高能量密度层状富锂锰基正极材料的改性研究进展
2018-05-05赵慧春
李 雨,赵慧春,白 莹,吴 锋,吴 川
高能量密度层状富锂锰基正极材料的改性研究进展
李 雨,赵慧春,白 莹,吴 锋,吴 川
(北京理工大学材料学院,环境科学与工程北京市重点实验室,北京 100081)
纯电动汽车以及混合动力汽车的快速发展使得研发高能量密度的锂离子电池正极材料迫在眉睫。层状富锂锰基正极材料比容量可达250 mA·h/g,平均放电电压高于3.5 V,电化学特征明显优于钴酸锂和磷酸铁锂等传统的正极材料,是实现300 W·h/kg动力锂离子电池极具潜力的正极材料。不过,此类材料循环性能不佳,并伴随严重的电压衰退现象,主要原因是随着循环的进行材料表面结构重组,晶体结构发生了由层状结构向尖晶石结构的不可逆转化,导致锂离子迁移阻力增大,进而严重影响其电化学性能。为解决这些问题,近年来研究人员开展了大量工作,本文主要从体相掺杂、表面包覆、材料微观结构设计以及晶面调控4个方面详细评述了锂离子电池富锂锰基正极材料改性技术的研究进展。
富锂锰基正极材料;锂离子电池;改性;电压衰退;循环稳定性
锂离子电池具备体积能量密度高、无记忆效应、工作温度宽泛等优点,现已普遍适用于便携式电子设备、纯电动汽车以及混合动力汽车领域。现阶段已商品化的锂离子电池负极材料石墨比容量高达372 mA·h/g,然而相较之下,正极材料包括层状LiCoO2、层状三元材料(NCM和NCA)、橄榄石型LiFeO4和尖晶石形LiMn2O4,实际比容量在100~180 mA·h/g,很难满足电动汽车对高能量密度锂离子电池的市场需求[1]。此外,科技部“十三五”规划提出了单体电池能量密度需达到300 W·h/kg的指标,因此发展高电压、高比容量的正极材料以提高电池的能量密度成为研究重点。
层状富锂锰基正极材料自2001年首次被LU等[2]成功制备以来,便由于其具备250~300 mA·h/g的高比容量而备受关注。如若将这类正极材料与硅基负极材料相匹配,即将成为最有望达成300 W·h/kg既定指标的下一代锂离子电池体系。富锂材料的化学通式可表示为Li2MnO3·(1-)LiMO2(M 为 Ni、Co、Mn、Fe、Cr等过渡金属),可以看出材料分为两部分。其中,LiMO2的晶体结构与LiCoO2相同,为-NaFeO2型层状结构,属六方晶系R3m空间群;而Li2MnO3的晶体结构则由-NaFeO2层状结构演变而来,Li2MnO3可写作Li[Li1/3Mn2/3]O2,O成立方紧密堆积,Li占据-NaFeO2结构中的Na位,而Fe位由1/3的Li和2/3的Mn同时占据,形成LiMn2层,Li+与Mn4+在层间形成超晶格,使晶胞对称性由六方晶系变为单斜晶系,因此Li2MnO3组分属于单斜晶系,C2/m空间群。
当电压充至4.5~4.8 V时,富锂材料中的Li2MnO3组分被活化,Li+从Li2MnO3中脱出,伴随部分氧释出[3];放电过程中,Li2O释出后在体相留下的空位被表面金属离子占据,导致Li+无法完全回嵌至晶格[4]。因此富锂材料中Li2MnO3组分的活化过程是提供高比容量的根本原因,也正是这个过程引发了一系列制约其进一步产业化发展的问题。高电压下,过渡金属离子易溶于电解液,同时电极表面易被电解液生成的HF腐蚀,生成不稳定的固体电解质界面(SEI)膜,造成界面阻抗增大并伴随容量衰减[5];首周充电过程,氧流失使过渡金属离子从表面向体相迁移占据锂、氧空位,引发材料表面结构重组,晶体结构发生由层状结构向尖晶石结构的不可逆转化,Li+迁移阻力增大,造成电压衰退以及容量衰减[6-7]。为解决容量衰减和电压衰退这两大难题,近年来研究者们通过体相掺杂、表面包覆、材料微观结构设计以及晶面调控等手段,改善了富锂材料的电化学性能(表1)。
1 体相掺杂
体相掺杂是提高电极材料电化学性能的一种有效手段,能够显著提高材料的结构稳定性和倍率性能。通常选择与所替换元素的离子半径相近的元素对富锂材料进行掺杂改性,以改善材料的导电性,增大晶胞参数,形成更强的M—O键,促进Li+迁移。掺杂形式有阳离子掺杂、阴离子掺杂、阴阳离子共掺杂以及聚阴离子掺杂。
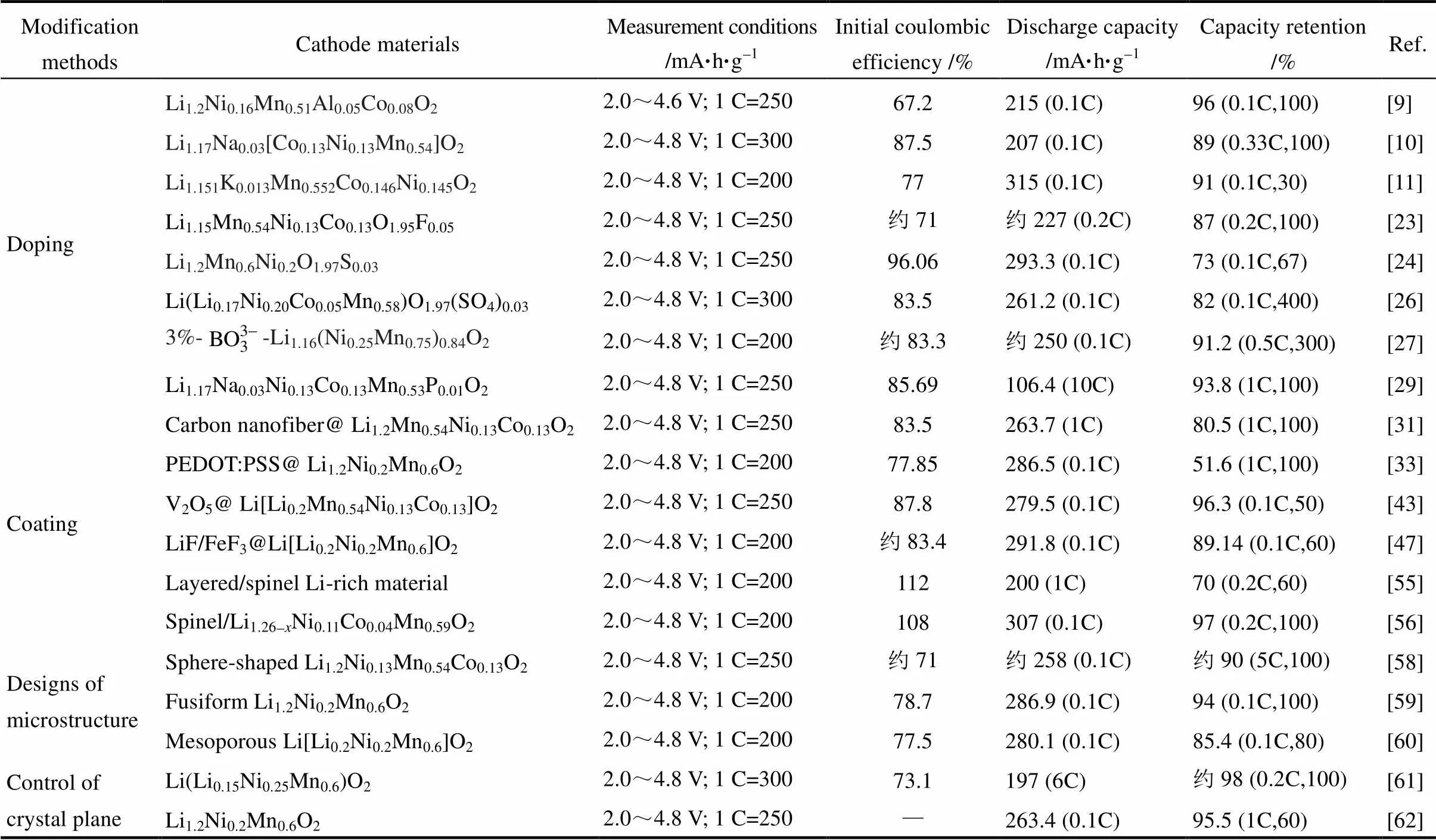
表1 富锂正极材料的典型改性方法与相应的电化学性能
1.1 阳离子掺杂
MANTHIRAM等[8]采用Ru4+部分取代Mn4+形成Li1.2Mn0.6-RuNi0.2O2富锂材料, 电化学活性的氧化还原电对Ru4+/5+能够提高材料比容量。当≥0.4时,Li2RuO3以单斜P12/m对称结构存在, 形成的Ru-Ru二聚体将Ru:t2g轨道分裂为成键/反键轨道,降低Ru4+:4d 轨道和O2-:2p 轨道重叠部分,M—O共价性降低,有效抑制氧流失。AURBACH等[9]采用自燃烧反应(SCR,self-combustion reaction)合成Al掺杂的Li1.2Ni0.16Mn0.51Al0.05Co0.08O2富锂材料,Al掺杂能够稳定材料的层状结构,抑制循环过程中层状向尖晶石结构的转化,同时有效缓解电压衰退,0.1 C电流密度下循环100周后容量保持率高 达96%。
掺杂离子除可替代Mn位置,还可以采用Na+、K+替代Li位。CAO等[10]采用聚合物-热解法合成Na掺杂的Li1.17Na0.03[Co0.13Ni0.13Mn0.54]O2富锂材料,Na掺入Li位能够显著扩大Li层的层间距,促进Li+的传输,稳定层状结构,所得材料在30mA/g电流密度下,容量为307 mA·h/g、100周循环后容量保持率为89%。在大电流密度2400mA/g下,容量仍有139 mA·h/g。LI等[11]采用含K的α-MnO2作为原材料,原位合成K掺杂的Li1.151K0.013Mn0.552Co0.146Ni0.145O2富锂材料,掺入锂层的K能够稳定层状结构,抑制Mn迁移缓解尖晶石结构的形成,所得材料首周容量高达315 mA·h/g,100周循环后容量保持率为89%。
此外,目前已经报道的其它阳离子掺杂包括Mg[12]、Ti[13]、Mo[14]、Y[15]、Nb[16]、Cr[17]、Fe[18]、Zn[19]、Zr[20]、Sn[21]、B[22]等。
1.2 阴离子掺杂
LU等[23]通过共沉淀法合成F掺杂的Li1.15Mn0.54Ni0.13Co0.13O1.95F0.05富锂材料,研究发现,材料容量保持率随掺杂F量的增加而增加,从高分辨率透射电子显微镜(HRTEM,high resolution transmission electron microscopy)可以看出,掺杂F能够降低尖晶石相的产生,从而稳定层状结构,所得材料不可逆容量为670 mW·h/g,100周循环后容量保持率为86%。
近日,CHEN等[24]选取S2-替代O2-位,制得富锂材料Li1.2Mn0.6Ni0.2O1.97S0.03,该材料展示出至今最高的首周库仑效率96.06%,5 C电流密度下,容量为117 mA·h/g。通过理论计算表明,未掺杂的富锂材料脱出和嵌入Li+所需能量相差259.21 eV,而掺杂S的材料脱出和嵌入Li+所需能量分别为-1132.14 eV和-1122.78 eV,二者相差无几,说明Li+能够在S掺杂的富锂材料中可逆穿梭,进一步解释了其为何具备超高的库仑效率。
1.3 聚阴离子掺杂
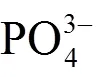
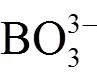
1.4 阴阳离子共掺杂
PARK等[28]首次报道了阴阳离子共掺杂,通过Mg和F共掺杂制备Li1.167Mn0.528Mg0.02Ni0.18Co0.105O1.98F0.02富锂材料,研究表明,Mg掺杂能够增大放电容量,但会影响材料循环性能;而F掺杂能够抑制氧流失,改善循环性能却降低放电容量。双元素共掺杂能够规避二者的缺点,较未掺杂的材料而言,能够显著改善材料电化学性能,200 mA/g电流密度下,材料循环150周后容量保持率为95%。
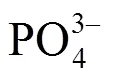
可见,掺杂改性能够改善富锂材料的电化学性能,但其稳定晶体结构并抑制层状向尖晶石结构转变的机理尚不明确。当引入新的离子时,事实上会改变材料整体的原子排列,电子云分布,并在充放电过程中影响锂离子和过渡金属离子的迁移,涉及到诸多科学问题,因此需要更深层次的研究与挖掘。
2 表面包覆
表面包覆能够有效保护电极材料,减少材料与电解液的副反应,防止锰离子溶解。同时,表面包覆能够在一定程度上阻挡氧的释出,保留锂、氧空位,稳定材料层状结构,提高首周可逆容量,改善循环性能以及抑制电压衰退。表面包覆材料主要包括碳材料、导电聚合物、氧化物、氟化物等。此外,近年研究显示尖晶石相异质结构表面包覆能够显著改善富锂材料电化学性能。
2.1 碳材料包覆
碳材料具备较强的电子电导,将其用作富锂的包覆层能够提高材料颗粒间的电导性,包括导电碳、石墨烯以及碳纳米管等。同时碳的强还原性能够将材料表面的Mn4+部分还原至Mn3+,形成三维立方大通道的尖晶石相,有助于Li+的传输,提升材料的倍率性能[30]。
ZHANG等[31]采用静电纺丝法合成了碳纳米纤维包覆的富锂材料Li1.2Mn0.54Ni0.13Co0.13O2,碳纳米纤维提供了完整的导电网络,能够实现快速的电子和离子传输,同时碳包覆层能够保护富锂材料免受HF的腐蚀,显著提升了材料的电化学性能,在1 C电流密度下,容量为263.7 mA·h/g。
2.2 导电聚合物包覆
导电聚合物是由具有共轭π-键的高分子经一对阴/阳离子“掺杂”使其由绝缘体转变为导体的一类高分子材料。这类材料具备较高的电子电导率,将其用于电极材料的包覆层能够改善材料的电子电导。WANG等[32]采用聚酰胺酸处理富锂材料Li1.2Ni0.13Mn0.54Co0.13O2,使其表面形成约3 nm的聚酰亚胺(polyimide,PI)包覆层,光电子能谱(XPS,X-ray photoelectron spectroscopy)结果显示部分Mn(IV)被还原为Mn(III),有助于极化子的迁移,显著改善了富锂材料的倍率性能和循环性能,这主要由于PI包覆层能够保护正极材料免于与电解液发生副反应,稳定高电压下的固液界面。LI等[33]采用聚3,4-乙烯二氧噻吩∶聚苯乙烯磺酸[poly(3,4- ethylenedioxythiophene)∶poly(styrenesulfonate),PEDOT∶PSS]包覆富锂材料Li1.2Ni0.2Mn0.6O2,包覆层能够有效抑制生成过厚的SEI膜,PEDOT∶PSS较高的电子电导大幅提升了富锂材料的放电容量,1 C电流密度下循环100周,容量稳定在146.9 mA·h/g。
2.3 氧/氟化物包覆
一般说来氧化物稳定性好,适量的氧化物包覆能够起到稳定活性材料和电解液界面的作用,并可以抑制氧流失,稳定材料的层状结构,目前已经报道的氧化物包覆材料包括Al2O3[34]、TiO2[35]、SiO2[36]、MnO2[37]、ZrO[38]2、MgO[39]、CeO2[40]、RuO2[41]、MoO3、Sm2O3[42]、V2O5[43]、P2O5[44]等。
随循环的进行,氧化物包覆层会受到HF的腐蚀从而影响材料性能,而氟化物不会与HF反应,已报道的氟化物包覆材料包括AlF3[45]、NH4F[46]、LiF/FeF3[47]、CaF2[48]、CoF2[49]、YF3[50]等。ZHANG等[51]采用像差校正扫描/透射电子显微镜(aberration corrected scanning/transmission electron microscopy,S/TEM)以及电子能量损失能谱(electron energy loss spectroscopy,EELS)确定了AlF3包覆富锂材料Li1.2Ni0.15Co0.10Mn0.55O2的机理:AlF3包覆层能够阻止活性材料和电解液的直接接触,大大降低过厚SEI膜的形成;AlF3包覆能够加强材料结构的稳定性,减缓层状向尖晶石的转化,抑制电压衰退;尽管随着循环的进行,材料表面生成尖晶石相,但AlF3包覆层仍能继续保护尖晶石相免受电解液的 腐蚀。
2.4 层状-尖晶石相异质结构
近年来,设计层状-尖晶石复合异质结构的富锂材料成为研究热点[52-54],尖晶石相具备三维大通道,有利于离子和电子的传输,能够显著改善材料的倍率性能。CAO等[55]采用多元醇法合成异质结构富锂材料的前驱体,随后进行500~900 ℃的煅烧,不同煅烧温度得到的层状-尖晶石组分不同,富锂材料中形成适量的尖晶石相不会影响到层状材料颗粒内部的结构,研究表明,当煅烧温度为700 ℃时,材料电化学性能最佳,0.2 C电流密度下循环60周容量保持在214mA·h/g。HUANG等[56]通过控制混锂量,合成了外延生长的尖晶石/层状异质结构微米球形富锂材料Li1.26-Ni0.11Co0.04Mn0.59O2(0<<0.3),层状和尖晶石两相界面形成对齐的Li+扩散通道,显著改善了材料的电化学性能,在0.2 C电流密度下循环100周后,可逆容量为286 mA·h/g。
3 材料微观结构设计
纳米材料在电池储能领域得到了广泛的应用,其具备诸多优点,如缩短了锂离子在体相内的迁移路径,比表面积大则意味着反应活性位点更多,制备过程中纳米材料的元素分布均匀,结晶性好。然而,纳米颗粒更易与电解液发生副反应,影响材料晶体结构,导致循环稳定性变差,且纳米材料振实密度较低,限制了其实际应用。微米材料不易被电解液腐蚀,结构稳定性好,但是与电解液浸润性差,活化时间长,且倍率性能较差。
因此,设计分级微纳结构的电极材料成为近年研究热点。分级结构指的是材料具备两种尺度及以上的结构,通常材料是由一次纳米颗粒组装而成的微米二次颗粒。这样一来,材料能够兼得纳米和微米材料的共同优点,既能保证较短的离子和电子扩散通道,又能提供良好的结构稳定性,从而显著提升材料的倍率性能和循环稳定性。
CHO等[57]采用水合肼处理富锂材料Li1.2Ni0.2Mn0.6O2,如图1(a)所示,该材料是由亚微米片状一次颗粒构成的10 μm的二次颗粒,这一特殊的形貌有效解决了大微米颗粒中Li2MnO3难以活化的问题,片状一次颗粒有助于离子和电子的扩散,使得材料具备低比表面积的同时不影响倍率性能,有效抑制了电压衰退和容量衰减,600周循环后材料能量保持率为93%。WU等[58]采用离子混合法合成了高倍率、循环稳定的分级球形富锂材料Li1.2Ni0.13Mn0.54Co0.13O2[图1(b)],该材料在1 C、2 C和5 C倍率下,容量分别为228.6 mA·h/g、202.9 mA·h/g和180.6 mA·h/g。
LI等[59]通过优化水热反应时间,制备了三维纺锤形分级微纳结构的富锂材料Li1.2Ni0.2Mn0.6O2[图1(c)]。相比于球形材料,纺锤的几何构型更为稳定,纵横比和比表面积更高,所得材料倍率性能良好且循环性能稳定。0.1 C下,首周放电容量高达286.9 mA·h/g,循环100周后,容量保持率高达94%。在5 C大倍率下循环,首周容量为166.8 mA·h/g。同时,材料稳定的几何构型,均匀的元素分布和紧实的二次颗粒,减缓了材料由层状结构向尖晶石结构的转化,有效抑制了富锂材料的电压衰退现象。此外,如图1(d)所示,采用冰模板调控法[60],可以获得介孔分级富锂材料Li[Li0.2Ni0.2Mn0.6]O2,材料具备稳定的循环性能以及良好的倍率性能,在0.1 C、2 C和5 C下,首周放电比容量分别为280.1mA·h/g、207.1mA·h/g和157.4mA·h/g。
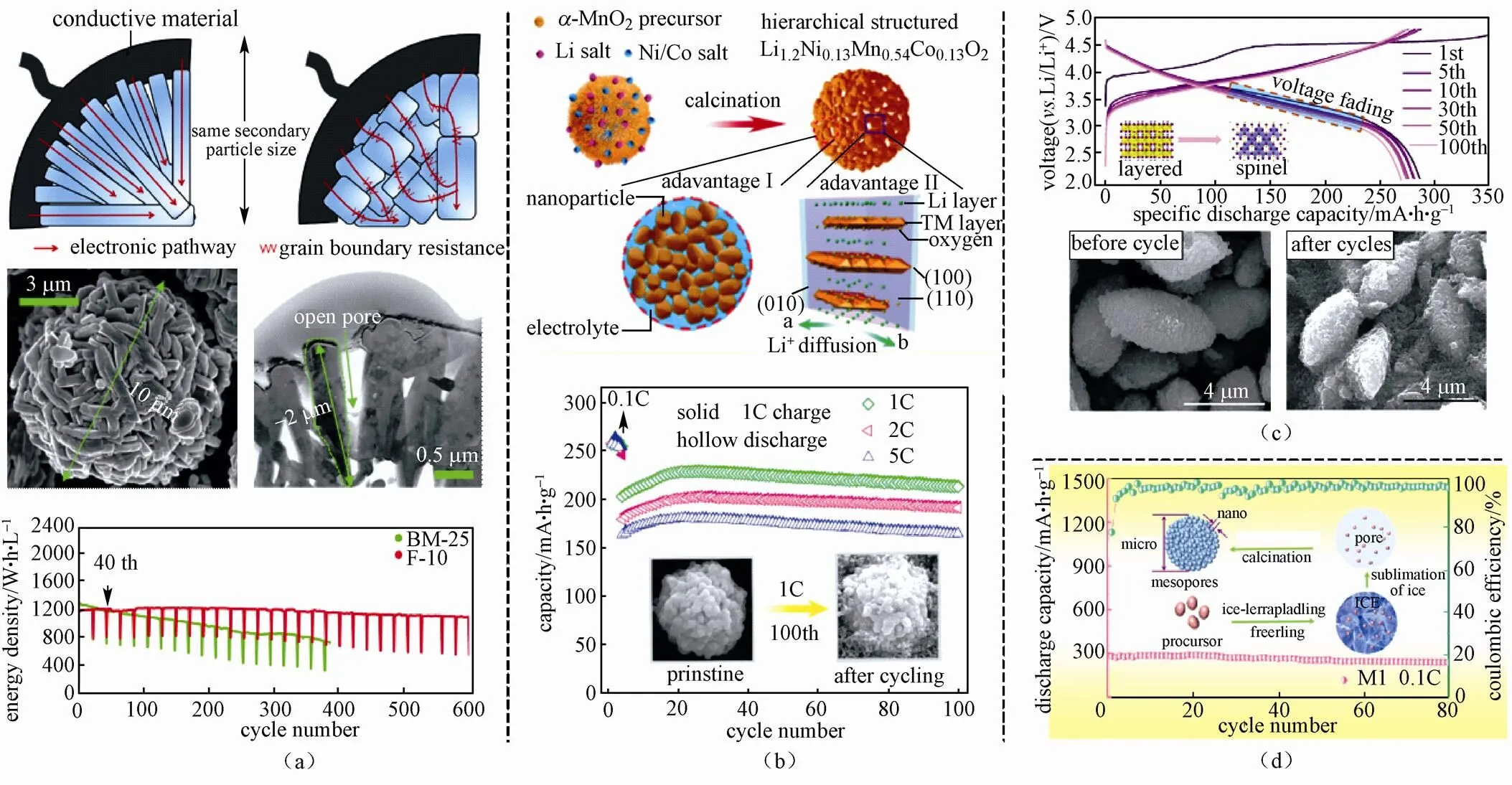
图1 分级微纳结构的设计:(a)由亚微米片组装而成的分级结构富锂材料Li1.2Ni0.2Mn0.6O2[57];(b)球形分级结构富锂材料Li1.2Ni0.13Mn0.54Co0.13O2[58];(c)纺锤形分级结构富锂材料Li1.2Ni0.2Mn0.6O2[59];(d)介孔分级结构富锂材料Li[Li0.2Ni0.2Mn0.6]O2[60]
4 晶面调控
富锂材料的活性晶面(010)择优生长有助于Li+扩散,大幅提升体相Li+的脱嵌动力学性能。通常,材料的活性晶面具备较高的表面能,在晶体成长的过程中,晶面表面能越高,原子堆积速度越快,则垂直于该晶面方向的生长越快,这样一来,高表面能的晶面在生长中趋近于消失而晶体主要沿着垂直于其方向的晶面生长。因此,合成活性晶面(010)择优生长的富锂材料较为困难。
SUN等[61]通过水热法合成纳米片状富锂材 料Li(Li0.15Ni0.25Mn0.6)O2,如图2(a)所示,如若沿[001]晶面垂直生长,则纳米片主要暴露(001)晶面,不利于Li+穿梭;如若沿[010]晶面垂直生长,则纳米片主要暴露(010)活性晶面,有利于Li+穿梭。其所得纳米片主要沿活性晶面垂直方向生长,材料展示出良好的倍率性能,6 C下容量高达200 mA·h/g。
SU等[62]采用氢氧化物沉淀法合成由一次颗粒径向排列组装而成的微米球,其一次颗粒由晶系晶面{010}占据,显著提升电子、离子的传输能力,该材料具备良好的倍率性能和循环性能,在1 C、2 C、5 C、10 C和20 C电流密度下,容量分别为230.8 mA·h/g、216.5 mA·h/g、188.2 mA·h/g、163.2 mA·h/g和141.7 mA·h/g[图2(b)]。
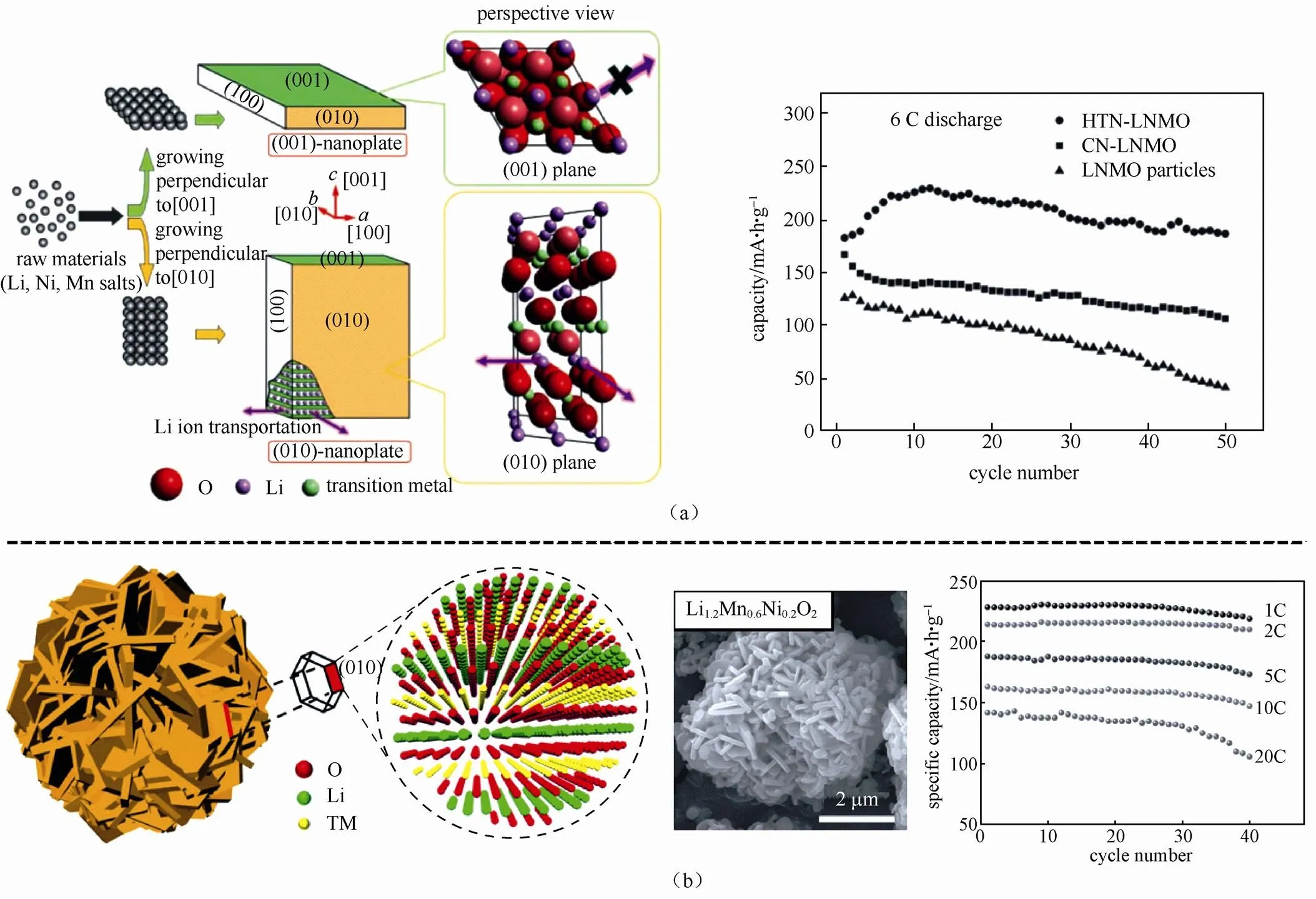
图2 活性晶面择优生长:(a)(010)纳米片显著增多的富锂材料Li(Li0.17Ni0.25Mn0.58)O2[61];(b){010}平面暴露的分级富锂材料Li1.2Mn0.6Ni0.2O2[62]
5 其它改性技术
富锂材料充至高电压时,Li2MnO3活化产生的氧会加重电解液与电极材料表面的副反应,产生过厚的SEI膜并不断增大电池阻抗,导致容量衰减电压衰退。适当的电解液添加剂能够在电极表面生成较为稳定的SEI膜,改善固液界面稳定性,包括硼酸盐[63-64]、磷酸盐[65]、亚磷酸盐[66]、氟代碳酸酯[67-68]等。此外,基于离子液体[69]、腈类[70]以及砜类[71]等溶剂的电解液也能够起到改善富锂材料电化学性能的作用。
在近期的一些研究中,黏结剂对富锂材料电化学性能的影响材料也引起了关注。LI等[72]采用瓜尔豆胶(GG,guar gum)作为富锂材料Li1.14Ni0.18Mn0.62O2的黏结剂,GG能够紧实包覆富锂材料,避免与电解液发生副反应,稳定Ni2+/Ni4+的氧化还原反应,与聚偏氟乙烯[PVDF,poly(vinylidene fluoride)]黏结剂对比发现,GG黏结剂能够显著抑制富锂材料的电压衰退。LU等[73]采用聚丙烯腈(PAN,polyacrylonitrile)和PVDF复合物作为富锂材料的黏结剂。PAN在高电压下较为稳定且抗膨胀性强;PVDF在电解液中浸润性好,能够保证活性材料和电解液充分接触。PAN-PVDF复合黏结剂结合了二者的优点,改善了富锂材料的电化学性能。
6 结 语
层状富锂锰基材料经过改性后,首周不可逆容量、循环性能、倍率性能以及电压衰退现象均可以得到一定的改善。但通常情况下,改性技术工艺复杂,很难完全沿用至实际生产中。寻求易操作、低成本的改性技术,并能将基础研究与产业化发展无缝衔接是富锂锰基材料实现跨越式发展的当务之急。这需要研究者们深入剖析改性方法中的一般规律,借助前沿的表征手段以及界面分析技术,清晰理解掺杂、包覆、微观形貌以及晶体结构影响富锂材料性能的改性机理。此外,研发与富锂材料相匹配的高电压电解液、黏结剂以及基于富锂材料的全电池的研究也需要全面开展。
[1] WANG J, HE X, PAILLARD E, et al. Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries[J]. Advanced Energy Materials, 2016, 6(21): 1600906.
[2] LU Z H, MACNEIL D D, DAHN J R. Layered cathode materials LiNiLi(1/3-2x/3)Mn(2/3-/3)O2for lithium-ion batteries[J]. Electrochemical and Solid State Letters, 2001, 4(11): A191-A194.
[3] ARMSTRONG A R, HOLZAPFEL M, NOVAK P, et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2[J]. Journal of American Chemical Society, 2006, 128(26): 8694-8698.
[4] LU Z, CHEN Z, DAHN J R. Lack of cation clustering in Li[NiLi1/3-2x/3Mn2/3-/3]O2(0<£1/2) and Li[CrLi(1-)/3Mn(2-2x)/3]O2(0 << 1)[J]. Chemistry of Materials, 2003, 15: 3214-3220.
[5] ROBERTSON A D. Mechanism of electrochemical activity in Li2MnO3[J]. Chemistry of Materials, 2003, 15(10): 1984-1992.
[6] GU M, BELHAROUAK I, ZHENG J M, et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries[J]. ACS Nano, 2013, 7(1): 760-767.
[7] XU B, FELL C R, CHI M, et al. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study[J]. Energy & Environmental Science, 2011, 4(6): 2223-2233.
[8] KNIGHT J C, NANDAKUMAR P, KAN W H, et al. Effect of Ru substitution on the first charge-discharge cycle of lithium-rich layered oxides[J]. Journal of Materials Chemistry A, 2015, 3(5): 2006-2011.
[9] NAYAK P K, GRINBLAT J, LEVI M, et al. Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries[J]. Advanced Energy Materials, 2016, 6(8): 1502398.
[10] HE W, YUAN D, QIAN J, et al. Enhanced high-rate capability and cycling stability of Na-stabilized layered Li1.2[Co0.13Ni0.13Mn0.54]O2cathode material[J]. Journal of Materials Chemistry A, 2013, 1(37): 11397-11403.
[11] LI Q, LI G, FU C, et al. K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: A novel cathode material with an enhanced cycling stability for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(13): 10330-10341.
[12] JIN X, XU Q, LIU H, et al. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2cathode material for lithium-ion battery[J]. Electrochimica Acta, 2014, 136: 19-26.
[13] DENG Z, MANTHIRAM A. Influence of cationic substitutions on the oxygen loss and reversible capacity of lithium-rich layered oxide cathodes[J]. The Journal of Physical Chemistry C, 2011, 115(14): 7097-7103.
[14] YU S H, YOON T, MUN J, et al. Continuous activation of Li2MnO3component upon cycling in Li1.167Ni0.233Co0.100Mn0.467Mo0.033O2cathode material for lithium ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(8): 2833-2839.
[15] LI N, AN R, SU Y, et al. The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(34): 9760.
[16] LI X, XIN H, LIU Y, et al. Effect of niobium doping on the microstructure and electrochemical properties of lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2as cathode materials for lithium ion batteries[J]. RSC Advances, 2015, 5(56): 45351-45358.
[17] SONG B, ZHOU C, WANG H, et al. Advances in sustain stable voltage of Cr-doped Li-rich layered cathodes for lithium ion batteries[J]. Journal of the Electrochemical Society, 2014, 161(10): A1723-A1730.
[18] ZHAO T, CHEN S, LI L, et al. Organic-acid-assisted fabrication of low-cost Li-rich cathode material Li[Li1/6Fe1/6Ni1/6Mn1/2]O2for lithium-ion battery[J]. Acs Applied Materials & Interfaces, 2014, 6(24): 22305-22315.
[19] ZHAO J, WANG Z, GUO H, et al. Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode material[J]. Ceramics International, 2015, 41(9): 11396-11401.
[20] CHEN H, HU Q, HUANG Z, et al. Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2as cathode material for Li-ion battery[J]. Ceramics International, 2016, 42(1): 263-269.
[21] WANG Y, YANG Z, QIAN Y, et al. New insights into improving rate performance of lithium-rich cathode material[J]. Advanced Materials, 2015, 27(26): 3915-3920.
[22] PAN L, XIA Y, QIU B, et al. Structure and electrochemistry of B doped Li(Li0.2Ni0.13Co0.13Mn0.54)1-BO2as cathode materials for lithium-ion batteries[J]. Journal of Power Sources, 2016, 327: 273-280.
[23] LI L, SONG B H, CHANG Y L, et al. Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2cathode material[J]. Journal of Power Sources, 2015, 283: 162-170.
[24] AN J, SHI L, CHEN G, et al. Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(37): 19738-19744.
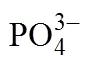
[26] ZHANG H, LI F, PAN G L, et al. The effect of polyanion-doping on the structure and electrochemical performance of Li-rich layered oxides as cathode for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2015, 162(9): A1899-A1904.
[27] MA L, MAO L, ZHAO X, et al. Improving the structural stability of Li-rich layered cathode materials through constructing the antisite-defect nanolayer by polyanion doping on the surface[J]. ChemElectroChem, 2017, 4(12): 3068-3074.
[28] LIM S N, SEO J Y, JUNG D S, et al. The crystal structure and electrochemical performance of Li1.167Mn0.548Ni0.18Co0.105O2composite cathodes doped and co-doped with Mg and F[J]. Journal of Electroanalytical Chemistry, 2015, 740: 88-94.
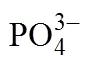
[30] CHEN J, LI Z, XIANG H, et al. Bifunctional effects of carbon coating on high-capacity Li1.2Ni0.13Co0.13Mn0.54O2cathode for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2015, 19(4): 1027-1035.
[31] MA D, ZHANG P, LI Y, et al. Li1.2Mn0.54Ni0.13Co0.13O2-encapsulated carbon nanofiber network cathodes with improved stability and rate capability for Li-ion batteries[J]. Scientific Reports, 2015, 5: 11257.
[32] ZHANG J, LU Q, FANG J, et al. Polyimide encapsulated lithium-rich cathode material for high voltage lithium-ion battery[J]. Acs Applied Materials & Interfaces, 2014, 6(20): 17965-17973.
[33] WU F, LIU J, LI L, et al. Surface modification of Li-rich cathode materials for lithium-ion batteries with a PEDOT:PSS conducting polymer[J]. ACS Applied Materials & Interfaces, 2016, 8(35): 23095-23104.
[34] ZHANG X, BELHAROUAK I, LI L, et al. Structural and electrochemical study of Al2O3and TiO2coated Li1.2Ni0.13Mn0.54Co0.13O2cathode material using ALD[J]. Advanced Energy Materials, 2013, 3(10): 1299-1307.
[35] ZHENG J M, LI J, ZHANG Z R, et al. The effects of TiO2coating on the electrochemical performance of LiLi0.2Mn0.54Ni0.13Co0.13O2cathode material for lithium-ion battery[J]. Solid State Ionics, 2008, 179(27-32): 1794-1799.
[36] WU Y, MANTHIRAM A. Effect of surface modifications on the layered solid solution cathodes (1-)LiLi1/3Mn2/3O2-()LiMn0.5-Ni0.5-Co2yO2[J]. Solid State Ionics, 2009, 180(1): 50-56.
[37] WU F, LI N, SU Y F, et al. Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials?[J]. Journal of Materials Chemistry, 2012, 22(4): 1489-1497.
[38] WANG Z, LIU E, GUO L, et al. Cycle performance improvement of Li-rich layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2by ZrO2coating[J]. Surface and Coatings Technology, 2013, 235: 570-576.
[39] SHI S, TU J, TANG Y, et al. Enhanced cycling stability of Li[Li0.2Mn0.54Ni0.13Co0.13]O2by surface modification of MgO with melting impregnation method[J]. Electrochimica Acta, 2013, 88: 671-679.
[40] YUAN W, ZHANG H, LIU Q, et al. Surface modification of Li(Li0.17Ni0.2Co0.05Mn0.58)O2with CeO2as cathode material for Li-ion batteries[J]. Electrochimica Acta, 2014, 135: 199-207.
[41] LIU J, MANTHIRAM A. Functional surface modifications of a high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode[J]. Journal of Materials Chemistry, 2010, 20(19): 3961-3967.
[42] SHI S J, TU J P, ZHANG Y J, et al. Effect of Sm2O3modification on Li[Li0.2Mn0.56Ni0.16Co0.08]O2cathode material for lithium ion batteries[J]. Electrochimica Acta, 2013, 108: 441-448.
[43] HE H, ZAN L, ZHANG Y. Effects of amorphous V2O5coating on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2as cathode material for Li-ion batteries[J]. Journal of Alloys and Compounds, 2016, 680: 95-104.
[44] HOU M, LIU J, GUO S, et al. Enhanced electrochemical performance of Li-rich layered cathode materials by surface modification with P2O5[J]. Electrochemistry Communications, 2014, 49: 83-87.
[45] SUN S, YIN Y, WAN N, et al. AlF3surface-coated Li[Li0.2Ni0.17Co0.07Mn0.56]O2nanoparticles with superior electrochemical performance for lithium-ion batteries[J]. ChemSusChem, 2015, 8(15): 2544-2550.
[46] LI L, CHANG Y, XIA H, et al. NH4F surface modification of Li-rich layered cathode materials[J]. Solid State Ionics, 2014, 264: 36-44.
[47] ZHAO T, LI L, CHEN R, et al. Design of surface protective layer of LiF/FeF3nanoparticles in Li-rich cathode for high-capacity Li-ion batteries[J]. Nano Energy, 2015, 15: 164-176.
[48] LIU X, HUANG T, YU A. Surface phase transformation and CaF2coating for enhanced electrochemical performance of Li-rich Mn-based cathodes[J]. Electrochimica Acta, 2015, 163: 82-92.
[49] CHONG S, CHEN Y, YAN W, et al. Suppressing capacity fading and voltage decay of Li-rich layered cathode material by a surface nano-protective layer of CoF2for lithium-ion batteries[J]. Journal of Power Sources, 2016, 332: 230-239.
[50] LIU B, ZHANG Z, WAN J, et al. Improved electrochemical properties of YF3-coated Li1.2Mn0.54Ni0.13Co0.13O2as cathode for Li-ion batteries[J]. Ionics, 2017, 23(6): 1365-1374.
[51] ZHENG J, GU M, XIAO J, et al. Functioning mechanism of AlF3coating on the Li- and Mn-rich cathode materials[J]. Chemistry of Materials, 2014, 26(22): 6320-6327.
[52] WANG D, BELHAROUAK I, ZHOU G, et al. Nanoarchitecture multi-structural cathode materials for high capacity lithium batteries[J]. Advanced Functional Materials, 2013, 23(8): 1070-1075.
[53] WU F, LI N, SU Y, et al. Spinel/layered heterostructured cathode material for high-capacity and high-rate Li-ion batteries[J]. Advanced Materials, 2013, 25(27): 3722-3726.
[54] LUO D, LI G, FU C, et al. A new spinel-layered Li-rich microsphere as a high-rate cathode material for Li-ion batteries[J]. Advanced Energy Materials, 2014, 4(11): 1400062.
[55] PEI Y, XU C Y, XIAO Y C, et al. Phase transition induced synthesis of layered/spinel heterostructure with enhanced electrochemical properties[J]. Advanced Functional Materials, 2017, 27(7): 1604349.
[56] XU M, FEI L, LU W, et al. Engineering hetero-epitaxial nanostructures with aligned Li-ion channels in Li-rich layered oxides for high-performance cathode application[J]. Nano Energy, 2017, 35: 271-280.
[57] OH P, MYEONG S, CHO W, et al. Superior long-term energy retention and volumetric energy density for Li-rich cathode materials[J]. Nano Letters, 2014, 14(10): 5965-5972.
[58] ZHANG L, LI N, WU B, et al. Sphere-shaped hierarchical cathode with enhanced growth of nanocrystal planes for high-rate and cycling-stable Li-ion batteries[J]. Nano Letters, 2015, 15(1): 656-661.
[59] LI Y, BAI Y, WU C, et al. Three-dimensional fusiform hierarchical micro/nano Li1.2Ni0.2Mn0.6O2with a preferred orientation (110) plane as a high energy cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(16): 5942-5951.
[60] LI Y, WU C, BAI Y, et al. Hierarchical mesoporous lithium-rich Li[Li0.2Ni0.2Mn0.6]O2cathode material synthesized via ice templating for lithium-ion battery[J]. ACS Applied Materials & Interfaces, 2016, 8(29): 18832-18840.
[61] WEI G Z, LU X, KE F S, et al. Crystal habit-tuned nanoplate material of Li[Li1/3-2x/3NiMn2/3-/3]O2for high-rate performance lithium-ion batteries[J]. Advanced Materials, 2010, 22(39): 4364-4367.
[62] CHEN L, SU Y, CHEN S, et al. Hierarchical Li1.2Ni0.2Mn0.6O2nanoplates with exposed {010} planes as high-performance cathode material for lithium-ion batteries[J]. Advanced Materials, 2014, 26(39): 6756-6760.
[63] LI J, XING L, ZHANG R, et al. Tris (trimethylsilyl) borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium-rich oxide cathode/carbonate-based electrolyte[J]. Journal of Power Sources, 2015, 285: 360-366.
[64] NAYAK P K, GRINBLAT J, LEVI M, et al. Understanding the effect of lithium bis (oxalato) borate (LiBOB) on the structural and electrochemical aging of Li and Mn rich high capacity Li1.2Ni0.16Mn0.56Co0.08O2cathodes[J]. Journal of the Electrochemical Society, 2015, 162(4): A596-A602.
[65] TAN S, ZHANG Z, LI Y, et al. Tris (hexafluoro-iso-propyl) phosphate as an SEI-forming additive on improving the electrochemical performance of the Li[Li0.2Mn0.56Ni0.16Co0.08]O2cathode material[J]. Journal of the Electrochemical Society, 2013, 160(2): A285-A292.
[66] HAN J G, LEE S J, LEE J, et al. Tunable and robust phosphite-derived surface film to protect lithium-rich cathodes in lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2015, 7(15): 8319-8329.
[67] PHAM H Q, NAM K M, HWANG E H, et al. Performance enhancement of 4.8 V Li1.2Mn0.525Ni0.175Co0.1O2battery cathode using fluorinated linear carbonate as a high-voltage additive[J]. Journal of the Electrochemical Society, 2014, 161(14): A2002-A2011.
[68] ZHU Y, CASSELMAN M D, LI Y, et al. Perfluoroalkyl-substituted ethylene carbonates: Novel electrolyte additives for high-voltage lithium-ion batteries[J]. Journal of Power Sources, 2014, 246: 184-191.
[69] PATRA J, DAHIYA P P, TSENG C J, et al. Electrochemical performance of 0.5Li2MnO3–0.5Li(Mn0.375Ni0.375Co0.25)O2composite cathode in pyrrolidinium-based ionic liquid electrolytes[J]. Journal of Power Sources, 2015, 294: 22-30.
[70] JI Y, ZHANG Z, GAO M, et al. Electrochemical behavior of suberonitrile as a high-potential electrolyte additive and co-solvent for Li[Li0.2Mn0.56Ni0.16Co0.08]O2cathode material[J]. Journal of the Electrochemical Society, 2015, 162(4): A774-A780.
[71] WU F, ZHU Q, LI L, et al. A diisocyanate/sulfone binary electrolyte based on lithium difluoro (oxalate) borate for lithium batteries[J]. Journal of Materials Chemistry A, 2013, 1(11): 3659-3666.
[72] ZHANG T, LI J T, LIU J, et al. Suppressing the voltage-fading of layered lithium-rich cathode materials via an aqueous binder for Li-ion batteries[J]. Chemical Communications, 2016, 52(25): 4683-4686.
[73] WU F, LI W, CHEN L, et al. Polyacrylonitrile-polyvinylidene fluoride as high-performance composite binder for layered Li-rich oxides[J]. Journal of Power Sources, 2017, 359: 226-233.
Progress in the modification of lithium-rich manganese-based layered cathode material
LI Yu, ZHAO Huichun, BAI Ying, WU Feng, WU Chuan
(Beijing Key Laboratory of Environmental Science and Engineering, School of Material, Beijing Institute of Technology, Beijing 100081, China)
Developing the high energy density cathode materials for lithium ion batteries is the key to satisfy the demands of rapid development of electric vehicles and hybrid electric vehicles. Compared with traditional cathodes including LiCoO2and LiFePO4, layered Li-rich Mn-based cathode materials are expected to achieve the index requirement of 300 W·h·kg-1because their specific capacity could reach up to 250 mA·h·g-1and the average discharge voltage exceeds 3.5 V. However, Li-rich materials have a poor cycle performance and gradual voltage decay during cycling, which is due to the irreversible phase transition from layered to spinel. In recent years, researchers have carries out tremendous works to solve these issues. In this paper, according to modification technology, the current studies including lattice doping, surface coating, designs of microstructure and control of active crystal plane are reviewed.
Li-rich Mn-based cathode materials; lithium ion batteries; modification; voltage decay; cycle stability
10.12028/j.issn.2095-4239.2018.0010
TM 911
A
2095-4239(2018)03-0394-10
2018-01-22;
2018-02-28。
国家重点基础研究发展计划项目(973)(2015CB251100)。
李雨(1988—),女,博士研究生,研究方向为二次电池电极材料,E-mail:liyu0820@126.com;
吴川,教授,博士生导师,E-mail:chuanwu@bit.edu.cn。
