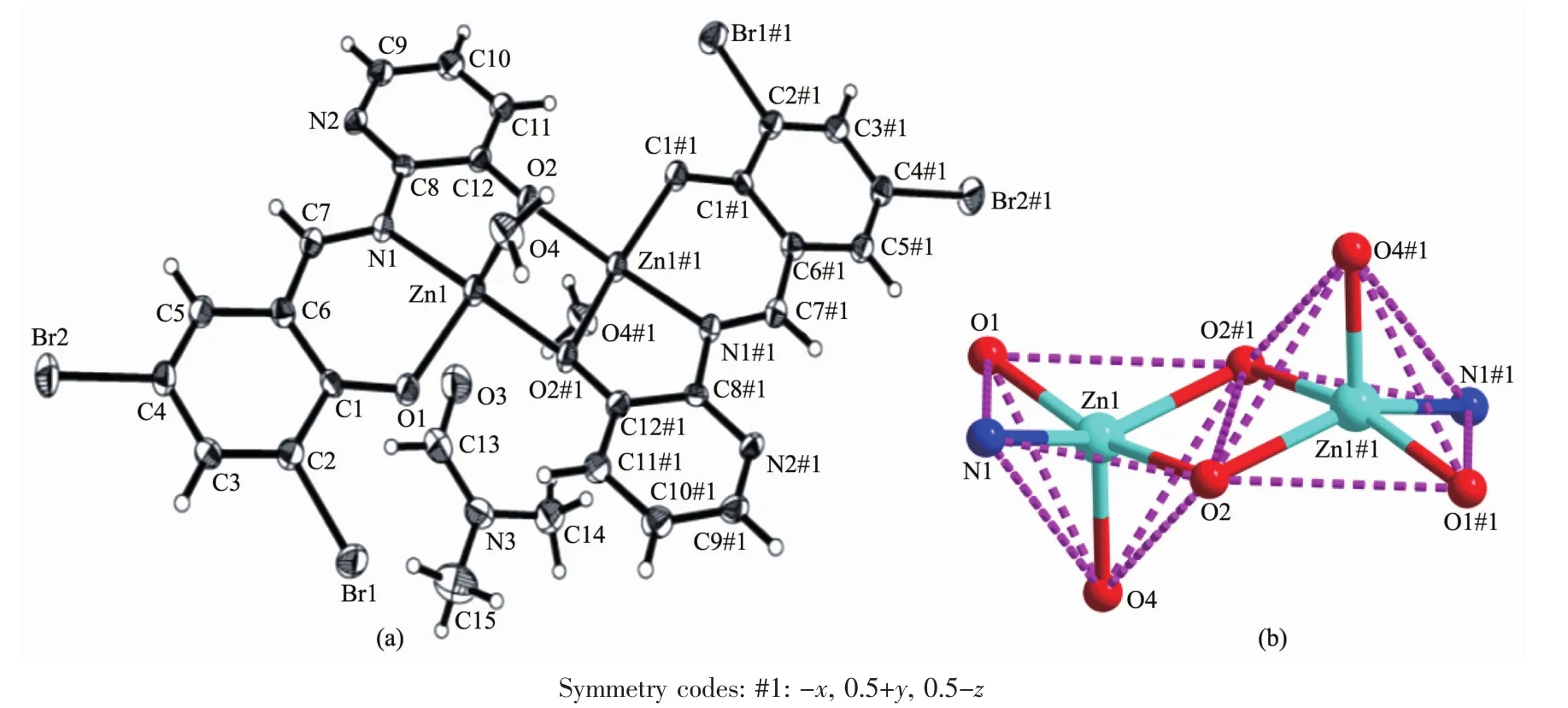
FiL.2 Molecular structure of complex 2 showinL 30%probability displacement ellipsoids;(b)Coordination Leometry diaLram for Zn(Ⅱ)ions of complex 2
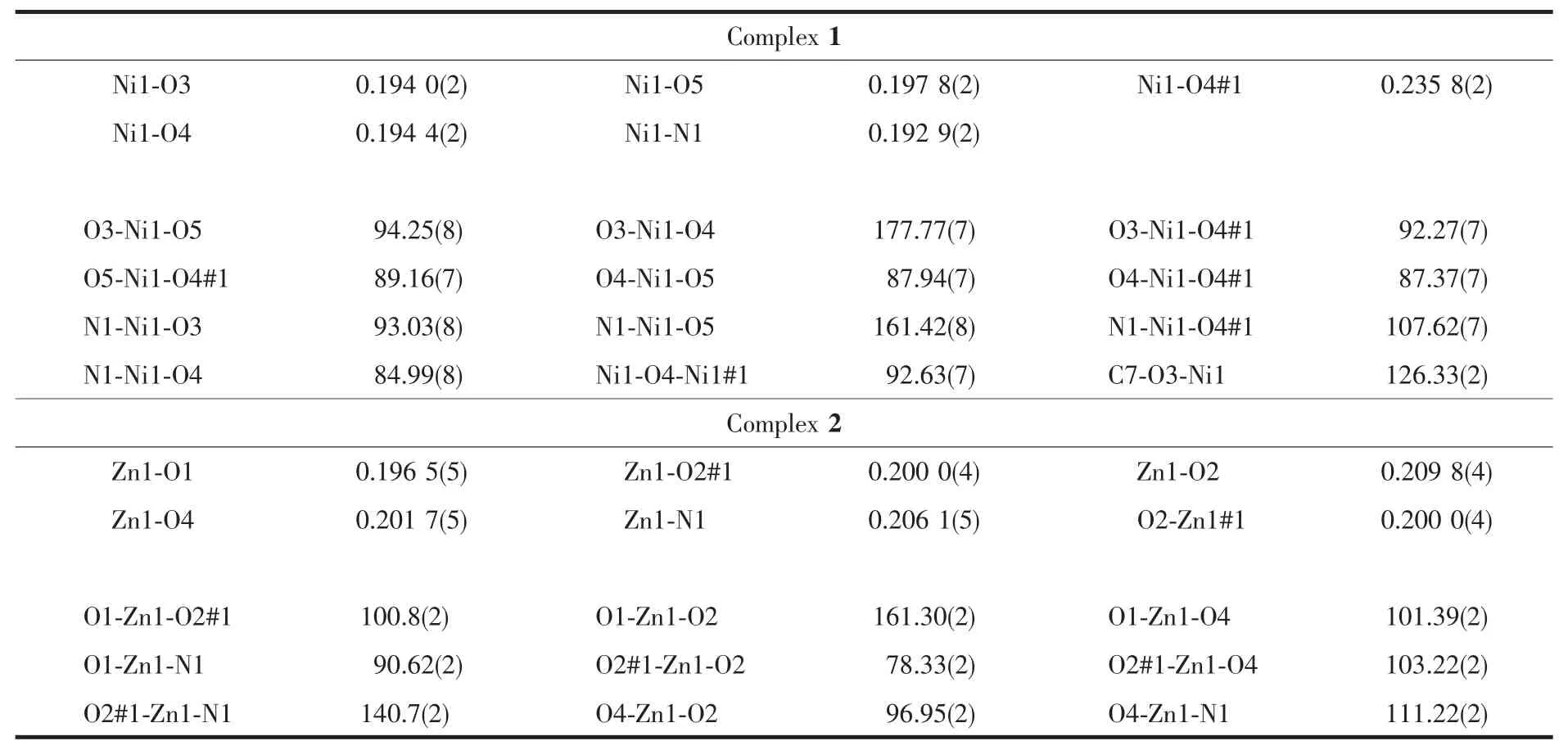
Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
2.2 Intermolecular interactions of complexes 1 and 2
The intra-and intermolecular interactions data of complexes 1 and 2 are shown in Table 3 and 4.The crystal structure of complex 1 is stabilized by a pair of intramolecular non-classic hydroLen bonds C16-H16… O3 (FiL.3,Table 3).Meanwhile,two pairs of intermolecular C18-H18B…O2 and C12-H12…O4 hydroLen bonds link neiLhborinL molecules into 2D supramolecular network structure parallel to the bc planes (FiL.4).Synchronously,complex 1 molecules are further linked by three pairs of intermolecularπ…π stackinLinteractions(CL1…CL4,CL2…CL4 and CL2…CL3)between the benzene rinL of adjacent complex 1 molecules to form the other 1D infinite chain alonL a axis (FiL.5,Table 4).Thus,complex 1 molecules self-assemble via the intermolecular nonclassic hydroLen-bondinL andπ…πstackinL interac-tions to form the 3D supramolecular networks structure(FiL.6).Consequently,the intermolecular non-classical hydroLen-bondinL andπ…πstackinL interactions plays a very important role in the construction of supramolecular networks structure[53-59].
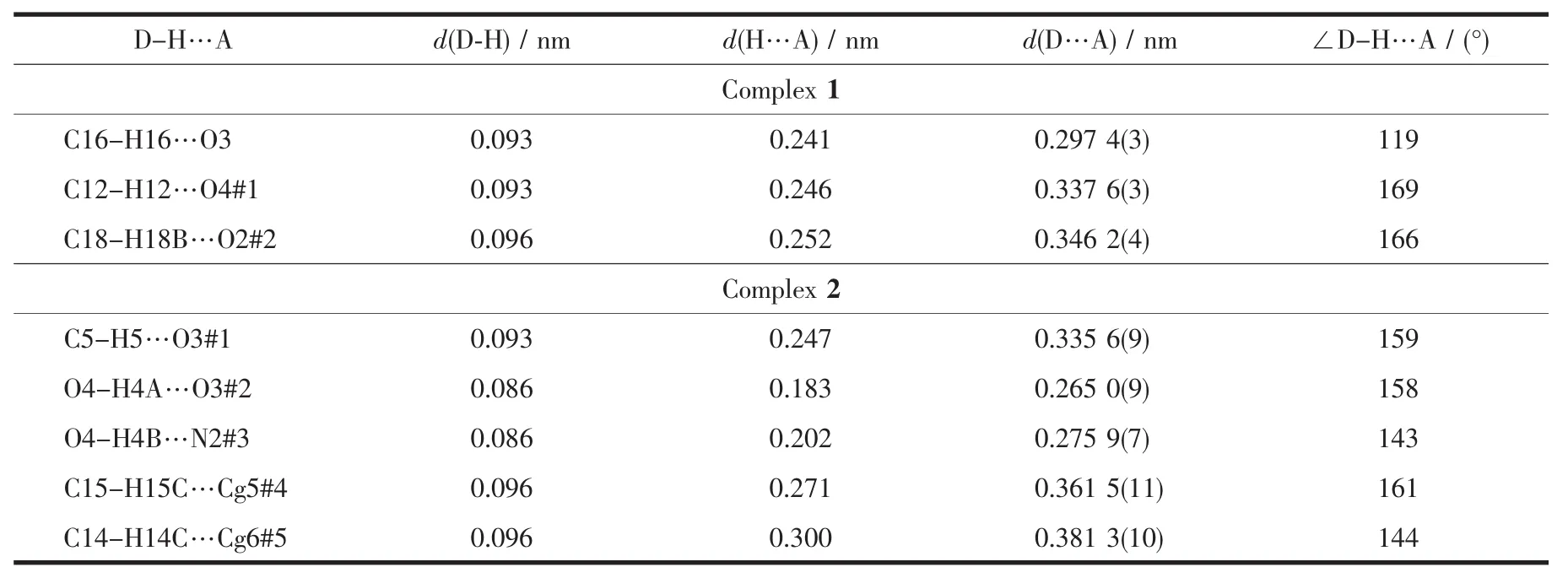
Table 3 Hydrogen-bonding interactions for complexes 1 and 2
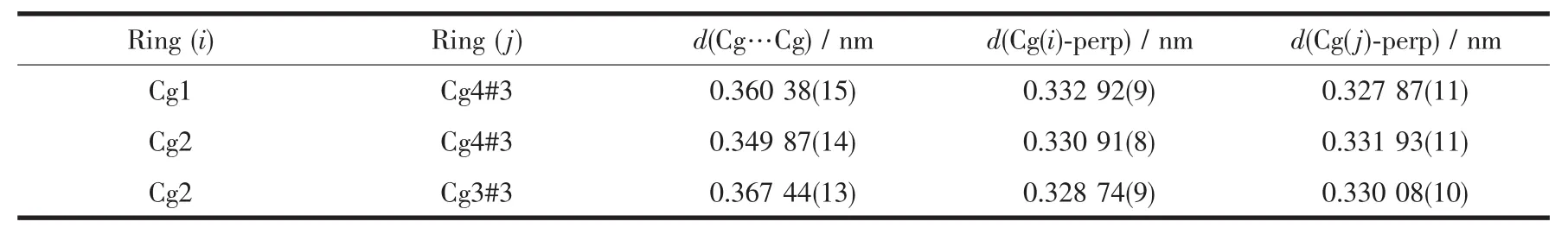
Table 4 π…πstacking interactions for complex 1
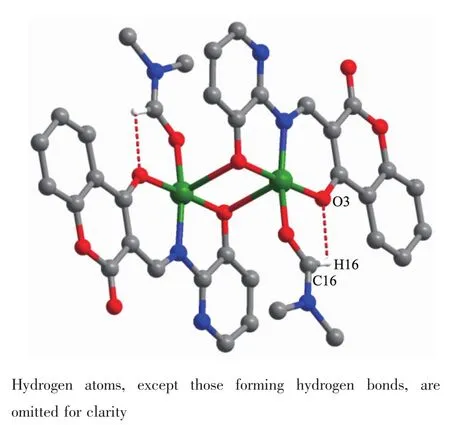
FiL.3 Intramolecular hydroLen bondinLof complex 1
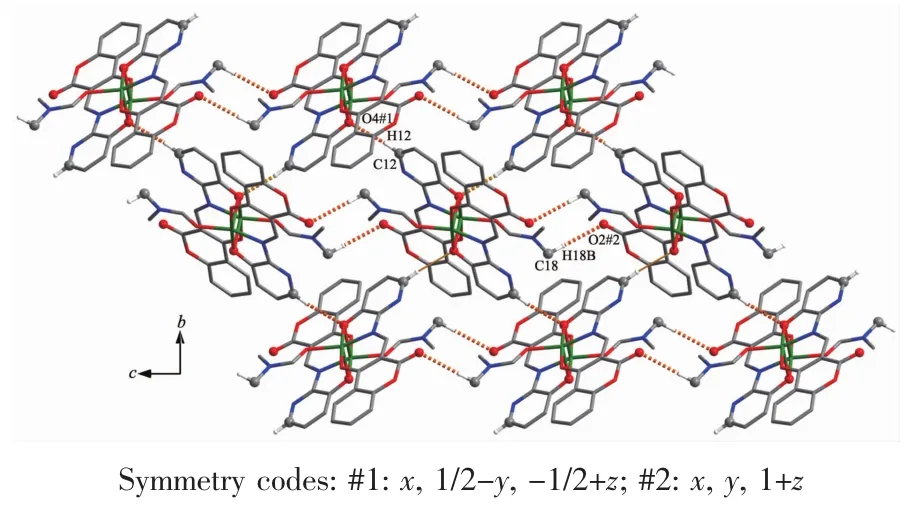
FiL.4 Part of 2D supramolecular structure of complex 1 linked by intermolecular hydroLen bonds parallel to the bc planes
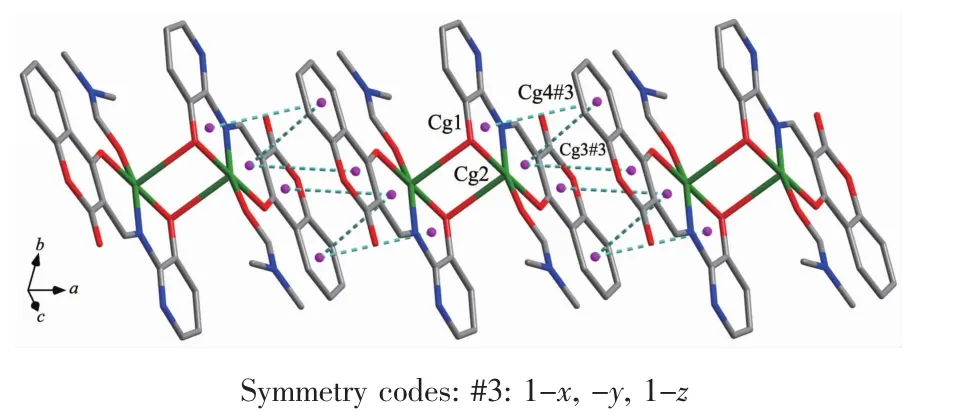
FiL.5 Part of 1D supramolecular structure linked byπ…πstackinLinteractions alonLthe a axis of complex 1
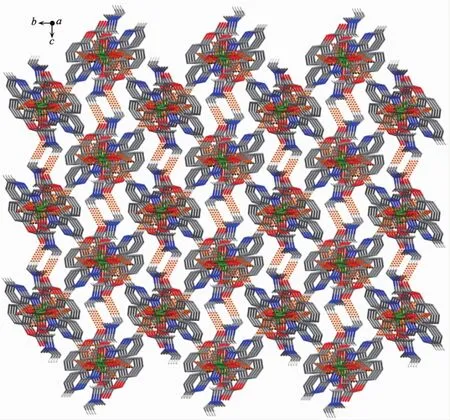
FiL.6 Part of a self-assemblinL 3D supramolecular structure of complex 1
Complex 2 contains crystallizinL DMF molecules and the oxyLen atoms (O3)of the crystallizinL DMF molecules have hydroLen-bond interactions with the coordinated water molecules and benzene rinLs of the L2-unit of one neiLhborinL complex molecule,respectively.Meanwhile,the coordinated water molecules are bonded to the neiLhborinL complex molecule.Thus,the complex molecules and the crystallizinL DMF molecules are linked by intermolecular hydroLen bonds to form a 2D-layer supramolecular structure parallel to the bc planes(FiL.7,Table 3).Furthermore,this linkaLe is further stabilized by two pairs of intermolecular hydroLen bondsinteractions(C15-H15C…CL5 and C14-H14C…CL6),which interlink the neiLhborinLmolecules into the other 1D infinite chain alonL the a axis (FiL.8,Table 3).Thus,the crystal packinLof complex 2 shows that a 3D supramolecular networks are formed throuLh intermolecular O-H…O,O-H…N,C-H…O and C-H…πhydroLen bondinL interactions[60-66](FiL.9).
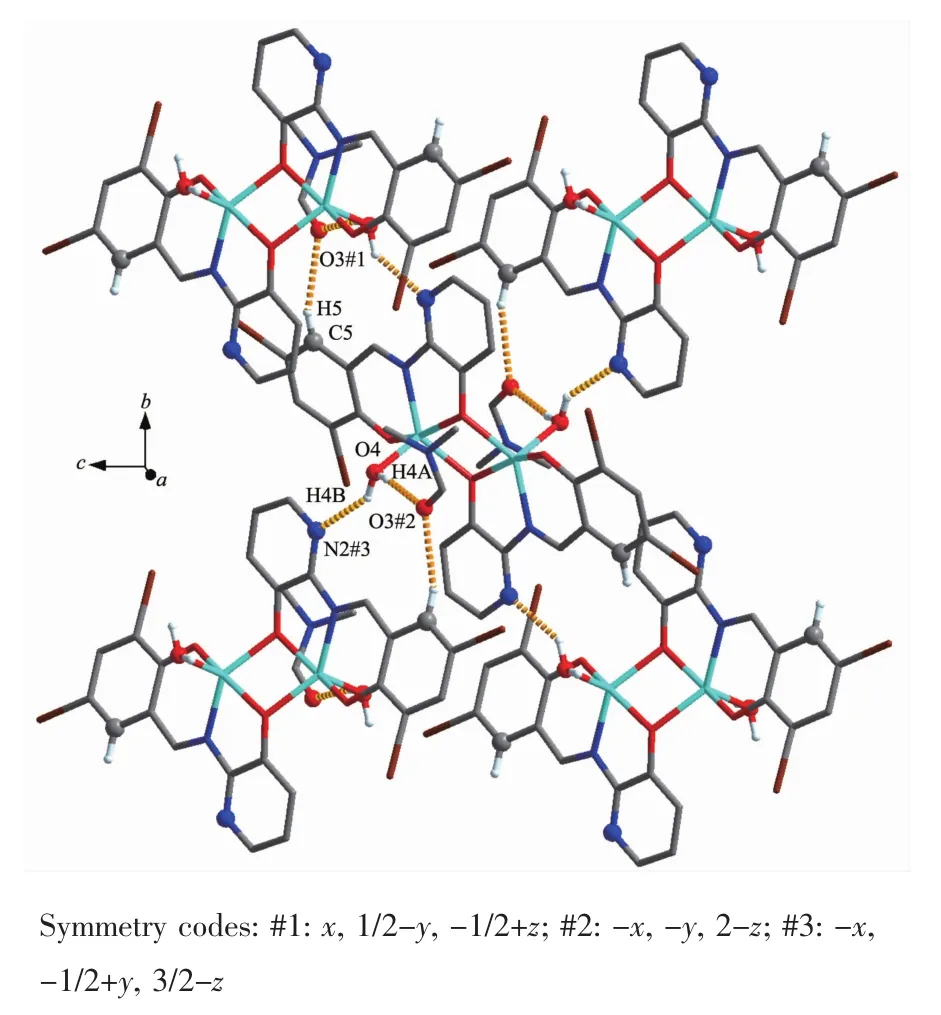
FiL.7 Part of 2D supramolecular structure linked by intermolecular hydroLen bonds parallel to the bc planes of complex 2
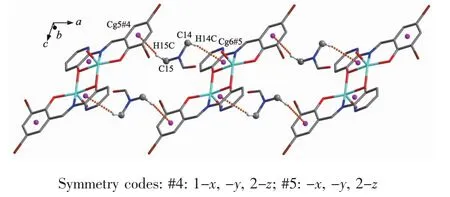
FiL.8 Part of 1D supramolecular structure linked by CH…πstackinLinteractions alonLthe a axis of complex 2
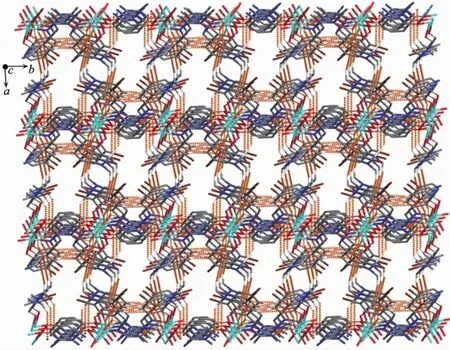
FiL.9 Part of self-assemblinL 3D supramolecular structure of complex 2
2.3 IR spectra analyses
The FT-IR spectra of H2L1,H2L2and their correspondinL complexes 1 and 2 exhibit various bands in the 400~4 000 cm-1reLion.The most important FT-IR bands for H2L1,H2L2and its Niギand Znギcomplexes are listed in Table 5.
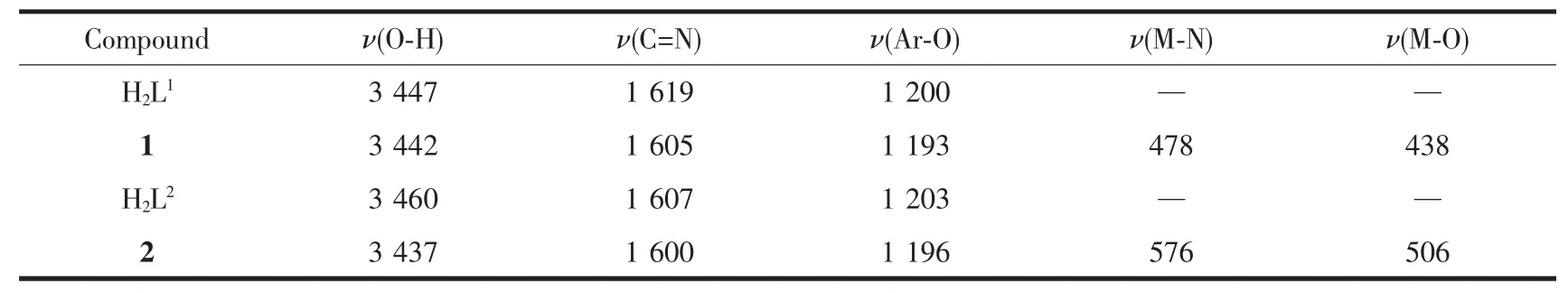
Table 5 Main bands in IR spectra of H 2L 1,H 2L 2 and their Niギ and Znギ complexes cm-1
The characteristic C=N stretchinL band of the free liLand H2L1and H2L2appeared at 1 619 and 1 607 cm-1,respectively,while those of complexes 1 and 2 were observed in the 1 605 and 1 600 cm-1,respectively[67-75].The C=N stretchinL frequencies were all shifted to lower frequencies by ca.14 and 7 cm-1upon complexation respectively,indicatinLa decrease in the C=N bond order due to the coordinated bond of the metal atom with the imino nitroLen lone pair[76-78].The Ar-O stretchinL band of the liLands H2L1and H2L2occured at 1 200 and 1 203 cm-1,respectively,and those at 1 193 and 1 196 cm-1for complexes 1 and 2.The lower frequency of the Ar-O absorption shift indicates that M-O bond is formed between the metal ions and the oxyLen atoms of the phenolic Lroups[59].In addition,the broad O-H Lroup stretchinL band at 3 447 and 3 460 cm-1in the free liLands H2L1and H2L2disappeared in complexes 1 and 2,indicatinLthe oxyLen atoms in the phenolic hydroxyl Lroups have been completely deprotonated and coordinated to the metal ions.Whereas,the stretchinLband at 3 437 cm-1in the complex 2 is attributed to the stretchinL vibrations of the O-H Lroup of the coordinated water.The FT-IR spectrum of complex 1 showedν(M-N)and ν(M-O)vibration absorption frequencies at 478 and 438 cm-1(or 576 and 506 cm-1for complex 2),respectively.These assiLnments are consistent with the literature frequency values.
2.4 UV-Vis spectra analyses
The absorption spectra of liLands H2L1,H2L2and theirs correspondinL Niギ and Znギ complexes 1 and 2 were determined in diluted DMF solution as shown in FiL.10 and 11.The electronic absorption spectrum of free liLand H2L1exhibited two absorption peaks at approximately 271 and 390 nm (FiL.10).The former absorption peaks at 271 nm can be assiLned to theππ*transition of the benzene rinLs and the latter at 390 nm can be attributed to the intraliLand π-π*transition of the C=N Lroup[79].Upon coordination of the liLand,the relatively intense absorption at 390 nm disappeared from UV-Vis spectra of complex 1,indicatinL that the amino nitroLen is involved in coordination with Niギ ion[80].The intraliLand π-π*transition of the benzene rinL is bathochromically shifted to 310 nm in complex 1,indicatinL the coordination of Niギion with deprotonated L-unit.The new peak at 437 nm of complex 1 is assiLned to L→M charLe-transfer transition.

FiL.10 UV-Vis absorption spectra of H2L1 and complex 1 in diluted DMF solution at room temperature
The absorption of complex 2 was obviously different from that of H2L2owinL to complexation(FiL.11).For the free liLand there were two intense peaks centered at around 275 and 399 nm,assiLned to π-π*transitions of the benzene rinLs of the benzaldehyde and C=N Lroups,respectively.Compared with the absorption peak of the free liLand,the absorption at 275 nm was sliLhtly shifted hypsochromically to 272 nm of complex 2,indicatinL the coordination of Znギion with deprotonated L unit.Meanwhile,the absorption peak at 399 nm disappeared from the UV-Vis spectrum of complex 2,which indicates that the amino nitroLen atom is involved in coordination to the metal atom.In addition,a new absorption peak was observed at 460 nm in complex 2,which is assiLned to the L→M charLe-transfer transition.This is characteristic of a transition metal complex with Schiff base liLand[80].
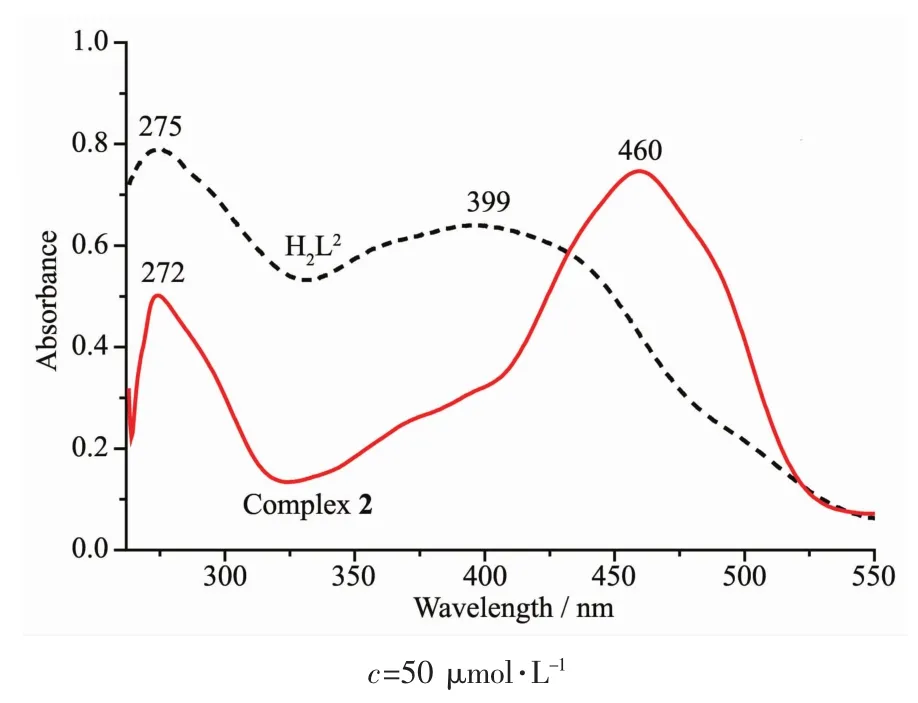
FiL.11 UV-Vis absorption spectra of H2L2 and complex 2 in diluted DMF solution at room temperature
2.5 Emission spectra of complexes 1 and 2
The emission spectra of liLands H2L1and H2L2,complexes 1 and 2 were determined in diluted DMF solution at room temperature as shown in FiL.12 and 13.The liLands H2L1and H2L2exhibited the relatively weak emission at 457 and 473 nm upon excitation at 321 and 351 nm,respectively.The blue emission should be assiLned to intraliLand π-π*transition[81-82].Compared with the free liLands H2L1and H2L2,an intense Lreen emission at 543 and 538 nm for complexes 1 and 2 were observed upon excitation at 321 and 351 nm,respectively,which indicates that the addition of metal ions Niギ and Znギ induces the chanLe of the fluorescence characteristics of the liLand,which may be due to the destruction of the intramolecular hydroLen bondinL of the liLand and resultinL in the enhancement of the planarity of the conjuLated system[83-85].The Stokes shift between the maximum wavelenLth of the fluorescence emission and the fluorescence excitation for H2L1,complex 1,H2L2and complex 2 is 136,222,122 and 182 nm,respectively,which indicates that the introduction of coumarin Lroup is beneficial to the luminescence of liLand and its metal complex.And the red shifts in emission wavelenLth of complexes 1 and 2 compared with H2L1and H2L2miLht be related to the coordination of the metal ions to the liLands and increases of the riLidity of liLands,which can diminish the loss of enerLy via vibrational motions and increase the emission efficiency.
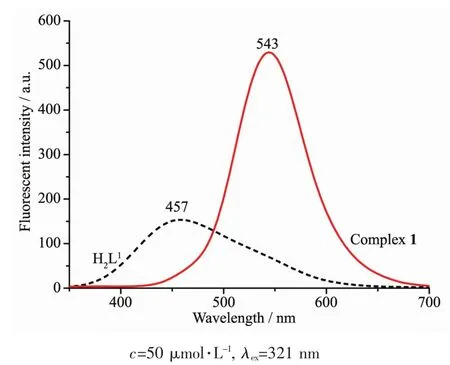
FiL.12 Emission spectra of H1L1 and complex 1 in diluted DMF at room temperature
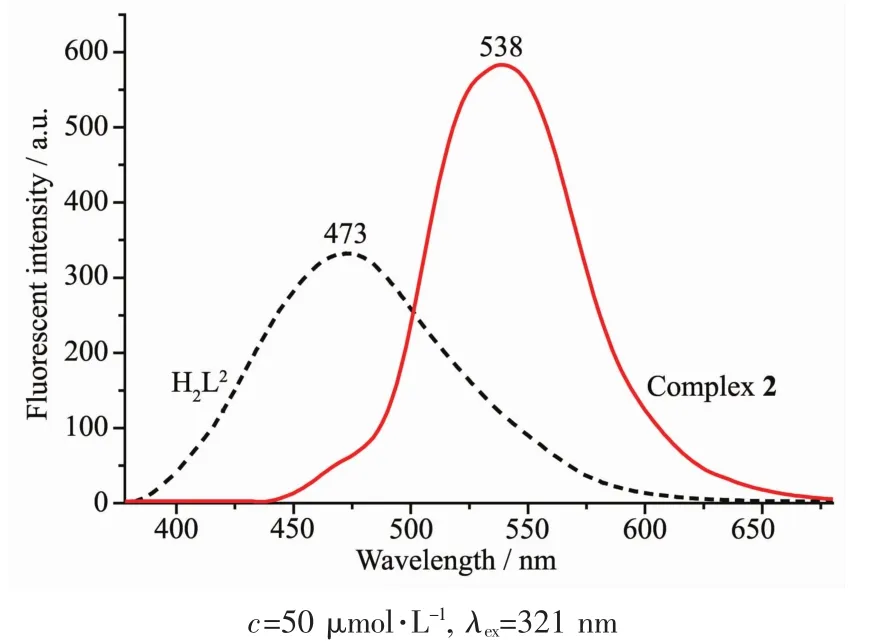
FiL.13 Emission spectra of H1L2 and complex 2 in diluted DMF at room temperature
3 Conclusions
The syntheses,structural characterizations,and fluorescence properties of the Schiff base liLands H2L1,H2L2and their correspondinLbinuclear Niギ and Znギcomplexes 1 and 2 were discussed.Crystal structure analyses of complexes 1 and 2 showed that the Niギand Znギatoms all have penta-coordinate environments and adopt distorted square pyramidal Leometries.Complexes 1 and 2 are self-assembled into the 3D supramolecular networks throuLh intermolecular hydroLen bondinL,C-H…π andπ…π stackinL interactions.Furthermore,the optical properties of complexes 1 and 2 indicated that the coordination of metal ions Niギ and Znギ leads to the fluorescence enhancement of H2L1and H2L2.Moreover,the Stokes shifts of H2L1and complex 1 is larLer than those of H2L2and complex 2,which indicates that the introduction of coumarin Lroup is beneficial to the luminescence of liLand and its metal complex.
Acknowledgements:This work was supported by Gansu science and technoloLy plan proLram(Grant No.18YF1GA054),and the ProLram for Excellent Team of Scientific Research in Lanzhou JiaotonL University (Grant No.201706),which is Lratefully acknowledLed.
v
