Two kinds of ketoprofen enteric gel beads(CA and CS-SA)using biopolymer alginate
2018-03-28BingchoChengDongyngLiQiyeHuoQinqinZhoQiLnMengsuoCuiWeisnPnXinggngYng
Bingcho Cheng,Dongyng Li,Qiye Huo,Qinqin Zho,Qi Ln,Mengsuo Cui,Weisn Pn,*,Xinggng Yng,b,**
aDepartment of Pharmaceutics,School of Pharmaceutical Sciences,Shenyang Pharmaceutical University,103 Wenhua Road 110016,Shenyang,China
bState Key Laboratory of New-tech for Chinese Medicine Pharmaceutical Process,222001,Jiangsu,Lianyungang,China
1. Introduction
Alginate,a type of hydrophilic biopolymer extracted from brown seaweed,consists of b-D-mannuronic acid(M)and a-L-guluronic acid(G)monomers[1].The uronic-acid-based monomers are joined together by 1,4-glycosidic linkages,and they exist in either homopolymeric(M-or G-residues)block or heteropolymeric(random arrangement of M and G residues)block[2].Natural polymer sodium alginate(SA)hasbiocompatibility and biodegradation,which has been widely applied in the field of food,medicine and other scientific research[3].What’s more,chitosan,the generic term for a family of linear polysaccharides which exists as copolymers of β-(1–4)-linked glucosamine and N-acetylglucosamine,is commercially obtained by partialde-acetylation of α-chitin produced from the exoskeletons of crustacean or the cell walls of fungi[4].Chitosan,a polycationic polysaccharide,could form polyelectrolyte complexes with polyanionic polymers such as alginate[5].
Alginate microparticlar drug delivery system with sodium alginate as the main material,is a newly developed oral sustained release carrier,which has been highly recommended to controlled-release of varieties of drugs[6].Alginate microparticlar drug delivery system offers not only therapeutic advantages,such as less gastro-intestinal tract irritation and lower side effects but also technological advantages[7].Compared to larger carriers,because of the small size and large specific surface area,alginate microparticlar is more suitable for oral drug delivery and could protect the encapsulated drugs against the harsh environment of the gastrointestinal tract.Alginate is able to form hydrogel easily and safely in addition of divalent cations,such as Ca2+,which bind to the carboxylates in the G-blocks to form the ‘egg-box’structures[8].The alginate gelation process has its origin in a capacity of the polysaccharide molecules specifically to bind divalent cations[9].Thus,a gentle and simple encapsulation technique was developed,involving the addition of a solution of sodium alginate dropwise through a syringe needle into a solution of divalent cation[10].
Ketoprofen is a non-steroidal anti-inflammatory drug(NSAID),which is an important drug widely used in treating musculo-skeletal disorders[11,12],such as rheumatoid arthritis and osteoarthritis,as well as some of the symptoms due to traumatic lesions[13].In addition,ketoprofen is used in the treatment of post-surgical and carcinous pain.NSAIDS’antiinflammatory and analgesic action are due to the inhibition of cyclooxigenase(COX)[14],an enzyme involved in production of prostaglandins[15,16].There are two subtypes of COX;subtype COX-1 occurs constantly in the body,while subtype COX-2 is induced by inflammation[17].Ketoprofen has shown a potent COX-1 inhibiting activity,therefore,it increases the risk of side effects if repeatedly taken orally[18].In addition,the use of ketoprofen could result in an increased risk of serious side effects,especially with regard to gastrointestinal[19].After encapsulation,on the one hand,the side effect of ketoprofen is reduced,on the other hand,it would be released in a controlled way.
In this paper,ketoprofen was selected as model drug to prepare two kinds of ketoprofen enteric preparation using calcium alginate(CA)gel beads and chitosan-alginate(CSSA)gel beads.Calcium alginate gel beads with pH-sensitivity was prepared utilizing the one-step method[20]which controls instant release of drug in artificial gastric fluid,and burst effect in artificial intestinal fluid.Thus,CA beads of ketoprofen could be applied in short-term release applications,such as instant analgesic for post-surgical and carcinous pain.
However,CA beads are very porous and exhibit a low retention capacity of encapsulated molecules.Even more,CA beads have a very short dissolution time when they are exposed to the intestinal fluid.CA beads with a prolonged dissolution time are desirable for sustained release of ketoprofen.In order to get long-acting analgesic,the microcapsules could be coated with a polycationic polymer to form a membrane at the surface of beads[21].To obtain expected sustained-release effect,this paper used biopolymer alginate to prepare CA-SA beads.On the basis of single factor investigation,optimized formulation was obtained through orthogonal design[22].The process parameters of CS-SA beads such as concentration of sodium alginate,the ratio of sodium alginate and drug,calcium chloride and concentration of chitosan were optimized.
The aim of the current study was to prepare the CA beads and CS-SA beads with biopolymer alginate,then,optimized the parameter and process and evaluated the beads.Two kinds of enteric preparation can serve as models for the future design of oral delivery systems.To obtain expected rapid-release and sustained-release of ketoprofen gel beads,much importance should be attached into the research on two kinds of ketoprofen enteric gel beads using biopolymer alginate.
2. Material and method
2.1. Materials
Sodium alginate with different molecular weight(Mw=3.00×105g/mol,Mw=2.49×105g/mol,Mw=1.93×105g/mol)were purchased from FMC.Chitosan was given by Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Calcium chloride was obtained from Tianjin Bodi Chemical Holding Co.,Ltd.(Tianjin,China).Keteprofen was from Zhejiang Jiuzhou Pharmaceutical Co.,Ltd.(Zhejiang,China).All other chemicals and reagents used were of analytical grade or better.
2.2. Preparation of CA beads
The gel beads containing a model drug keteprofen were prepared by one-step method.Brie fly,keteprofen was dissolved in sodium alginate solution and then extruded as droplets through a plastic needle(inner diameter of 1.0 mm)into a continuously stirred solution of calcium chloride at 25°C[23].The needle was positioned at 5 cm above the surface of calcium chloride solution.The extrusion rate of sodium alginate solution was approximately 2.5 ml/min.The beads formed were stirred continuously in the solution for 4 h,separated and washed with 300 ml of distilled water(3 min)for 3 times and air-dried at 25°C.
2.3. Preparation of CS-SA beads
By a one-step procedure,CA beads with pH-sensitivity were obtained,which controlled the instant release condition of ketoprofen in artificial gastric fluid,nevertheless,the release of that in artificial intestinal fluid was torrential.In order to overcome the burst effect in artificial intestinal fluid,CA beads coated with chitosan were obtained utilizing improved onestep method,which could control the instant release condition of ketoprofen in artificial intestinal fluid and gastric fluid.The beads were prepared by one-step method,then,they were transferred into the chitosan solution,the beads formed were stirred continuously in the solution for 4 h.Finally,they were separated and washed with 300 ml of distilled water(3 min)for 3 times and air-dried at 25°C.
2.4. Optimization of formulation parameters of CS-SA beads
The CS-SA beads were prepared by improved one-step method.The following formulation variables,molecular weights of sodium alginate(Mw=3.00×105g/mol,Mw=2.49×105g/mol,Mw=1.93×105g/mol),concentration of sodium alginate(1.0%,1.5%,2.0%,w/v),concentration of calcium chloride(1.0%,1.5%,2.0%,w/v),the ratio of sodium alginate and drug(1:1,2:1,3:1),concentration of chitosan(1%,1.2%,1.4%,w/v),were investigated.Different batches of the beads were analyzed for entrapment efficiency,drug loading and the release of ketoprofen from the beads.
2.5. Orthogonal experiment
On the basis of the single factor investigation of the release of ketoprofen from CA beads and CS-SA beads,we initially determined four main factors:the concentration of sodium alginate(A),the ratio of SA:drug(B),the concentration of calcium chloride(C),the concentration of chitosan(D).In this paper,we adopted comprehensive score method,taking the characteristics of the preparation into account at the same time[24].Due to the release of ketoprofen from the beads for 12 h was above 95%,which indicated that it could release completely,the cumulative release of 2 h and 6 h were chosen as evaluation indexes.
2.6. Drug loading and entrapment efficiency
Drug loading and entrapment efficiency were estimated by UV–vis spectrophotometer(Shimadzu,Japan).20 mg of beads were added into the sodium citrate solution until stirred to be dissolved completely..Methanol was added into the sodium citrate solution to get the gel,this solution was then filtered with 0.45µm filter to get the drug solution.Absorbance was taken at 260 nm.All the measurements were done in triplicate.
The drug loading and entrapment efficiency were calculated using following formulae:

where,Ma is the actual drug content in weighed quantity of beads,Mbis the weighed quantity of dried beads and Mcis the theoretical amount of drug in beads calculated from the quantity added in the process[25].
2.7. Scanning electron microscopy(SEM)
Scanning electron microscopy(SEM)is an electron optical imaging technique that provides photographic images and elemental information[26].SEM is useful for characterizing the morphology and size of microscopic specimens with particle size as low as nanometer[27].It is used to determine particle size distribution,surface topography,texture and examine the morphology of fractured or sectioned surface[26].The morphology of microbeads was characterized by scanning electron microscopy(SEM)[28].Prepared beads were examined under an argon atmosphere at room temperature with SEM operating at 15 kV.
2.8. Determination of bead size
Particle size of 100 dried beads from each batch was measured by optical microscopic method.The ocular micrometer was previously calibrated by stage micrometer[29].
2.9. Swelling behavior measurement
Swelling measurement of optimized CA beads and CS-SA beads containing keteprofen was carried out in five different aqueous media:pH1.0,pH4.5,pH6.8,pH7.4 and water.100 mg beads were placed in vessels of dissolution apparatus(Campbell Electronics,India)containing 500 ml respective media.The experiment was carried out at 37±1°C under 100 rpm paddle speed.The swelled beads were removed at predetermined time interval and weighed after drying the surface using tissue paper[30].
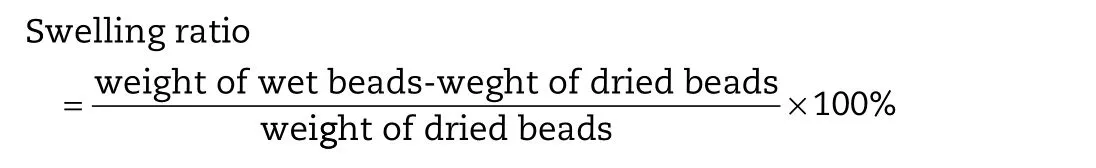
2.10. In vitro drug release studies
The release of the keteprofen from CA beads and CS-SA beads was tested by dissolution testers.The dissolution rates were measured at 37±1°C under 100 rpm paddle speed.Weighed quantities of CA beads or CS-SA beads containing keteprofen equivalent to 75 mg keteprofen were added to 900 ml of simulated gastric fluid(pH 1.0).The test was carried out in simulated gastric fluid(pH 1.0)for 2 h,and then transferred into intestinal fluid(pH 6.8)for next 12 h.5 ml of sample was collected at regular time intervals,and the same amount of fresh dissolution medium was replaced into dissolution vessel to maintain the sink condition throughout the experiment.The collected sample were filtered,and suitably diluted to determine the absorbance using a UV–vis spectrophotometer(Shimadzu,Japan)at max of 260 nm.The cumulative drug release of beads was calculated using the following formula:
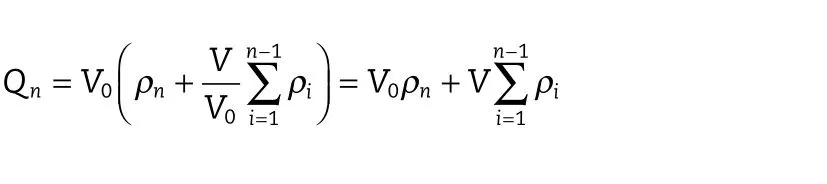
where,Qnis cumulative drug release,ρnis the concentration of drug at the time oft,ρiis the concentration of drug before the time oft,V0is the total volume of dissolution medium and V is the sampling volume collected at regular time intervals[31].
2.11. Kinetic analysis of drug release
The release pro files of the ketoprofen were up to 60%,which fitted to the kinetic models commonly applied in drug release studies.The models used are as follows:
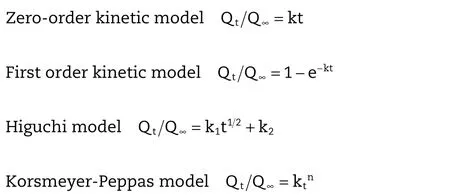
where,tis the duration of release(min),Qtis the amount of drug released at time t(mg/mL),Q∞is the total amount of drug released(mg/mL),k is the kinetic constant,andnis the release exponent indicating the mechanism of drug release[32].Whenn≤0.43,the release mechanism is based on Fickian diffusion.When 0.43<n<0.85,the release mechanism is based on an anomalous(non-Fickian)transport(i.e.,mixed mechanisms of diffusion and polymer relaxation).Whenn≥0.85,the release mechanism is based on a case-II transport which reflects the influence of polymer relaxation on the drug molecules movement within the matrix[33].The correlation coefficient was used to evaluate the fitness[34].
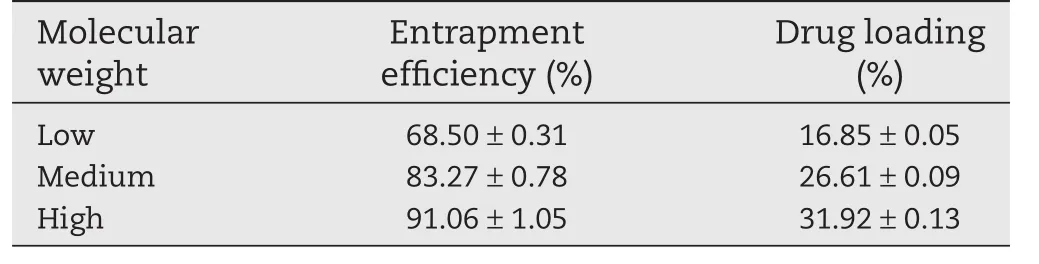
Table 1–Influence on entrapment efficiency and drug loading of different molecular weights of SA.
3. Results and discussion
3.1. Optimization of formulation parameters of chitosancalcium alginate gel beads
As shown in Tables 1 to 5 and Fig.1,different formulation parameters of calcium alginate gel beads could influence the entrapment efficiency,drug loading and release characteristics of the beads.
3.1.1.Molecular weights of SA
As shown in Table 1,we could see that with the increase of molecular weight of sodium alginate,the drug loading and entrapment efficiency of CS-SA gel beads increased significantly.As the concentration of sodium alginate used in the preparation was relatively low,the molecular weight of sodium alginate played an important role in the viscosity of the solution,the higher the molecular weight of sodium alginate,the greater the viscosity of the solution,which lead to better drug suspension and less drug leakage in the process of forming film.Moreover,the G/M value of polymer sodium alginate used in the experiments was relative high,so it was easy to form three dimensional network structure with Ca2+,leading to the decrease of film forming time.
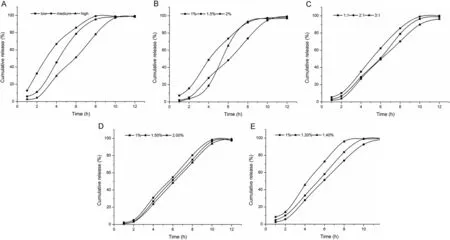
Fig.1–Influence on release from CS-SA beads in the artificial gastric and intestinal juice:(A)molecular weights of SA,(B)concentrations of SA,(C)the ratio of the SA:drug,(D)the concentrations of CaCl2and(E)the concentrations of CS.
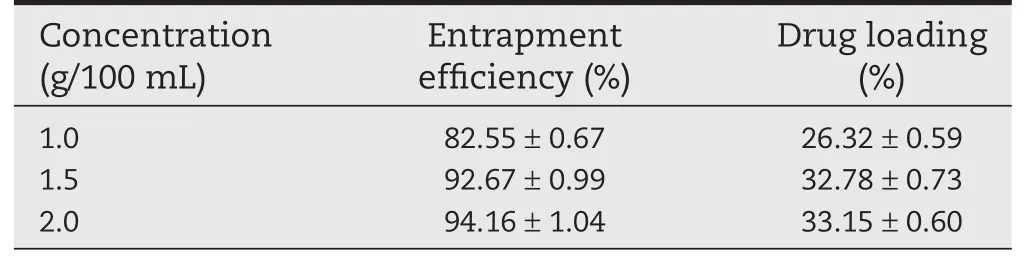
Table 2–Influence on entrapment efficiency and drug loading of different concentrations of SA.
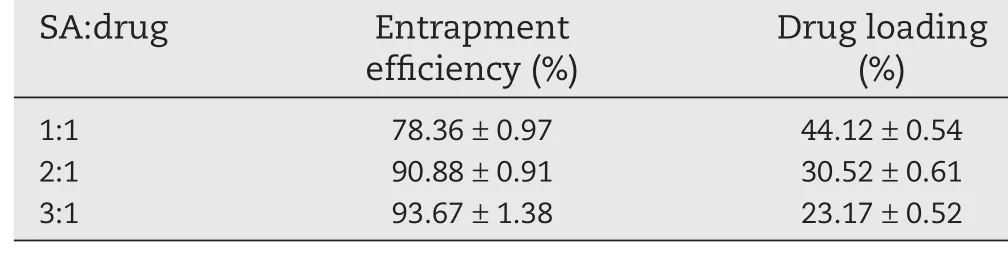
Table 3–Influence on entrapment efficiency and drug loading of different ratio of the SA:drug.
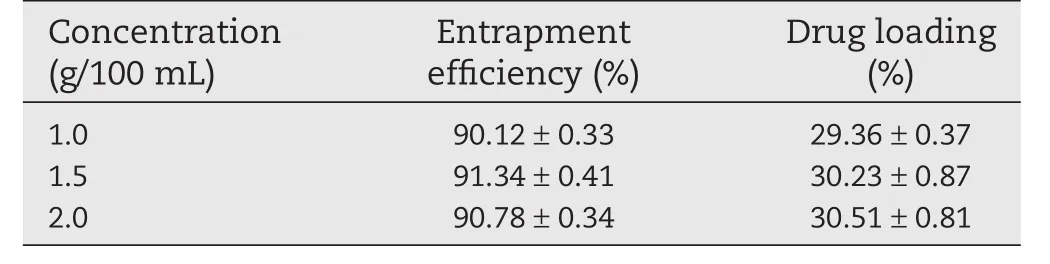
Table 4–Influence on entrapment efficiency and drug loading of different concentrations of CaCl2.
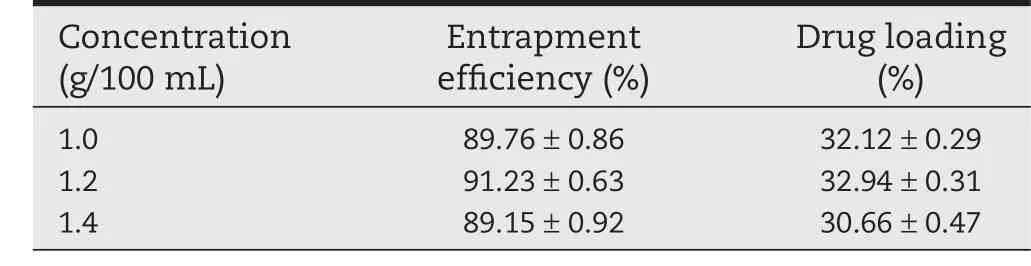
Table 5–Influence on entrapment efficiency and drug loading of different concentrations of CS.
As Fig.1A indicated,the molecular weight of sodium alginate had great influence on sustained release.The higher the molecular weight of sodium alginate,the lower the release rate.Because high molecular weight of sodium alginate containing a large number of negatively charged carboxyl was more likely to react with positively charged chitosan,leading to the formation of tense film.
Considering the Table 1 and Fig.1A,we chose high molecular weight sodium alginate to prepare the beads for further study.
3.1.2.Concentrations of SA
As shown in Table 2,we could see that with the increase of concentration of sodium alginate,the drug loading and entrapment efficiency of CS-SA gel beads increased.When the concentration of sodium alginate was 2%,the solution was so viscous that a large amount of‘tadpole shape’beads formed.Fig.1B revealed that when the concentration of sodium alginate solution increased from 1%to 1.5%,the release rate of ketoprofen from CS-SA gel beads decreased significantly.But when the concentration increased to 2%,the release in artificial gastric juice was slow,while in artificial intestinal juice,the drug release increased suddenly for the reason that it was not propitious for further reaction of chitosan molecules and high concentration of sodium alginate.
Considering the Table 2 and Fig.1B,we chose the concentration of 1.5%sodium alginate solution to prepare beads for further research.
3.1.3.SA:drug
As we could see in Table 3,the higher the ratio of sodium alginate and drug,the greater the entrapment efficiency,the lower the drug loading.Because the higher the content of sodium alginate,the greater the uniformity of drug suspension,the faster the reaction of sodium alginate and chitosan.
As shown in Fig.1C,the greater the ratio of sodium alginate and drug,the slower the drug release,but not significantly.
Considering Table 3 and Fig.1C,when the ratio of sodium alginate and drug was 2:1,the drug loading,entrapment efficiency and release characteristics of the beads were better.
3.1.4.Concentrations of CaCl2
Table 4 indicated that the concentration of calcium chloride had no significant effect on drug loading and entrapment efficiency of the CS-SA beads.The solubility of calcium chloride in chitosan acetic acid solution was low,but the concentration of the calcium chloride could meet the requirements of formation of the capsule membrane.However,the concentration of calcium chloride should not be too low,otherwise the tense of the gel beads would decrease,meanwhile,the beads would deform after drying.
As shown in Fig.1D,the concentration of calcium chloride had no significant influence on the release of ketoprofen from the beads.
Considering the Table 4 and Fig.1D,1.5%was chosen as the concentration of calcium chloride for further study.
3.1.5.Concentrations of chitosan
As shown in Table 5,we could see that when the concentration of chitosan increased from 1.0%to 1.2%,drug loading and entrapment efficiency of the CS-SA gel beads increased.However,when its concentration increased to 1.4%,the drug loading and entrapment efficiency decreased.In the experiment,we found that the higher the concentration of chitosan,the higher the viscosity of the solution.When the solution was dropped into chitosan and calcium chloride solution by peristaltic pump,due to the resistance of high viscosity of the solution,part of the droplets appeared flat.The higher the viscosity of chitosan and calcium chloride solution,the higher the probability of getting flat beads.
Fig.1E indicated that when the concentration of chitosan increased from 1.0%to 1.2%,the release of ketoprofen from the gel beads decreased.During the increase of the concentration of chitosan solution,the number of electrostatic attraction between the carboxyl on sodium alginate and the amino on the molecule chain of chitosan increased,thus enhanced the strength of the capsule membrane and delayed the release of drug.
Taken the Table 5 and Fig.1E into account,1.2%was selected as the concentration of chitosan for further research.
It was found that the optimum concentration of sodium alginate[1.5%],calcium chloride[1.5%]and concentration of chitosan[1.2%]could influence the beads entrapment efficiency,drug loading and the release characteristics.Different batches of beads were then prepared with the optimized process variables.
3.2. Orthogonal experiment
L9(34)orthogonal experiment was applied to optimize the formulation,the factors and levels were shown in Table 6[35].This study referred to the following cumulative release of two time points:the calculating formula of accumulative release within 2 h was|P2h|and the calculating formula of accumulative release within 6 h was|P6h-50%|.The calculation formula of the total score(L):L=|P2h|+|P6h-50%|[36].The smaller the value of L,the closer the release quantity to the prescribed standard.
R was the reaction to the influence of various factors on the determination of index.Table 7 showed that the order of four factors affecting the determination of index was A>C>B>D.As shown in Table 8,F-value of A,B,C and D were 19.72,9.85,15.67 and 1,respectively.While F1-0.05(2,2)=19.00,the P-value of A was less than 0.05.So,further variance analysis of various factors indicated that the concentration of sodium alginate had remarkable influences on the drug release[37].
Due to the ratio of sodium alginate and drug had less effect on the degree of the release,when the ratio of sodium alginate and drug were 2:1 and 3:1,the release of drug met the requirements.However,drug loading should be taken into consideration at the same time,the ratio of sodium alginate and drug were 2:1 and 3:1 with the drug loading 30%and 20%,respectively.Therefore,considering the drug loading and thecumulative release,we finally chose the A2B2C2D3as the optimal formulation,namely,the concentration of sodium alginate was 1.5%,the ratio of sodium alginate and drug was 2:1,the concentration of chitosan was 1.2%and the concentration of CaCl2was 2%.
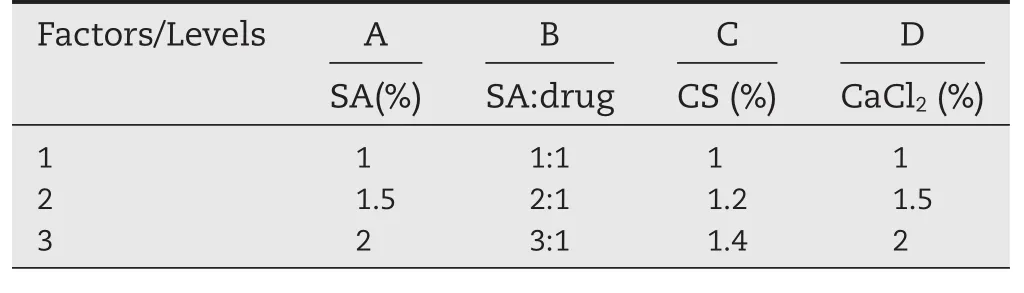
Table 6–The factors and levels of orthogonal design.
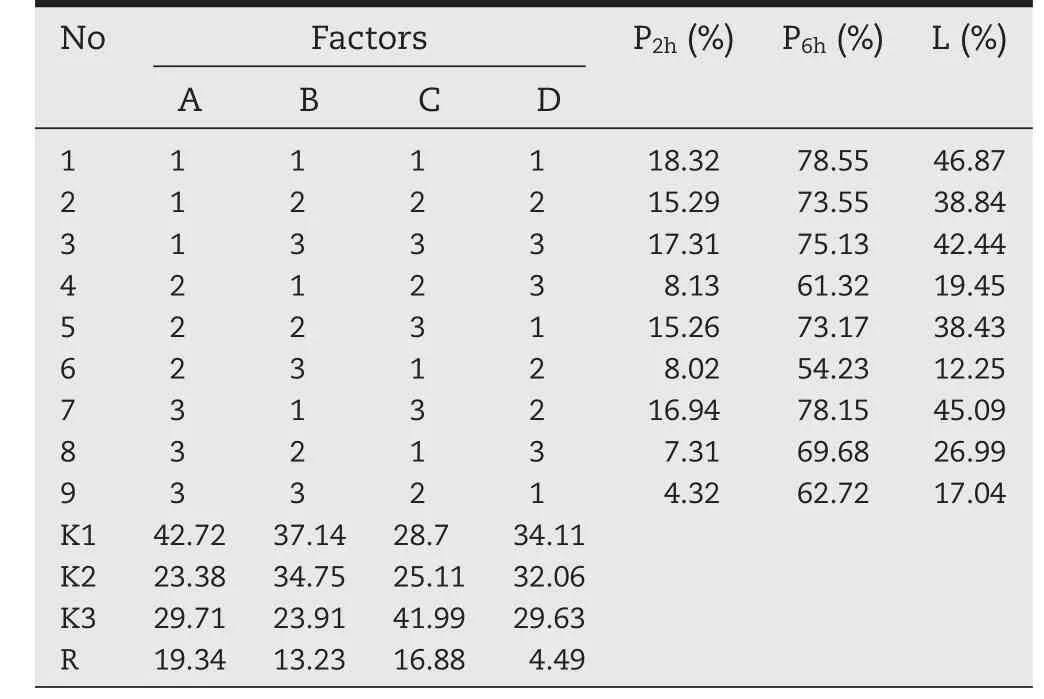
Table 7–The results of orthogonal design.
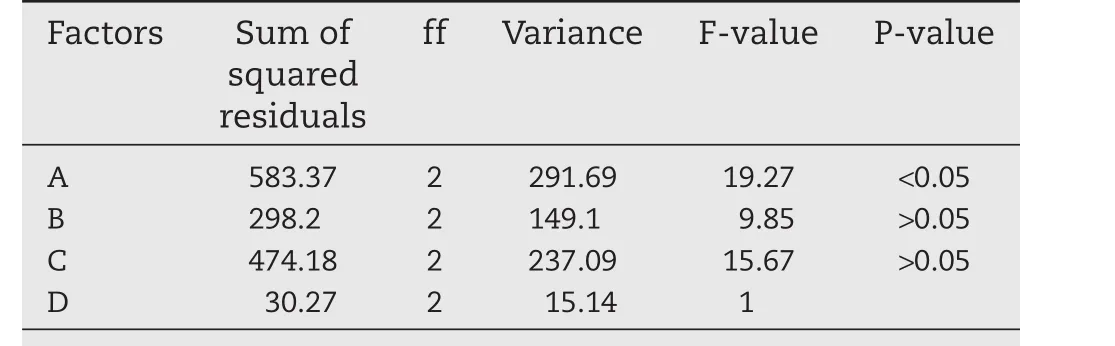
Table 8–The results of variance analysis.
3.3. Surface morphology
Fig.2 indicated that the surface morphology of the CA beads and CS-SA beads,respectively.As shown in Fig.2A,the scale bar was 0.25 mm and 50µm,respectively.The surface of the CA beads were fold and stack due to shrank substantially after drying.During the cross-linking process,a large amount of water would be expelled from the cross linked polymer matrices.A portion of water would be expelled into the inner core.The folded surface was resulted from the removal of water in the inner core.While,the CS-SA beads had a smoother surface compared to the CA beads,which was due to the coat of chitosan at the surface showed in Fig.2B,the scale bar was 0.25 mm and 50µm,respectively.The polyelectrolyte complex produced on the surface of gel beads could effectively improve the crack structure on the surface of sodium alginate gel beads.The formation of thick coat on outer surface of the CA beads indicated drug was completely entraped into interior polymer network.
3.4. Size
As shown in the Table 9,the average size of CA gel beads containing keteprofen was about 1.0 mm.However,the average size of CS-SA gel beads containing keteprofen was about 0.75 mm.The beads coated with chitosan had a strong and tightness structure,which led to the smaller size of CS-SA gel beads than CA gel beads.
3.5. Swelling behavior
As shown in Fig.3A,CA beads swelled in different pH.The results showed that the beads in pH1.0 and water had no swelling,while in pH 6.8 and pH 7.4 the beads had the strongability of swelling.The swelling of the beads were very quick,which reached saturated state in 20 min,but after 20 min,the beads dissolved and gradually disappeared completely.CA beads were not disintegrating dissolved in the stomach,but in the artificial intestinal juice,calcium and phosphate radical anions combined quickly,causing the gel beads swelling and eventually dissolved completely.Therefore,the release from CA beads indicated that it was suitable for ketoprofen to release in stomach slowly.
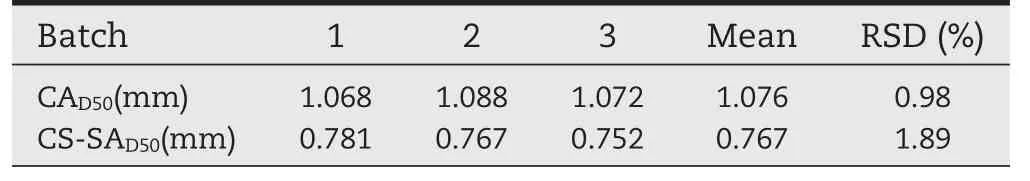
Table 9–The properties of CA and CS-SA gel beads.
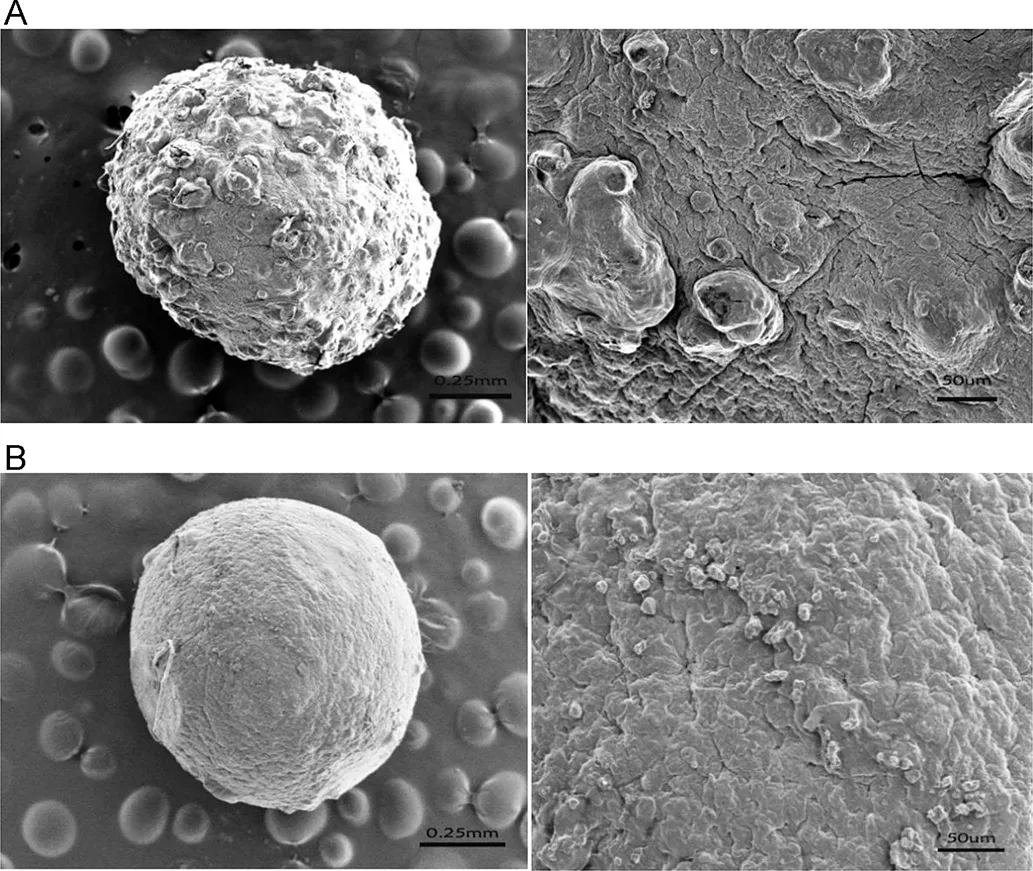
Fig.2–Scanning electron micrographs of(A)the SA gel beads and(B)the CS-SA gel beads,the scale bar was 0.25 mm and 50 μm,respectively.
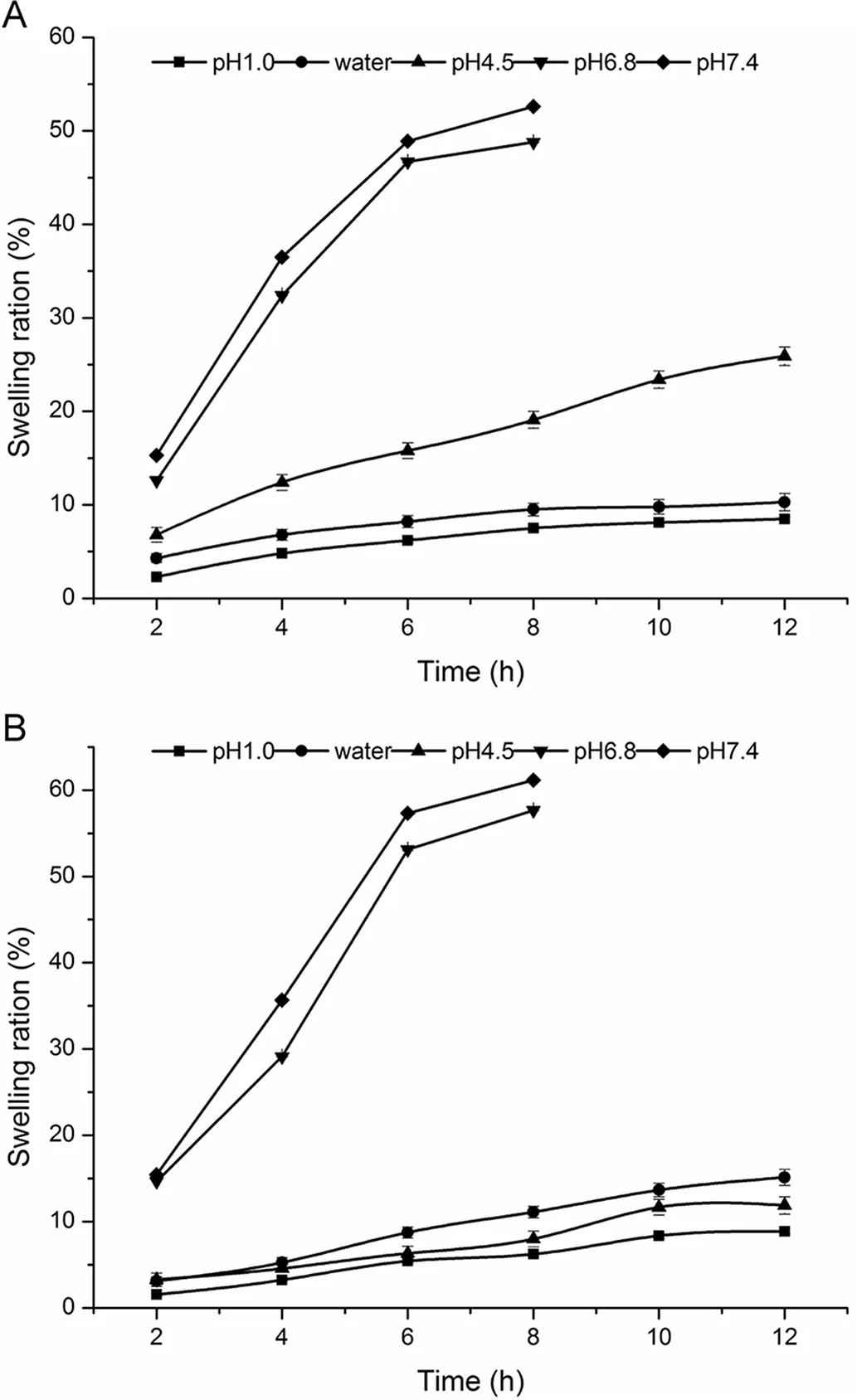
Fig.3–The swelling ration of(A)CA gel beads and(B)CS-SA gel beads in different mediums.
Fig.3B indicated that the swelling properties of the CS-SA beads had a pH dependency.The swelling of beads in acidic medium were poor,while,with the increase of pH,the beads swelling rate increased,swelling in artificial intestinal juice.Compared with CA beads,swelling of the two curves are similar,but the CS-SA beads swelling rate slowed significantly.In the artificial intestinal juice,the beads swelling at the equilibrium is within 8 h.
The results of swelling were in line with the release of the CS-SA gel beads in different medium.Almost no drug released in the artificial gastric juice,while ketoprofen released slowly in artificial intestinal juice by swelling.
3.6. In vitro drug release
The release of CA and CS-SA beads were researched in different dissolution medium(pH 1.0,pure water,pH 4.5,pH 6.8 and pH 7.4),then the results were shown in Fig.4,respectively.
As shown in Fig.4A,the pH of dissolution medium had significant influence on the drug release behavior of CA beads.Under the condition of pH1.0,the release of the beads was slow and the cumulative release amount was low in 12 h.The cumulative release amount of the beads in pH4.5 and water could reach 40%and 80%in 12 h,respectively.However,under the condition of pH 6.8 and pH 7.4,the release within 45 min was around 95%,the beads swelled rapidly, finally dissolved completely.
Showed in Fig.4B,we could see that the pH of dissolution medium had a significant effect on the release of CA beads.The cumulative release of the beads in 12 h was no more than 30%in pH 1.0 and water.What’s more,the cumulative release increased in pH 4.5.However,in pH6.8 and pH7.4,the cumulative release in artificial intestinal juice reached the requirement of the controlled-release,which suggested that the addition of chitosan could effectively reduce the pH sensitivity of the CA beads,enhancing the dependence of the pH of the beads,which had a good sustained-release behavior.
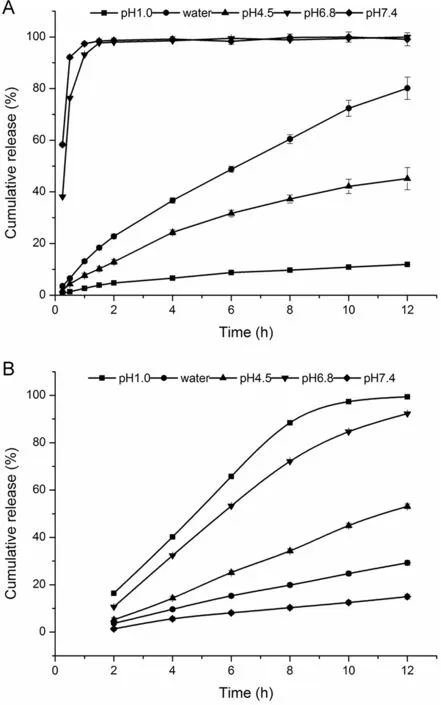
Fig.4–The release profile of Ketoprofen from(A)CA gel beads and(B)CS-SA gel beads in different media.
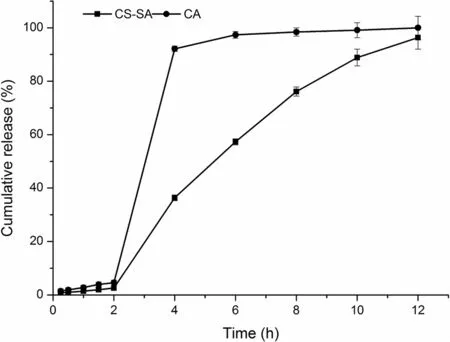
Fig.5–The release profile of CA and CS-SA gel beads in the artificial gastric and intestinal juice.
The release profile of CA and CS-SA gel beads in the artificial gastric and intestinal juice were shown in Fig.5,under the condition of pH 1.0,the release of CA and CS-SA were slow and the cumulative release amount was low in 2 h.While,under the condition of pH 6.8,the release of CA and CS-SA were 99%and 96%in 12 h,respectively.However,the cumulative release of CS-SA in artificial intestinal juice was slower than that of CA beads,which met the requirement of the controlled-release.
3.7. Modeling of drug release kinetics
The release pro files of CA beads and CS-SA beads were shown in Fig.5,which were fitted with the kinetic models as shown in Table 10 and Table 11.The fitness was evaluated using thervalues.In general,the release kinetics of keteprofen from the CA beads fitted well with the Korsmeyer–Peppas model(r> 0.98).n=1.061,judging from thenvalues,it could be concluded that the release of keteprofen from alginate beads followed a case-II transport mechanism(n≥0.85).
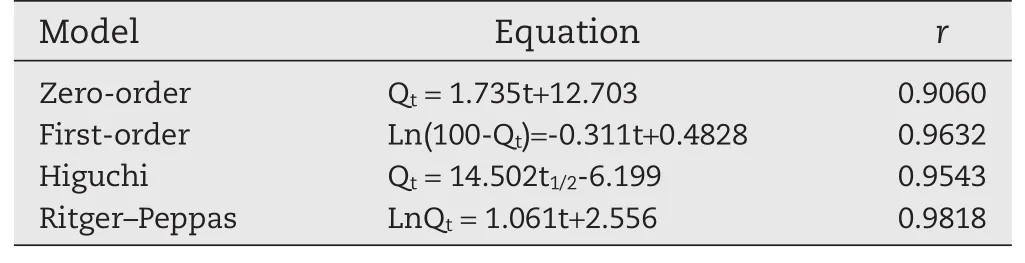
Table 10–Regression equation of different models for CA gel beads.
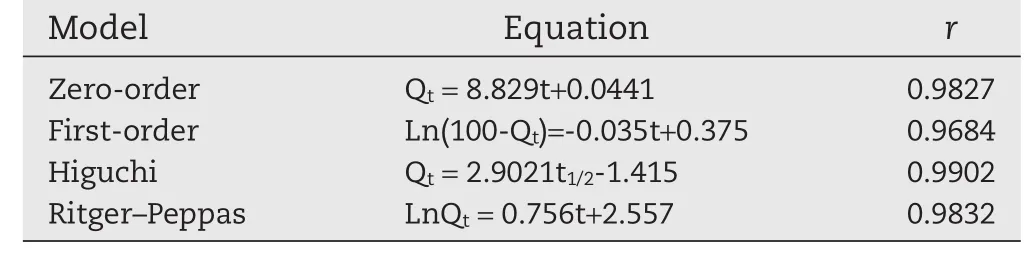
Table 11–Regression equation of different models for CS-SA gel beads.
In addition,the release kinetics of drug from the CS-SA beads might be fitted to the Higuchi model(r>0.99).n=0.756,the release of keteprofen from the CS-SA beads exhibited a non-Fickian mechanism(0.43<n<0.85).The keteprofen was released based on the mixed mechanisms of diffusion and polymer relaxation.
4. Conclusions
In this work,we have successfully prepared two kinds of ketoprofen enteric preparation using biopolymer alginate.In addition,the release pro files of CA beads indicated that it was suitable for ketoprofen to be released in stomach slowly;while,the release of ketoprofen from CS-SA beads had a good sustained-release behavior in gastrointestinal tract.Thus,the result demonstrated that addition of chitosan controlled drug release effectively.Furthermore,the investigation on the drug release mechanisms indicated that drug release from CA beads followed the erosion mechanism and the drug release from CS-SA beads accorded with non-Fick diffusion model.In conclusion,this study showed that ketoprofen loaded CA beads could relieve pain immediately,which could be applied in short-term release applications,such as the instant analgesic of post-surgical and carcinous pain,while CS-SA beads could control the release of ketoprofen during gastro-intestinal tract,prolong the drug’s action time,decrease administration times and lower gastro-intestinal irritation,which reached the expected result.Based on the results we can conclude that two novel formulations have a potential to be applied as drug delivery systems for several oral routes.Hence,there is a scope for improvement in microparticlar drug delivery system so as to achieve different goals,which may be economical,technical or commercial.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgment
This study was supported by the program of supporting career development of young and middle-aged teachers from Shenyang Pharmaceutical University(ZQN2015011)and the Open fund of Key Laboratory of Ministry of Education for TCM Viscera-State Theory and Applications,Liaoning University of Traditional Chinese Medicine(zyzx1608).The authors confirm that there are no known conflicts of interest associated with this publication.
[1]Pathak R,Prasad DR,Misra M,et al.Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route.Acta Pharm Sin B 2014;4:151–60.
[2]Giri TK,Thakur A,Alexander A,et al.Modified chitosan hydrogels as drug delivery and tissue engineering systems:present status and applications.Acta Pharm Sin B 2012;2:439–49.
[3]Kumari S,Panesar PS,Bera MB,et al.Comparative studies on physico-chemical characterization of yeast cells entrapped with alginate and hybrid beads.Iran Polym J 2014;23:111–9.
[4]Smith A,Perelman M,Hinchcliffe M.Chitosan:a promising safe and immune-enhancing adjuvant for intranasal vaccines.Hum Vaccines Immunotherapeutics 2014;10:797.
[5]Yan H,Chen X,Feng Y,et al.Modification of montmorillonite by ball-milling method for immobilization and delivery of acetamiprid based on alginate/exfoliated montmorillonite nanocomposite.Polym Bull 2015;73:1185–206.
[6]Ahmad M,Panda BP.Alginate immobilization of escherichia coli MTCC 1652 whole cells for bioconversion of glycyrrhizinic acid and into 18-β glycyrrhetinic acid.Pak J Biol Sci 2013;16:2046–9.
[7]Sun L,Chen Y,Zhou Y,et al.Preparation of 5- fluorouracilloaded chitosan nanoparticles and study the sustained release in vitro and in vivo.Asian J Pharm Sci 2017;12:418–23.
[8]Vecino X,Devesa-Rey R,de Lima Stebbins DM,et al.Evaluation of a cactus mucilage biocomposite to remove total arsenic from water.Environ Technol Innov 2016;6:69–79.
[9]Tasdighi E,Azar ZJ,Mortazavi SA.Development and in-vitro evaluation of a contraceptive vagino-adhesive propranolol hydrochloride gel.Iran J Pharm Res 2012;11:13.
[10]Ghaffari S,Varshosaz J,Haririan I,et al.Cipro floxacin loaded alginate/chitosan and solid lipid nanoparticles,preparation,and characterization.J Dispersion Sci Technoly 2012;33:685–9.
[11]Huguet ML,Neufeld RJ,Dellacherie E.Calcium-alginate beads coated with polycationic polymers:comparison of chitosan and deae-dextran.Process Biochem 1996;31:347–53.
[12]Huguet ML,Dellacherie E.Calcium alginate beads coated with chitosan:effect of the structure of encapsulated materials on their release.Process Biochem 1996;31:745–51.
[13]Amiri M,Salavati-Niasari M,Pardakhty A,et al.Caffeine:a novel green precursor for synthesis of magnetic CoFe2O4nanoparticles and pH-sensitive magnetic alginate beads for drug delivery.Mater Sci Eng:C 2017;76:1085–93.
[14]Voo W-P,Ooi C-W,Islam A,et al.Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour.Eur Polym J 2016;75:343–53.
[15]Sriamornsak P,Kennedy RA.A novel gel formation method,microstructure and mechanical properties of calcium polysaccharide gel films.Int J Pharm 2006;323:72–80.
[16]Favre E,Leonard M,Laurent A,et al.Diffusion of polyethyleneglycols in calcium alginate hydrogels.Colloids Surf A Physicochem Eng Asp 2001;194:197–206.
[17]Barquilha CER,Cossich ES,Tavares CRG,et al.Biosorption of nickel(ii)and copper(ii)ions in batch and fixed-bed columns by free and immobilized marine algae sargassum sp.J Cleaner Prod 2017;150:58–64.
[18]Sriamornsak P,Kennedy RA.Effect of sodium fluorescein on release characteristics of a macromolecule from calcium alginate gel beads.Carbohydr Polym 2011;84:1208–12.
[19]Al-Taani B,Khanfar MS,Salem MS,et al.Release behaviour of diclofenac sodium dispersed in gelucire and encapsulated with alginate beads.J Microencapsul 2010;27:10–3.
[20]Praveen R,Singh SK,Verma PRP,et al.Sustained delivery of cefdinir to upper gastrointestinal tract using calcium alginate beads:a formulation by design.J Pharm Investig 2014;44:455–63.
[21]Pawar HA,Lalitha KG,Ruckmani K.Alginate beads of captopril using galactomannan containing senna tora gum,guar gum and locust bean gum.Int J Biol Macromol 2015;76:119–31.
[22]Bertagnolli C,Grishin A,Vincent T,et al.Recovering heavy metal ions from complex solutions using polyethylenimine derivatives encapsulated in alginate matrix.Ind Eng Chem Res 2016;55:2461–70.
[23]Belhouchat N,Zaghouane-Boudiaf H,Viseras C.Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads.Appl Clay Sci 2017;135:9–15.
[24]Tomovic N,Trifkovic K,Rakin M,et al.Influence of compression speed and deformation percentage on mechanical properties of calcium alginate particles.Chem Ind Chem Eng Q 2015;21:411–7.
[25]Barzegar-Jalali M,Hanaee J,Omidi Y,et al.Preparation and evaluation of sustained release calcium alginate beads and matrix tablets of acetazolamide.Drug Res(Stuttg)2013;63:60–4.
[26]Ashfaque M,Solomon S,Pathak N.Kinetic study of immobilized cellobiase produced from immobilized wildtype trichoderma longibrachiatum.Sugar Tech 2015;18:340–6.
[27]Aceval Arriola ND,de Medeiros PM,Prudencio ES,et al.Encapsulation of aqueous leaf extract of stevia rebaudiana bertoni with sodium alginate and its impact on phenolic content.Food Biosci 2016;13:32–40.
[28]Kamaraj N,Rajaguru PY,Issac PK,et al.Fabrication,characterization,in vitro drug release and glucose uptake activity of 14-deoxy,11,12-didehydroandrographolide loaded polycaprolactone nanoparticles.Asian J Pharm Sci 2017;12.
[29]Campañone L,Bruno E,Martino M.Effect of microwave treatment on metal-alginate beads.J Food Eng 2014;135:26–30.
[30]Fu YC,Ho ML,Wu SC,et al.Porous bioceramic bead prepared by calcium phosphate with sodium alginate gel and PE powder.Mater Sci Eng:C 2008;28:1149–58.
[31]Gupta VK,Yola ML,Eren T,et al.Catalytic activity of Fe@Ag nanoparticle involved calcium alginate beads for the reduction of nitrophenols.J Mol Liq 2014;190:133–8.
[32]Cheewatanakornkool K,Niratisai S,Manchun S,et al.Characterization and in vitro release studies of oral microbeads containing thiolated pectin–doxorubicin conjugates for colorectal cancer treatment.Asian J Pharm Sci 2017;12(6):509–20.
[33]Fan R,Sun Y,Li B,et al.Preparation and stability investigation of tamsulosin hydrochloride sustained release pellets containing acrylic resin polymers with two different techniques.Asian J Pharm Sci 2016;12.
[34]Rocher V,Siaugue J-M,Cabuil V,et al.Removal of organic dyes by magnetic alginate beads.Water Res 2008;42:1290–8.
[35]Angelescu DG,Anastasescu M,Anghel DF.Synthesis and modeling of calcium alginate nanoparticles in quaternary water-in-oil microemulsions.Colloids Surf A Physicochem Eng Asp 2014;460:95–103.
[36]Zhang ZH,Sun YS,Pang H,et al.Preparation and evaluation of berberine alginate beads for stomach-specific delivery.Molecules 2011;16:10347–56.
[37]Mahou R,Meier R,Bühler L,et al.Alginate-poly(ethylene glycol)hybrid microspheres for primary cell microencapsulation.Materials 2014;7:275–86.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- ROS-responsive drug delivery systems for biomedical applications
- Formulation design of granules prepared by wet granulation method using a multi-functional single-punch tablet press to avoid tableting failures
- Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel
- Tablets of paliperidone using compression-coated technology for controlled ascending release
- Quantification and spatial distribution of salicylic acid in film tablets using FT-Raman mapping with multivariate curve resolution
- Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery
