Tablets of paliperidone using compression-coated technology for controlled ascending release
2018-03-28
School of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
1. Introduction
Schizophrenia is a chronic disorder that affects many people,and mainly manifests as cognitive dysfunction,decreased interpersonal ability and decline of social functions,and the incidence and the resulting suicide rate is increasing year by year.Although currently available antipsychotics can improve the onset of symptoms,traditional antipsychotic drugs have a high incidence of adverse reactions,require frequent dosing,and are not tolerated by or complied with by all patients.Paliperidone(PAL)is an advanced second-generation atypical antipsychotic,with smaller fluctuations in blood concentration and less adverse reactions,approved by the FDA for the treatment of central nervous system(CNS)disease schizophrenia[1–4].Owing to the production of the osmotic pump tablet(OPT),paliperidone extended-release(ER)is an atypical antipsychotic that delivers 9-hydroxyrisperidone using OROS®technology[5].Class II drugs possess the characteristics of high permeability and poor solubility according to the Biopharmaceutical Classification System(BCS).It has been found that,a smooth drug release of poorly soluble drugs can be achieved through pushepull osmotic pump(PPOP)[6]which works as follows.In the gastrointestinal tract,water is absorbed by the osmotic activity gradient,and the rate of water absorbed is determined by the thickness and composition of the membrane.As the gastrointestinal fluid ions do not penetrate the semipermeable membrane,the drug release rate of the osmotic pump is not influenced by the pH.As the drug layer begins to be hydrated and the push layer begins to swell,osmotic pressure is the driving force to control drug release,and the drug is discharged through the pores by the expanded bottom layer.The drug concentration of the second drug layer is higher than the first layer,and therefore ascending release can be achieved through the drug concentration gradient.Initially the drug contained within the first compartment is released,and once the majority of drug from the first compartment has released,the drug within the second compartment begins to release at an ascending rate[7].The ascending release and delayed sustained-release PPOP show great advantages for achieving stable and slow drug release rates,for avoiding high initial plasma concentrations to remain effective and safe,and also for maintaining a controlled and prolonged treatment window,which reduces adverse effects and improves compliance[8].However,the osmotic pump process is complex,high cost,time-consuming,dangerous of conspicuous burst release,requires cumbersome quality inspection,and has strict processes.
Compression coating is an old technique that was first proposed by Noyes in an 1896 patent[9].Although it is an old concept,compression coating is a novel technology for formulation of new DDS systems.It has been used in the pharmaceutical field for many purposes,such as separating incompatible drugs and combining drugs to achieve different therapeutic effects[10],protecting drugs with oxygen labile,acid-labile,light-sensitive or hygroscopic characteristics[11],for delayed release[12],pulsatile release[13],colon-specific release[14],programmable release,to modify pH sensitivity[15,16]and more.This can be achieved as CC tablets possess many characteristics that cannot be matched by general tablets.At the time of administration,the outer component of the CC tablets can distribute on the surface of the intestinal mucosa,and the core components are thus concentrated on the surface of the gastric mucosa,allowing for complete absorption of the drug and improved bioavailability.A better drug release rate can be obtained through the combination of the two different release rates,to achieve the desired plasma concentration,to maintain a stable,long effective concentration,reduce the toxic effects of drugs,avoid the stimulation of gastric mucosa and other side effects[17].When sustained-release tablets are formed by pressing on the outer layer,they are more reliable than when directly coating on the surface of the tablet,successfully avoiding problems such as drug leakage and other issues caused by uneven coatings.Further,the stability of the drug can be increased by compressing two different components without mixing.More combinations of controlled release requirements can be met through the technology of CC tablet,and thus can overcome limitations of using single sustainedrelease pellets,and achieve more requirements during administration.In the CC tablet system,the entire surface of an inner core is completely surrounded by the coating,which is able to delay drug release from the core until the polymeric coat has begun to erode,dissolve or is removed.For functional coatings,it is typical to use organic solvents,however these processes have a longer production cycle and limited drug loading,and it is desired to reduce environmental impact.Dry powder tablet coating can avoid these drawbacks,can be prepared with incompatible compound ingredients,and is much more efficient than solution coating.Thick coatings can be applied rapidly and the coating process is solvent-free,and the process is more desirable to industry because of the simple manufacturing methods.Current commercially available compression-coated tablets include glipizide marketed tablets(Glucotrol XL®,P fizer of USA),nifedipine controlled release tablets(Adalat,Bayer of Germany),amoxicillin and potassium clavulanate compression-coated tablets(Moxicle TM,Korea)and more.
The aim of this work was to design paliperidone compression-coated tablets for ascending release,using HPC and HPMC as the main functional material for control of the release rate.The factors affecting the drug release behaviorin vitrowere investigated to optimize the formulations.The drug release mechanism of the paliperidone CC tablets was studied by model fitting,as well as uptake and erosion tests.The rate of drug release is dependent on the rate of polymer swelling,the solubility of the drug,and the soluble fraction of the gel matrix over time.As the rate of erosion of the matrix accelerated,the drug release also presented ascending release.In the later stages of drug release,although the matrix erosion rate slowed,the majority of the matrix had dissolved,thus reducing the barrier and so the drug release rate was still increasing.This work can function to establish a foundation for compression coating technology,and as such is the first one to apply the compression coating technique to obtain ascending release of paliperidone Fig.1.
2. Materials and methods
2.1. Materials
Paliperidone(PAL)was synthesized by Jinchang Pharmaceutical Co.,Ltd.Commercial paliperidone extended-release tablets Invega®(6 mg)as references in this study were purchased from Janssen Cilag Manufacturing L.L.C.(Xian-janssen,China).Eudragit RL-PO was supplied by Evonik in Germany.Sodium alginate was kindly gifted by FMC Corporation.High viscosity hydroxypropyl cellulose(HPC-H)was purchased from Nippon Soda(Tokyo,Japan).Hydroxy-propyl methyl cellulose(HPMC)was a gift from Colorcon Asia Paci fic Pte.Ltd.Citric acid was purchased from Tianjin Hengxing Chemical factory.Glyceryl behenate and microcrystalline cellulose were from Zhejiang Huzhou Looking Pharmaceutical Co.,Ltd.
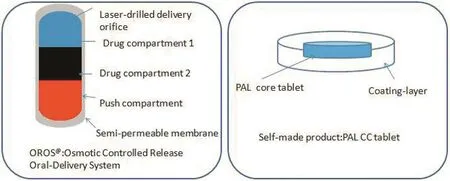
Fig.1–The structure of Invega®and PAL CC tablet.
2.2. Methods
2.2.1.Pre-treatment of paliperidone
Paliperidone is a BSC II drug,with low solubility and high penetration,and thus the solubility is the dissolution rate limiting step.In order to improve the solubility and bioavailability,a micronization process is required to reduce the particle size.Two methods were attempted:dry grinding(DG)and jet milling(JM).In the JM process,air was used as the grinding gas and the temperature was set to 20°C.For the DG process,the raw materials were directly placed in a ball mill and ground for 24 h.The pulverized products obtained through each method were then studied,and the optimum pretreatment method was selected,with the dissolution rate and the particle size as the evaluation indices.
2.2.2.Preparation of paliperidone CC tablets
The formula of the paliperidone CC tablets is shown inTable 1.The core tablets were prepared by the wetting granulation method,with 3%(w/w)Sodium alginate water solution with citric acid(pH 1.0)used as the binder to knead the powder of the micronized paliperidone,Eudragit RL-PO,and microcrystalline cellulose for 10 min.The final mixture was forced through a 40 mesh sieve.The granules were dried at 40°C for 6 h and pushed once more through a 40 mesh sieve.HPC-H was mixed with the granules for 5 min in a sealed container.Finally,glyceryl behenate was added and mixed for another1 min.The tableting was performed using a TDP-5 Single Punch Tablet Press(Shanghai TianFan Drug-manufacturing Plant,China.)equipped with a 5 mm diameter punch with concave surface.The hardness of the core tablets was controlled to approximately 50±10 N.The mass of each core tablet was in the range of 50±2.25 mg.
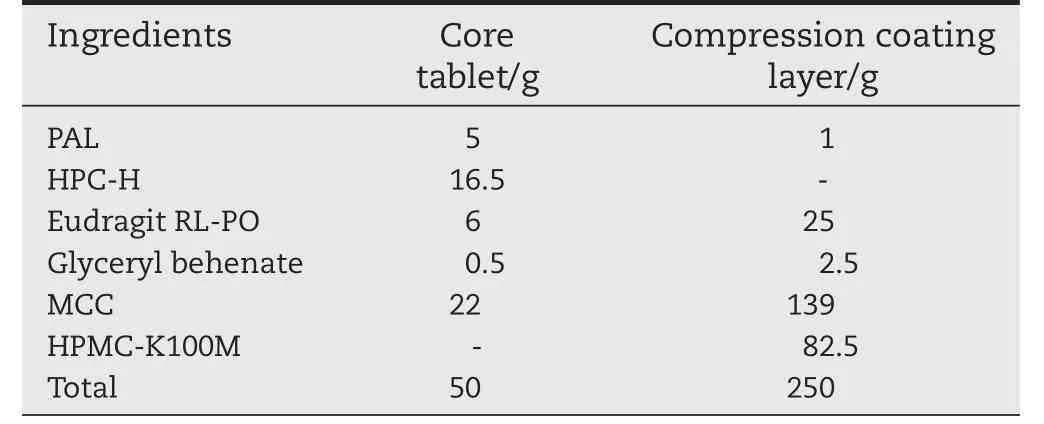
Table 1–The core tablet and coating layer composition of the CC tablets of paliperidone(1000 pills).
The same method was applied in preparing the compression-coated layer.The final mixture was comprised of micronized paliperidone,Eudragit RL-PO,and microcrystalline cellulose,and was forced through a 40 mesh sieve,granules were dried at 40°C and pushed through a 40 mesh sieve again.HPMC-K100M was mixed with the granules for 10 min in a sealed container and glyceryl behenate was added and mixed for another 2 min.The paliperidone CC tablets were prepared by first filling some of outer mixture in the die cavity,then centrally positioning the core tablet on the powder bed followed by filling the remainder of the mixture on top,and finally the tablets were pressed at 120±10 N.The weight of each paliperidone CC tablet was 300±5 mg.Finally,5 mm diameter core tablets were press-coated into 9 mm diameter tablets.
The axial position of the core tablets was changed by adjusting the ratio of the powder bed and the top mixture.The weight ratio of the mixture of the powder bed and the top is shown in Table 2.The radial position of core tablets was adjusted by the placement of the core tablets in the center of the powder bed.
2.2.3.In vitro dissolution study and evaluation of in vitro conformance
Anin vitrodissolution test was carried out in a dissolution apparatus(ZRS-8G Test Dissolution Tester,Tianjin TiandaTianfa Technology Co.,Ltd.China).The study was performed at 50 rpm according to the USP paddle method[18].A volume of 500 ml NaCl 2 g/l(0.2%w/w)in HCl(pH 1.0±0.5)was selected as the dissolution fluid at 37±0.5°C.The CC tablets and themarketed Invega®were separately added into dissolution medium,and samples were withdrawn at predetermined sampling time points.Fresh dissolution medium was added to replace the volume removed after each sampling.Prior to analysis,all samples were filtered through a 0.45µm membrane filter.All samples were analyzed by HPLC(LC-2010A HT,SHIMADZU,Japan),with the oven maintained at 35 C and detector at 275 nm.Prior to conducting the dissolution test,a methodological study was performed to assess the suitability of the analysis protocols,and con firmed no interference in the UV absorption between excipients and the drug,and that the analytical methods were satisfactory for the concentration range of samples from 1 to 24 h.Separation was performed on a C18column(4.6 mm×150 mm,particle size 5 mm,Thermo Fisher Scientific,USA).The mobile phase was 0.05 mol/l ammonium formate buffer solution(pH 3.3±0.1)acetonitrile–methanol(75:8:17,v/v)at a flow rate of 1.5 ml/min,and was filtered through a 0.45 um membrane and degassed by ultrasonic filtration before use.Drug release studies were performed six times in parallel for each formulation tested as well as the commercially available tablets Invega®.
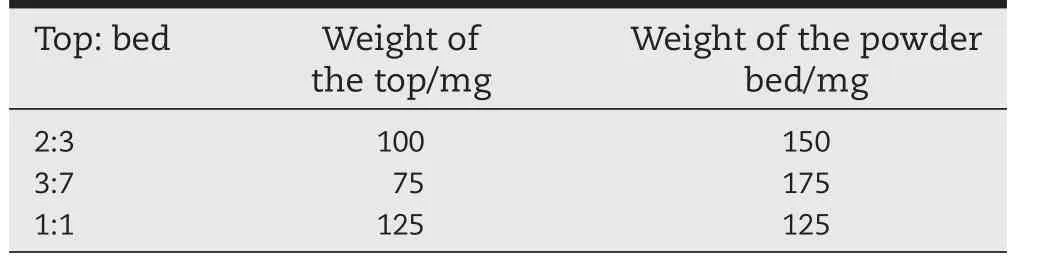
Table 2–The different weight ratios of the top and powder bed.
Paliperidone is a weakly alkaline drug with a pH-dependent solubility,the dissolution rate increases with decreasing pH.It was found that self made CC tablets dissolve the fastest in dissolution medium of pH 1.0,with the slowest rate when the pH was 6.8.The tablets could completely release the drug when the media pH was 1,however the tablets only released less than 70%after 24 h when the media pH was 6.8.The commercial Invega®are osmotic pump tablets,and thus show no pH dependency.In systems with pH sensitivity and water insoluble drugs,pH adjusting agents are usually added to increase the solubility of the drug[19].In order to achievein vitrodissolution conformance, first a buffer system was used to ensure that the drug release environment maintained a stable local pH to maintain stability.After this,a small amount of enteric materials were added to balance the pH dependency of the drug.Finally,citric acid solution(pH 1.0)was chosen as the pH-modifying agent and sodium alginate as pH dependent materials added into the tablets.To examine the effects of different pH,0.2%sodium chloride hydrochloric acid medium(pH 1.0),acetate medium(pH 4.5),phosphate medium(pH 6.8)and pure water were used as dissolution media,and the similarity was investigated according to the similarity factors(f2).
2.2.4.Uptake and erosion studies
The tablets were evaluated by gravimetric analysis,and the uptake and erosion tests were performed as reported by Efentakis et al[20].Tests were conducted using the above dissolution apparatus,the samples were placed in 500 ml NaCl 2 g/l(0.2%w/w)in HCl(pH 1.0±0.5)buffer solutions at 37±0.5°C and the paddle stirred at 50 rpm.At various time points,the tablets were withdrawn from the medium and the excess liquid removed and then weighed.Samples were dried at 60°C until a constant weight was reached.Six different tablets were measured at each time point,and fresh tablets were used at each individual time point.
For calculation erosion,the percentage of the removing mass(RM)was used as an indicator.The water absorption(WA)was used to explain the process of water uptake and expansion,as weight of the tablets increased due to absorbed liquid.RM and WA were calculated according to the following formula:
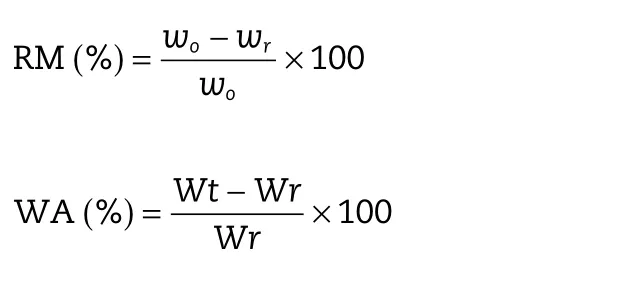
whereW0is the original weight of the dry tablet;Wris the weight of remaining dried tablet after entering the media at time t;Wtis the weight of tablet without water on the surface at time t before drying.
2.2.5.In vitro drug release kinetics
To evaluate the mechanism of drug release,the Korsmeyer–Peppas model was used[21,22].The Peppas equation was a simple analysis that revealed a complex release mechanism.The data was calculated according to the following equation:
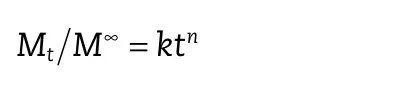
whereMtis the time t drug releasing,M∞is the total drug releasing,kis a constant and the exponentnis used as indicators of the drug release mechanism.A different release mechanism was determined by the value ofn.The correlation coefficient(R)was used as the indicator of the models.
3. Results and discussion
3.1. Pre-treatment of paliperidone
Solubility played an important role in modifying the drug release behavior.As PAL is an insoluble drug,it was difficult to achieve controlled release,and therefore,a pre-treatment of the drug was required.In this way the particle size could be reduced,and thus the solubility of PAL improved,and this was attempted by two different methods.A reduced particle size also helped to reduce the difference of dissolution behavior of the drug in different pH media.Particle size analysis was carried by a BT-9300S Laser particle size distribution analyzer(Dandong Baxter Instrument Co.,Ltd.Liaoning,China),using water as the medium for measurements.The real part of the refractive index of substance was set to 1.584,and the imaginary part was set to zero and each sample was performed in triplicate.Fig.2 shows the results of particle size analysis,which showed the median diameter(D50)of raw materials,DG product and JM product as 31.98µm,18.96µm and 3.895µm respectively.The particle size of the JM method was smaller and the size distribution was more uniform than for the DG method.In terms of the dissolution rate,all micronized PAL samples were faster than the raw material.As shown in Fig.3,micronized PAL by the DG method dissolved slower than for the JM method.Through the JM method,micronized PAL could dissolve completely in 1 h,and the method was simple and high yielding,and thus jet milling was chosen as the final pretreatment method.
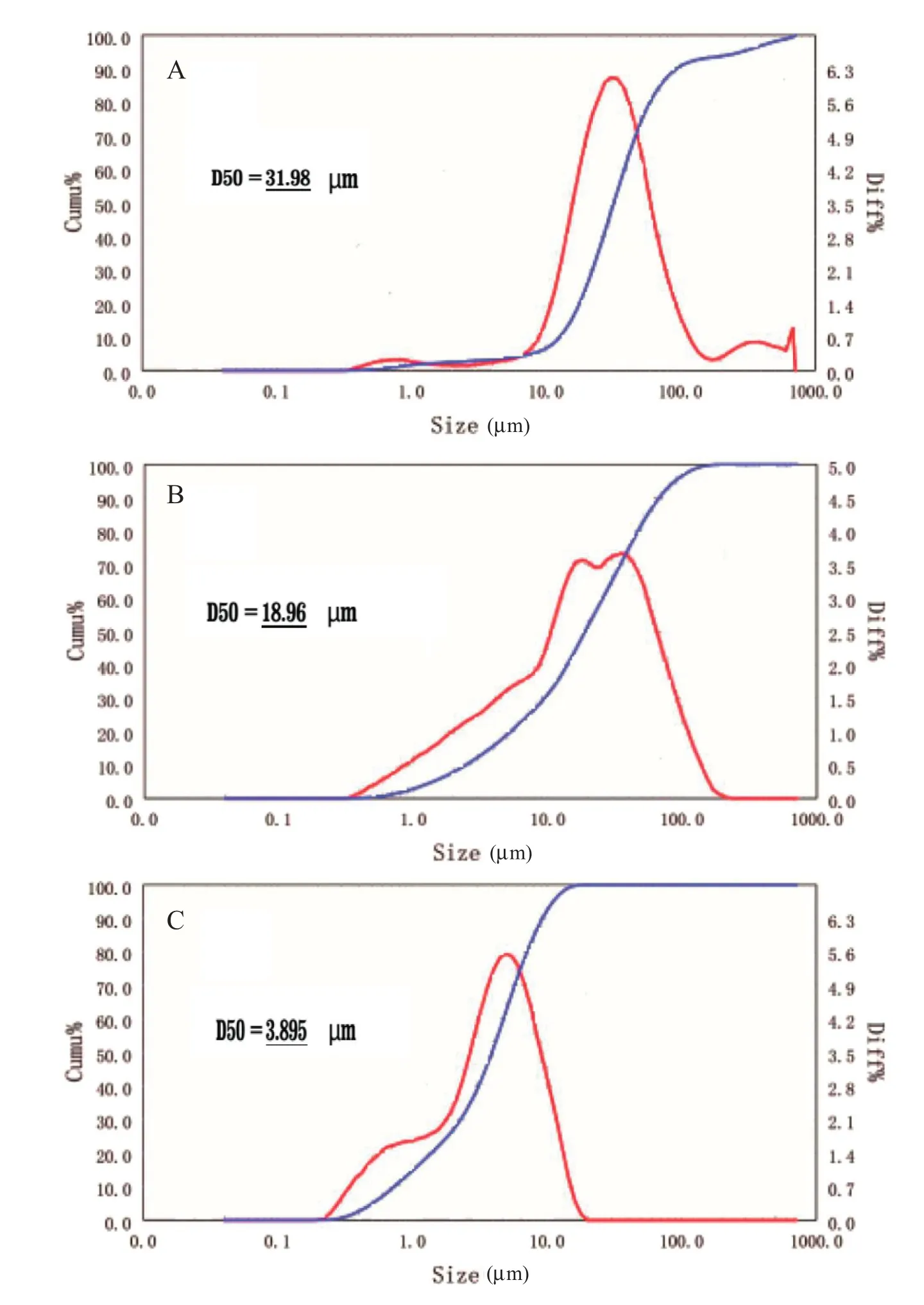
Fig.2–Particle size distribution of different powders,(A)original PAL,(B)dry grinding PAL,(C)jet milling PAL.
3.2. Effects of manufacturing tablets
3.2.1.Screening of retardation materials
Different types of matrix material were selected for testing in this study:PEO,HPMC,HPC.The highest drug release rate was achieved with PEO,and the lowest release rate was attained by HPMC.PEO could not meet the controlled release requirements in this work.HPMC had a more controlled release abilit,showing controlled release over 24 h,with full drug release.However,it was observed that the initial 6 h had a low release of drug,and a suddenly large release at the 12 h point,and thus could only control drug release over the first few hours.It was found that HPC could control drug release for the full 24 h,with no sudden release phenomenon.However under established prescription conditions,if HPC used as the block material of the outer layer,the tablet would disintegrate very fast,but when HPMC is used,this can be avoided.Based on this,HPC was chosen as the retardation material of the core layer and HPMC was used as the block material of the outer layer.
3.2.2.Influence of the viscosity grade and the amount of HPC and HPMC
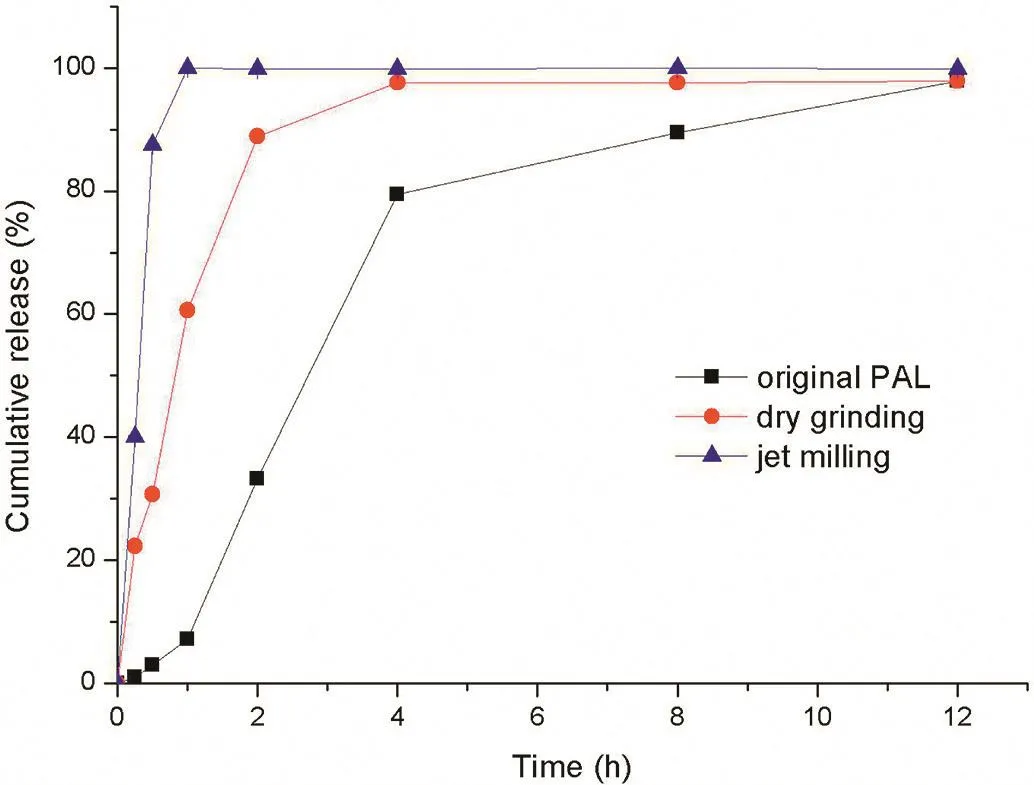
Fig.3–Release pro files of different powders by different pretreatments in pure water at 37°C(Mean ±SD,n=3).
HPMC and HPC are hydrophilic polymers and the main pharmaceutical excipients of controlled release at present.It has been reported that the drug releasing rate can be further suppressed as the viscosity of the polymer is increased[23].The release rate is reduced in the order of HPMC-K4M,K15M and K100M,HPC-L,M and H.As the viscosity grade of HPMCK4M and HPC-L is low,the dissolution of the hydrogel layer was rapid with these materials.In comparison the dissolution rate of HPMC-K15M and HPMC-K100M was slower and the gel layer could remain on the surface for a longer period of time,and a similar effect was observed for HPC-M and HPC-H.The ascending release rate was found to be significantly influenced by the viscosity grade of HPMC and HPC,and as shown in Fig.4A and 4B,the release rate decreased along with the increasing viscosity grade of HPMC and HPC.This can be explained by the higher viscosity grade of HPMC and HPC requiring more water to reach a hydration state of the compression and core layer.As the water permeability decreased with increasing viscosity of materials,this resulted in lower speeds of erosion and drug release.The results showed that the most suitable release rate was achieved,with complete dissolution at 24h,when using HPMC-K100M and HPC-H as the materials.The influence of the amount of HPMC and HPC is demonstrated in Fig.4C and 4D,where the drug release rate decreased with an increase of the amount of HPMC and HPC.However,dissolution was not complete by 24 h when the amount of material was too large,and so the optimum amount of HPMC and HPC was determined as 33%.
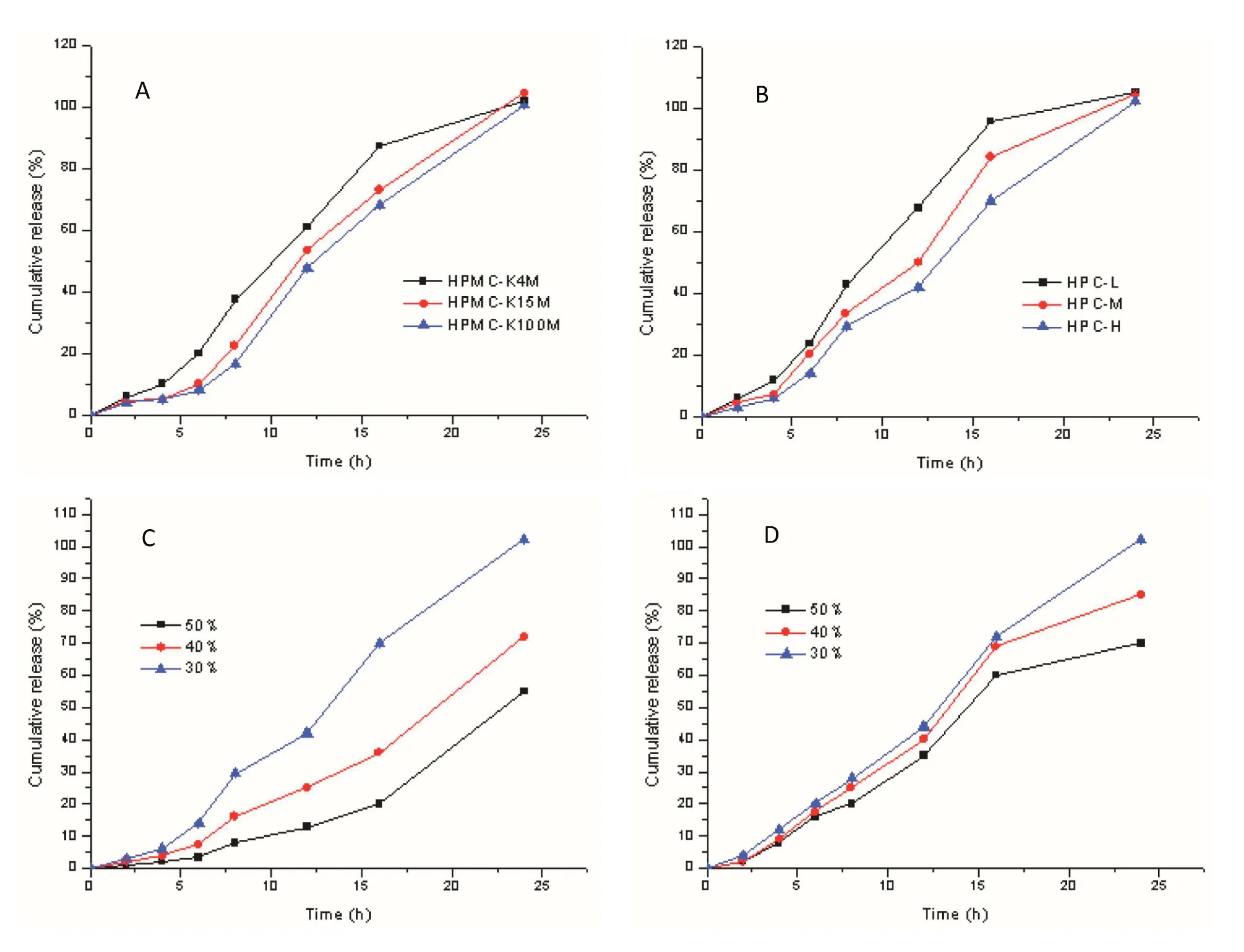
Fig.4–The effect of(A)the viscosity grade of HPMC,(B)the viscosity grade of HPC,(C)the amount of HPMC and(D)the amount of HPC on in vitro drug ascending release pro files(Mean±SD,n=6).
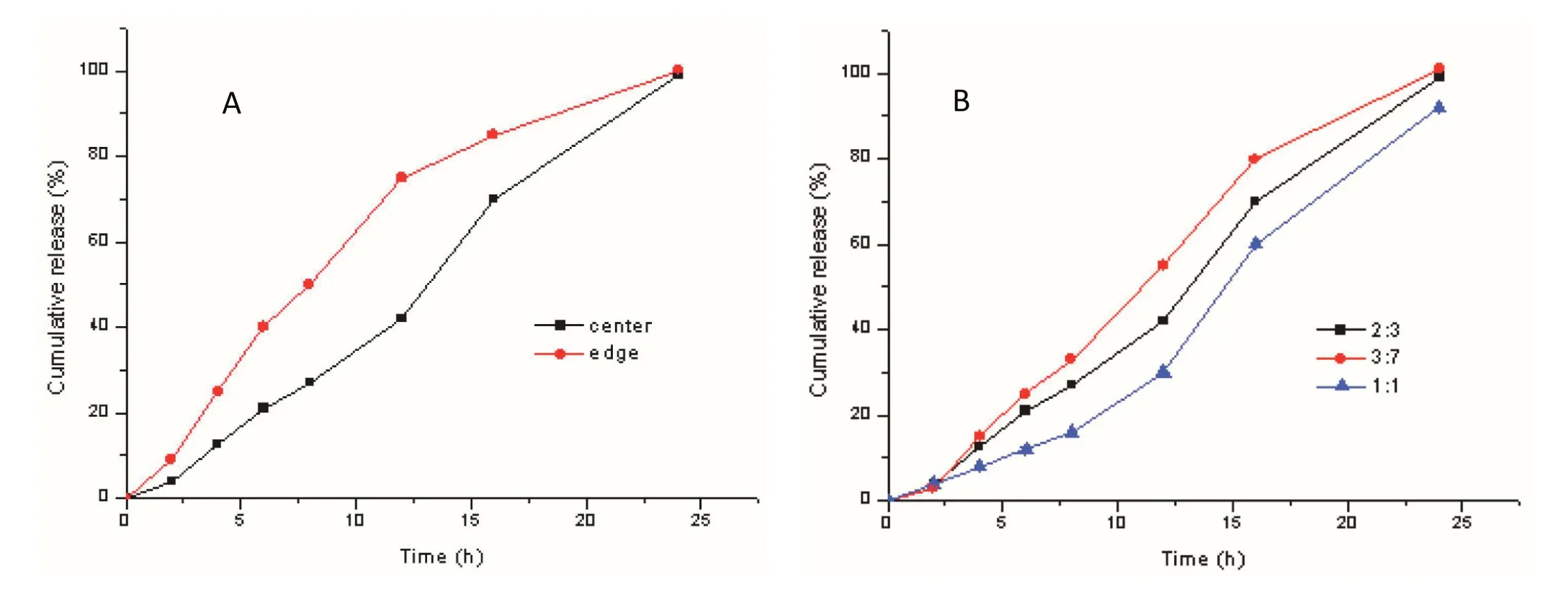
Fig.5–Release pro files of different position of core tablet(A)axial position,(B)radial position under the conditions of 0.2%NaCl pH 1.0 hydrochloric acid medium at 37°C(Mean ± SD,n=6).
3.2.3.Effect of the position of the core tablets
Besides the prescription investigation,the influence of the tableting process was also studied,with the position of the core tablet as an important indicator.Fig.5A shows the effect of an axial position on the drug release,while Fig.5B shows the effect of a radial position on release.When the core tablet was deviated from the center,it was exposed to medium earlier,leading to faster release and total release over a shorter time period.Then the fast release brought forward and total release get faster.Considering the effect of these two aspects,it was determined that the position of the core tablet in the outer layer should be monitored.The highest ascending releasing rate could be achieved when the weight ratio of the top and powder bed was 3:7,however the drug risked being released too quickly.For this work,it was concluded that an appropriate ascending releasing rate could be obtained when the weight ratio of the top and powder bed was 2:3.
3.3. In vitro dissolution study
Both commercially available Invega®and the prepared formulations showed an ascending drug release rate.The release characteristics of the tablets were compared by calculating the statistically derived mathematical parameter“similarity factor”(f2)[24].Fraction released data for this purpose to standardize the percentage of drug release for the labeled amount of paliperidone present in the tablets.If the similarity factor(f2)was≥50,it was considered there were similarities between the two dissolution pro files.
In thein vitrodissolution study,the self-made compressioncoated tablets and the marketed tablets Invega®had a good similarity,and so could be considered equivalent to Invega®in vitro.In order to study the effect of different pH values on drug release behaviors of the marketed tablets and the compression-coated tablets,the dissolution test in different pH values was also studied.Fig.6 and Fig.7 shows dissolution profiles of the compression-coated tablets and the commercially available tablets under different conditions reported in standard documents.
3.4. Weight gain and erosion behavior
Studying matrix hydration and erosion by gravity analysis is a valuable method for directly understanding the relative importance of the mechanism of release and participation parameters[25].Calculating the matrix erosion rate according to the formula ‘2.2.4’,the first step in the dissolution process should be solvent in filtration,where the compression-coated layer is hydrated and expanded.The resultant change of thickness of the swollen rubbery region of the tablets(the change of tablet volume with time)is shown in Fig.9.In general,swelling properties could be attributed to water absorption,which resulted in polymer swelling and led to a decrease in polymer concentration and increased macromolecule mobility[26].When water penetrates into the tablets,the water molecules are inserted into the hydrogen bonds between the molecular chains of the material,and as more water inserts between the chains,the interaction between the chains is gradually reduced.The molecular chains are then able to freely rotate and occupy more space,resulting in swelling of the tablet.The slow erosion of the tablets made with HPMC can be attributed to their wetting,porosity and or structure characteristics which retard water diffusion into the polymer.For PAL CC tablets,from 0 h to 4 h,there was concurrent swelling and erosion.From 4 to 12 h,the matrix erosion increased significantly,however at this point absorption of water by the matrix slows down,which may be due to thickening of the gel layer,which reduces the rate of further diffusion of water to the core.The hydrophilic gel matrix initially was solid polymer,however in filtration of water slowly turned the polymer into a semi-solid to form a gel layer,and with the continuous in filtration of water,the polymer material partly transformed into a solution state dissolved in the releasing medium,and thus erosion.The gel eroded to approximately 42%at 12 h.After 12 h,although the trend of matrix erosion had slowed,the trend of water absorption increased.With a hydrophilic material used as the matrix,when the prepared tablet was in contact with water,the water could continuously diffuse into the film due to the large water content around the sheet and the inside.The tablet surface hydration formed a gel layer,and so the matrix gradually swelled,showing a gradual increase in water absorption.Fig.8 shows the results of the erosion and absorption studies of the compressioncoated tablets.
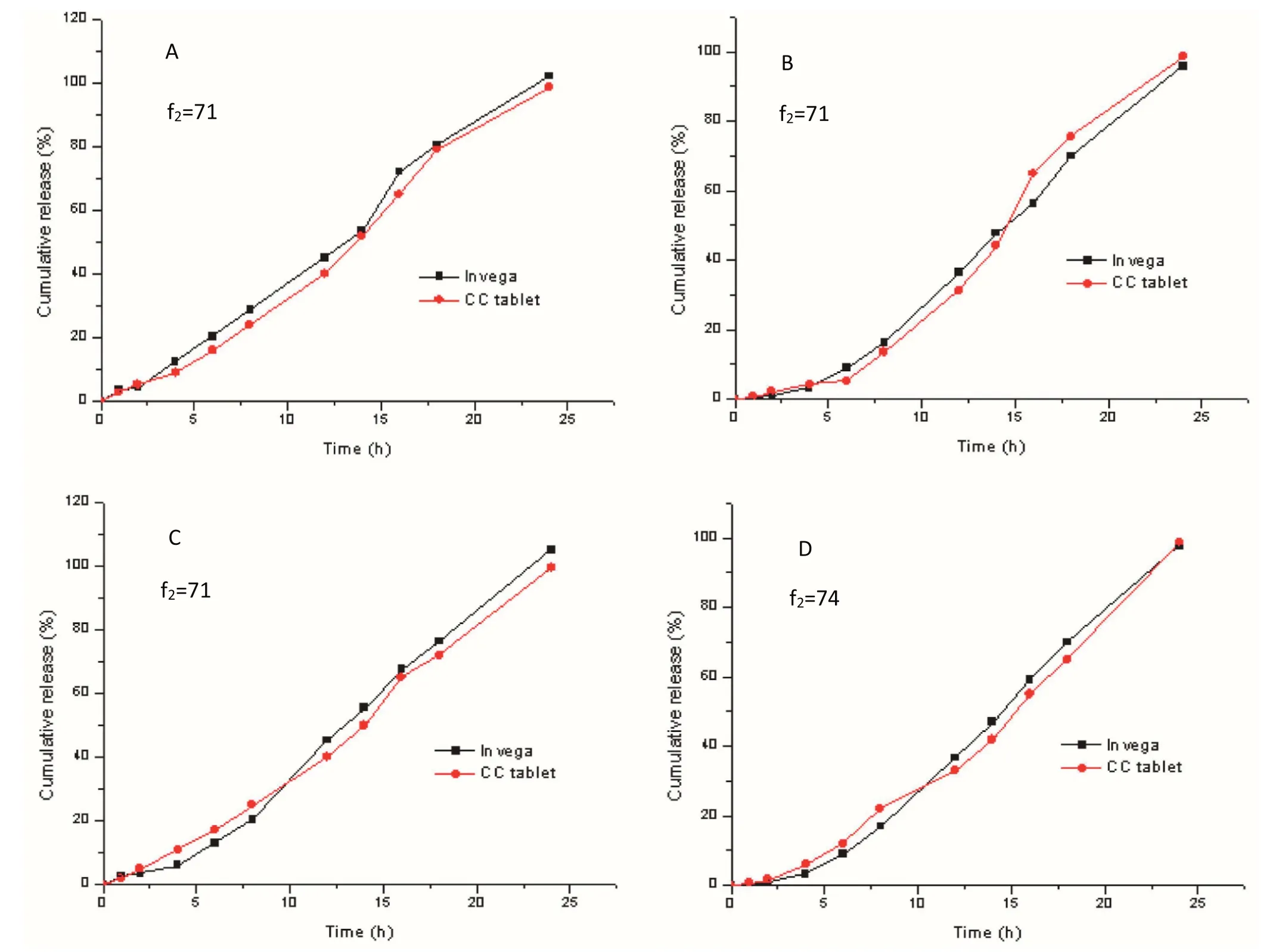
Fig.6–Dissolution pro files comparing the compression-coated tablets and Invega®at different pH dissolution mediums:(A)0.2%NaCl,pH 1.0 HCl medium,(B)pH 4.5 acetate medium,(C)pH 6.8 phosphate medium and(D)distilled water(Mean±SD,n=12).
3.5. Drug release mechanism
When water-soluble polymer was used as the gel material for the sustained-release tablets,the drug was wrapped,dissolved and dispersed in the macromolecule polymer.The release rate of the drug was determined by the erosion rate of the polymer and the rate of drug diffusion.In the process of drug release,along with the change of the matrix shape,the dissolution of the matrix material,the thickness of the gel layer,the length of the diffusion path,and the dynamic process is complicated.The hydration and swelling of HPC and HPMC play an important role in controlling drug release rate[27].The drug release curve shows that there is no burst release in the early stages with the water-soluble hydrophilic gel matrix sustainedrelease tablets[28].It is apparent that the drug release mechanism was erosion and diffusion based on the above dissolution release results,however in order to investigate the mechanism exactly,thein vitrorelease curve of PAL sustained release tablets was further studied by the Korsmeyer–Peppas equation.Korsmeyer–Peppas can analyze both Fickian and non-Fickian drug release from swelling as well as nonswelling[29].The results of release kinetics of PAL from the CC tablets are shown in Table 3(The cumulative release of Q<0.6 was used to calculate the n value).For a cylindrical skeleton,when n < 0.45,the release was controlled by Fick’s diffusion,while for n>0.89,the release was controlled by erosion,with a synergistic effect of drug diffusion and matrix erosion when 0.45<n<0.89.In this case,the exponent n was 0.767,and thus,the drug release of PAL CC tablets was controlled by erosion and diffusion with possibly more contribution from erosion.
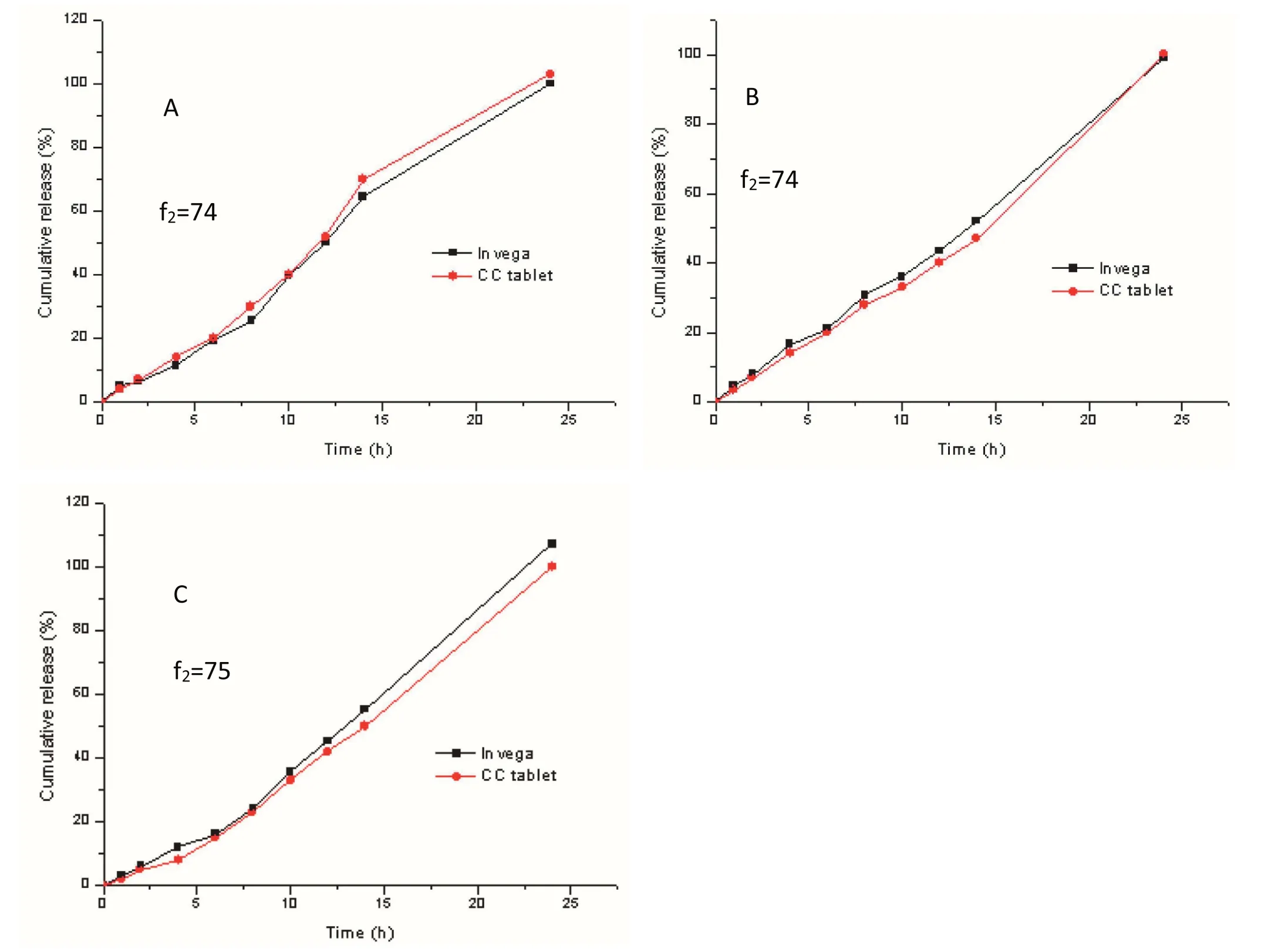
Fig.7 – The dissolution pro files of the compression-coated tablets and Invega® in 0.2%NaCl,pH=1.0 HCl medium at 37°C:(A)for paddle method 100 rpm and 500 ml,(B)for paddle method 50 rpm and 900 ml,(C)for basket method 50 rpm and500 ml(Mean±SD,n=12).
For PAL CC tablets,the drug content of the core layer and the coated layer was different,with the outer layer containing less drug than the inner layer.Initially,the outer drug began to release,when the tablets hydrated to a certain extent afterapproximately 4 h,did the inner drugs begin to gradually release into the medium.When the sustained-release tablets came into contact with water,drugs contained within the tablets surface released into the medium,HPMC hydration resulted in a gellike state,the tablets swelled and formed a gel barrier layer around the outside,and thus the drug diffusion path increased.Therefore,before the tablets were fully hydrated in the first 4 h,there was decreasing drug release.As the gel layer continued to hydrate,at around 4 h,the tablets swelled to the maximum volume,after which erosion was the main action.Although the matrix expanded at this stage increasing resistance to drug release,the tablet matrix gradually hydrated and eroded and so part of the drug could slowly spread to the gel layer and dissolve in the medium.Over 4 to 12 h period,the rate of erosion increased,however because of the previous hydration of tablets and volume expansion,at this stage there was ascending release overall.Between 6–10 h erosion rate was relatively stable,in the 10–12 h period,the rate of erosion continued to increase,and the water absorption capacity also increased.In 6 to12 h,the outer gel was in the continuous erosion,but the core hydrated,swelled,so this stage of drug release rate unchanged.It was proved in Fig.10 as well that the slope kept unchanging.At 12 h,the core also swelled to the maximum volume.After 12 h,the core tablets also began to erode,and the drug concentration in the tablets increased.Although the erosion rate decreased,the structure of the sustained-release material was relatively inactive,and so tended to have the effect of matrix overall erosion.The rate of water penetrating into the material was much faster than the rate of molecular chain decomposition of materials.At this time,the tablet was rapidly in filtrated by water,and erosion not only affected the tablet surface but also acted on the inside of the tablets.The gel layer transformed from the initial wound state to a gradually increasing solvated state,due to the relaxation of the polymer,blocking of the release was poor,and so the drug release rate accelerated significantly in the later stage.Fig.10 shows the slope kept increasing during this period.The mechanism of drug release was the combination of diffusion and erosion.The key point of ascending release was increasing rate of erosion and the water absorption capacity of gel.
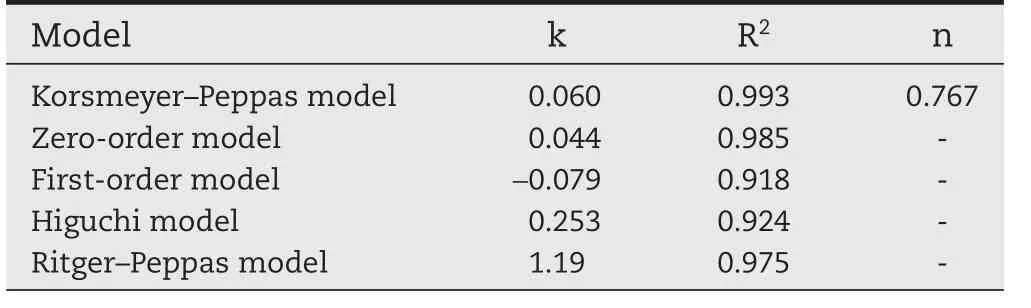
Table 3–Model fitting for the release mechanism of drug from the CC tablets.
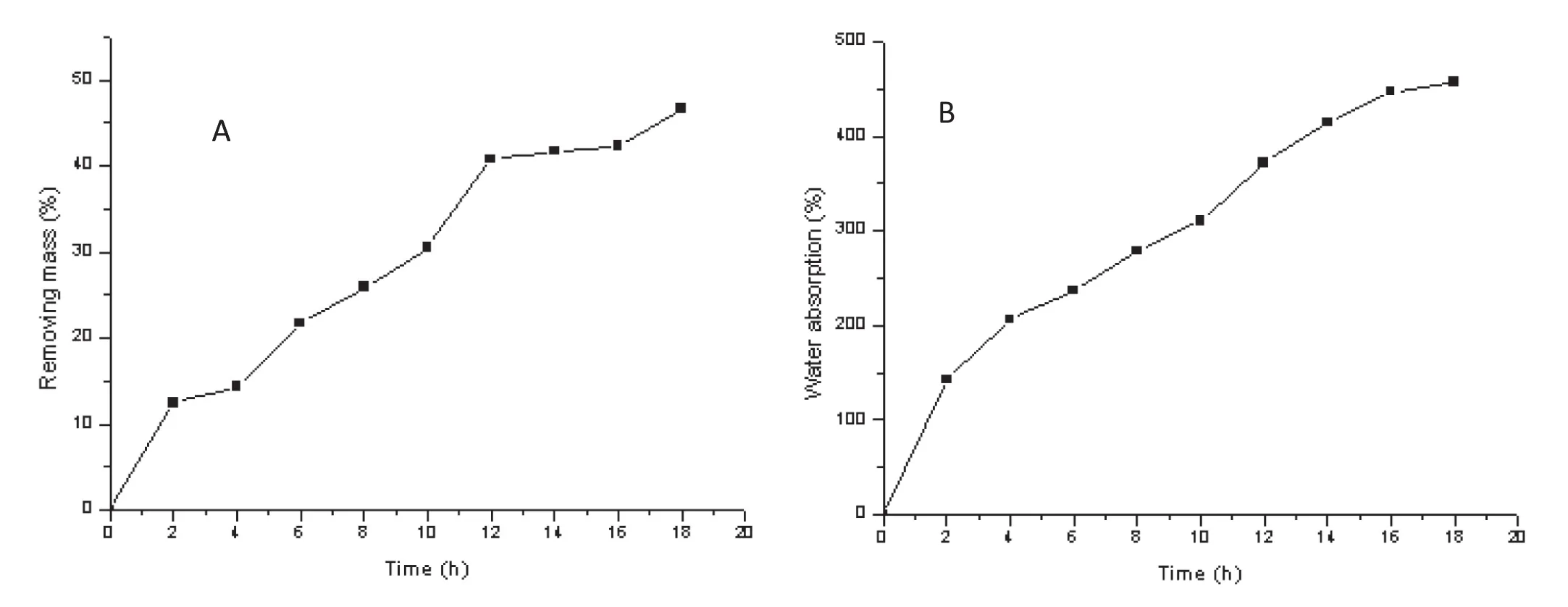
Fig.8–The behavior of(A)matrix erosion and(B)water uptake study of the compression-coated tablets under the conditions of pH 1.0 hydrochloric acid medium at 37°C(Mean±SD,n=6).
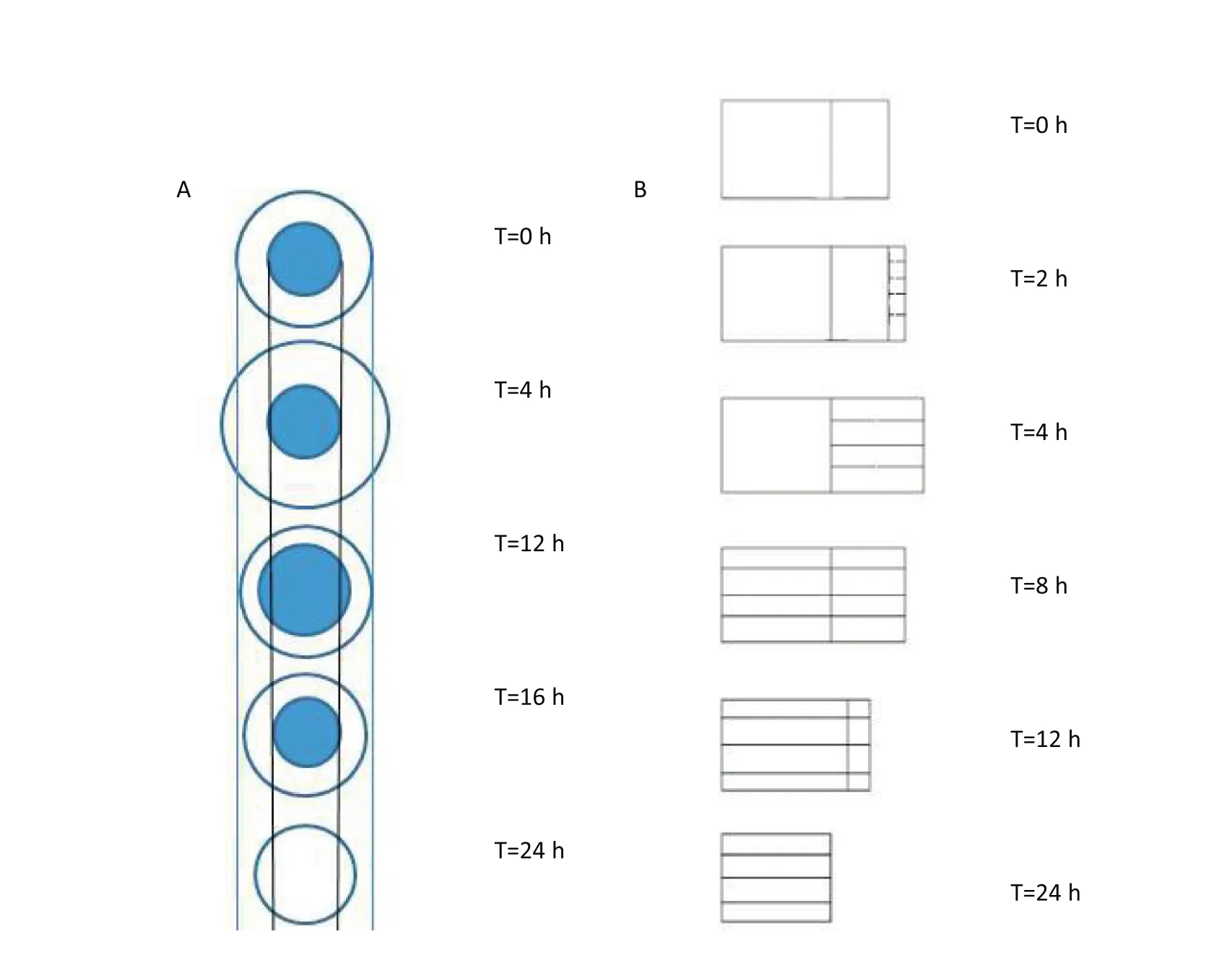
Fig.9–The change of thickness of the swollen rubbery region and erosion of tablets with time(The shaded portion of Fig.9B represented the hydrated portion of the gel).
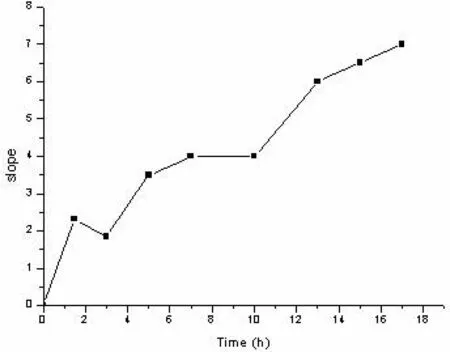
Fig.10–The changing rate of slope for PAL CC tablets dissolution under the conditions of pH 1.0 hydrochloric acid medium at 37°C.
4. Conclusion
This work successfully used the compression-coated technique to obtain PAL ascending release tablets,utilizing HPC-H as the inner polymer and HPMC-K100M as the outer polymer,with Eudragit RL-PO to control drug release.A series of influencing factors were investigated.It was observed that the particle size of PAL,the viscosity grade and amount of HPC and HPMC,and the position of the core tablets affected the PAL release ratein vitro.Forin vitrodissolution,PAL CC tablets had medium pH independence,medium volume independence and dissolution method and speed independence.The use of citric acid and sodium alginate was able to overcome the pH dependency of PAL.The tablets could achieve similar release behavior with osmotic pump tablets and required only a simple production process.The ascending release mechanism of these compression-coated tablets was found to be solvent penetration into the CC tablets and drug dissolution from the erosion of the gelatinous HPC and HPMC matrix.In conclusion,the compression-coated tablets are a potential drug delivery system for achieving ascending release.With the development of innovative compression equipment,the CC tablet can achieve industrial production.
Conflict of interest
There is no potential conflict of interest.
This work was funded by National Natural Science Foundation of China(No.81673378).
[1]Citrome L.Oral paliperidone extended-release:chemistry,pharmacodynamics,pharmacokinetics and metabolism,clinical efficacy,safety and tolerability.Expert Opin Drug Metab Toxicol 2012;8:873–88.
[2]Nussbaum AM,Stroup TS.Oral paliperidone for schizophrenia.Cochrane Database Syst Rev 2012;(3):CD006369.
[3]Yang LPH.Oral paliperidone.CNS Drugs 2011;25:523–38.
[4]Marino J,Caballero J.Paliperidone extend-release for the treatment of schizophrenia.Pharmacotherapy 2008;28:1283–98.
[5]Conley R,Gupta SK,Sathyan G.Clinical spectrum of the osmotic-controlled release oral delivery system(OROS),an advanced oral delivery form.Curr Med Res Opin 2006;22:1879–92.
[6]Malaterre V,Ogorka J,Loggia N,et al.Approach to design pushepull osmotic pumps.Int J Pharm 2009;376:56–62.
[7]Li G,Wang Y,Chen H.Can semipermeable membranes coating materials influence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet:In vitro evaluation and in vivo pharmacokinetics study.A J Pharm Sci 2015;10:128–37.
[8]Owen RT.Extended-release paliperidone:efficacy,safety and tolerability pro file of a new atypical antipsychotic.Drugs Today(Barc)2007;43:249–58.
[9]Lin SY,Kawashima Y.Current status and approaches to developing press-coated chronodelivery drug systems.J Control Release 2012;157:331–53.
[10]Kohlrausch A.Multilayer tablet.US Patent 2005;0(186):274.
[11]Picker KM.Influence of tableting on the enzymatic activity of different[alpha]-amylases using various excipients.Eur J Pharm Biopharm 2002;53:181–5.
[12]El-Malah Y,Nazzal S.Preparation of delayed release tablet dosage forms by compression coating:Effect of coating material on theophylline release.Pharm Dev Technol 2010;15:305–10.
[13]Riddhish P,Chintan V,Karan M.Quality by design empowered development and optimisation of timecontrolled pulsatile release platform formulation employing compression coating technology.AAPS Pharm Sci Tech 2017;18:1213–27.
[14]Wei X,Lu Y,Qi J,et al.An in situ crosslinked compression coat comprised of pectin and calcium chloride for colonspecific delivery of indomethacin.Drug Deliv 2015;22:298–305.
[15]Lopes CM,Sousa Lobo JM,Pinto JF,et al.Compressed matrix core tablet as a quick/slow dual-component delivery system containing ibuprofen.AAPS Pharm sci tech 2007;8:195–202.
[16]Siepe S,Lueckel B,Kramer A,et al.Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH.Int J Pharm 2006;316:14–20.
[17]Huang H,Wu Z,Qi X.Compression-coated tablets of glipizide using hydroxypropylcellulose for zero-order release:In vitro and in vivo evaluation.Int J Pharm 2013;446:211–8.
[18]Nagata S,Jin-nai A,Hirai K,et al.Evaluation of dissolution of osmotic-controlled release paliperidone tablets using the reciprocating cylinder method.Yakugaku Zasshi 2012;133:405–10.
[19]Lu E,Jiang Z,Jiang X.Preparation of controlled release coated tablets of naproxen sodium.Chin Pharm J 2002;37:841–4.
[20]Efentakis M,Koligliati S,Vlachou M.Design and evaluation of a dry coated drug delivery system with an impermeable cup,swellable top layer and pulsatile release.Int J Pharm 2006;311:147–56.
[21]Ritger PL,Peppas NA.A simple equation for description of solute release.II.Fickian and anomalous release from swellable devices.J Control Release 1987;5:37–42.
[22]Siepmann J,Peppas NA.Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose(HPMC).Adv Drug Deliv Rev 2001;48:139–57.
[23]Nakano M,Ohmori N,Ogata A,et al.Sustained release of theophylline from hydroxypropylcellulose tablets.J Pharm Sci 1983;72:378–80.
[24]Costa P,Sousa Lobo JM.Modeling and comparison of dissolution pro files.Eur J Pharm Sci 2001;13:123–33.
[25]Durig T,Fassihi R.Guar-based monolithic matrix systems:effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics.J Control Release 2002;80:45–56.
[26]Siepmann J,Streubel A,Peppas NA.Understanding and prediction drug delivery from hydrophilic matrix tablets using the sequential layer model.Pharm Res 2002;19:306–13.
[27]Alderman DA.A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms.Int J Pharm Tech Prod Mfr 1984;5:1–9.
[28]Xiao H,Brazel CS.On the importance and mechanism of burse release in matrixcon-trolled drug delivery systerm.J Controlled Release 2001;73:121–36.
[29]Korsmeyer RW,Gurny R,Doelker EM,et al.Mechanisms of solute release from porous hydrophilic polymers.Int J Pharm 1983;15:25–35.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Combretastatin A4/poly(L-glutamic acid)-graft-PEG conjugates self-assembled to nanoparticles
- Development of lamellar gel phase emulsion containing baru oil(Dipteryx alata Vog.)as a prospective delivery system for cutaneous application
- Preparation and toxicity evaluation of a novel nattokinase-tauroursodeoxycholate complex
- Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery
- Quantification and spatial distribution of salicylic acid in film tablets using FT-Raman mapping with multivariate curve resolution
- Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel
