Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel
2018-03-28ThwtchiPhechmudSiMyoThureinbTkronChntdee
Thwtchi Phechmud*,Si Myo Thureinb,c,Tkron Chntdee
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000,Thailand
bDepartment Pharmaceutical Engineering,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000,Thailand
cDepartment of Pharmacognosy,University of Pharmacy,Mandalay,Myanmar
1. Introduction
The periodontitis treatment is highly challenging and it needs an effective pharmaceutical dosage form because drug partitioning into the periodontal pockets is generally not very pronounced and gingival crevicular fluid flows rapidly to eliminate the drug from the site of action[1].Injectablein situforming gel(ISG)is a particularly effective drug delivery system.Before administration,thisin situforming system is a sol form and when administered gradually it alters to a gel form or solid-like depot[2].ISG is one of the promising local delivery systems because of the prospect of maintaining effective high levels of drug in the gingival crevicular fluid for a prolonged period of time to gain the desirable clinical benefits[3].
For solvent exchange-induced ISG,the aqueous insoluble polymer and drug are initially dissolved in a water-miscible organic solvent with low toxicity[4].The model drug used in the present study is doxycycline hyclate(DH).DH is a broad spectrum antibiotic and used widely in periodontal treatment.It is bacteriostatic and it disturbs bacterial protein synthesis[5].It is actively against Gram-negative and Grampositive bacteria,including beta-lactamase producing strains which occurred in deep periodontal pockets.Atrigel®is the commercial injectable system used for a periodontal therapy containing PLA or PLGA dissolved inN-methyl pyrrolidone(NMP)([6,7].The challenge in the ISG system is to modulate the burst release effect.The high burst release can be minimized by adding the hydrophobic plasticizers[8].In this study,clove oil(CO)was selected as a hydrophobic substance of ISG.CO is essential oil extracted fromEugenia caryophyllata(Syzygium aromaticum)which includes the main properties such as antioxidant,insecticidal,antifungal and antibacterial properties.Its major components,eugenol,has been reported for an antimicrobial activity[9].Furthermore,CO has been used as a natural antibacterial agent against cariogenic and periodontopathogenic bacteria[10].CO-loaded orabase preparations showed a biphasic controlled release[11].When CO was added into the zinc oxide gel,the antimicrobial activity of the prepared gel was increased as the CO amount was increased[12].
Eudragit RS(ERS),a copolymer composed of poly(ethyl acrylate,methyl methacrylate,trimethyl ammonioethyl methacrylate chloride)(1:2:0.1),was used as polymer in formulation of ISG of this study.This compound has a low permeability property and it can swell in aqueous media independently of pH without dissolving[13].Moreover,5%w/w quaternary ammonium groups present in this compound play an important role for its hydration:attracting water into the system.It is generally regarded as a nontoxic and nonirritant material[13].Therefore,this quaternary polyacrylate positively charged polymer has been widely used to form a waterinsoluble film for the sustained-release dosage forms and enteric coated tablets.In situnovel intragastric controlledrelease formulation of hydrochlorothiazide was prepared using Eudragit as polymer[14].Moreover,ERS was used as matrix former of extended-release aspirin tablets[15].In addition,ERS had been used as a polymer in the formulation of sustained release matrix prepared by a hot-melt extrusion technique[16,17]or microsphere[18].Dexamethasone-loaded ERS nanoparticles sustained the drug to the skin and the corneal epithelium with no toxicity potentialsex vivo[19].However,the burst drug release was evident in ERS solutions comprising benzoyl peroxide,DH and metronidazole as the active compounds[20,21].We found that ESR could dissolve in NMP and also CO;therefore,this was interesting to investigate the influence of CO on the physical properties of injectable DH-loaded solvent exchange-induced ISG system using ERS as a polymer.
This investigation applied ERS as a polymeric matrix for DH-loaded solvent exchange induced-ISGs for periodontitis treatment.The influence of CO incorporation on the DH-loaded ISGs was investigated for physical properties and a biological action including pH,viscosity,rheology,syringeability,gel formation,rate of water diffusion into the gels and antimicrobial activities againstStaphylococcus aureus,Escherichia coli,Candida albicans,Streptococcus mutansandPorphyromonas gingivalis.
2. Materials and methods
2.1. Materials
ERS(EVONIK RöhmGsmbH,Germany)and CO(PC Drug,Bangkok,Thailand)were used as received.DH(batch no.20071121;Huashu Pharmaceutical Corporation,Shijiazhuang,China)was used as model drug.NMP(lot no.A0251390;Fluka,New Jersey,USA)were used as solvent for ERS and CO.Agarose(Lot H7014-14,Vivantis Technologies Sdn,Bhd,Malaysia)was used as received.For antibacterial susceptibility test,the disc containing ampicillin 10µg/disc(Becton Dickinson&Company,USA)was used as positive control and clotrimazole(kindly supported from T.MAN Pharma Ltd.,Part,Bangkok,Thailand)of 10µg/cup was used as a positive control for an antifungal test.Brain Heart Infusion(BHI)(lot no.0270845;BactoTM,USA),Brain Heart Infusion Agar(BHA)(lot no.0298038;BactoTM,USA),MitisSalivarius Agar(MSA)(lot no.0118681;DifcoTM,USA),Sabouraud Dextrose Agar(SDA)(lot no.7312647;DifcoTM,USA),Sabouraud Dextrose Broth(SDB)(lot no.6345690;DifcoTM,USA),Tryptic Soy Agar(TSA)(lot no.7341698;DifcoTM,USA),andTryptic Soy Broth(TSB)(lot no.8091999;DifcoTM,USA)were used as media for an antimicrobial test.Potassium dihydrogen orthophosphate(lot no.E23W60;Ajax Finechem,Australia)and sodium hydroxide(lot no.AF 310204;Ajax Finechem,Australia)were used as component in phosphate buffer pH 6.8 to simulate the gingival crevicular fluid[22].Dialysis tube(Spectra/Por® membrane,molecular weight cut-off 6000–8000,lot no.32644;Spectrum Laboratories,Inc.,CA,USA)was used as received.
2.2. Preparation of ISGs
The 30%w/w ERS was dissolved in NMP and CO individually.The different concentrations of ERS(30,35,40%w/w)were also dissolved in different ratios of NMP and CO containing 5%DH.Then these polymer solutions were kept for 24 h to obtain the clear solutions.The 5%DH dissolved in NMP and CO without addition of ERS was used as the control formulation in this study.The composition of the formulations is shown in Table 1.
2.3. Evaluations
2.3.1.Appearance
The appearance of the prepared formulations including color and homogeneity was observed by visual observation.
2.3.2.Viscosity and rheological behavior studies
Viscosity properties of the prepared systems were determined using Brook field DV-III Ultra programmable rheometer(Brook field Engineering Laboratories Inc.,Middleboro,MA,USA).The CP-40 spindles with different shear rates of 20–180 1/sec were used with a 15 1/sec equilibration time at every shear rate(n=3).This test was performed at 25 and 37°C,which was of the room and physiological temperature,respectively.The shear stress of samples was measured at various shear rates at 25 and 37 °C.The temperature was maintained within ±0.1 °C by water bath(Buchi Heating bath B-490,New Hampshire,USA)connected to the sample cup of viscometer.The samples were equilibrated on the plate to reach the running temperature prior to each measurement.
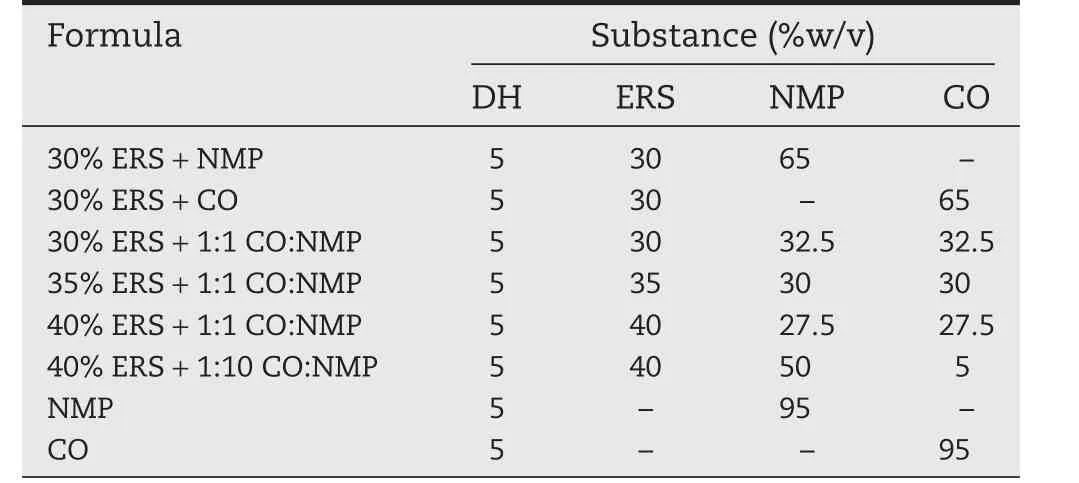
Table 1–Composition of DH-loaded solvent exchange induced-ERS ISGs.
2.3.3.In vitro gel formation
The determination ofin vitrogel formation was the change of clear solution formulation into turbid gel or a solid-like form when injecting the sample into the simulated gingival crevicular fluid.The gel formation was assessed by injecting the 1 ml sample into 5 ml phosphate buffer solution pH 6.8(to simulate the gingival crevicular fluid)[23]with 18-gauge needle and observed this transformation visually at various times(0,0.5,1,3,5,7 and 9 min).
2.3.4.Syringeability test
Because of administration with injection ease of administration was carried out by syringeability test.Syringeability of the systems is an important factor to consider for the ease of administration by injection which is the force required expelling the prepared product via a needle.Syringeability test was performed by using texture analyzer(TA.XT plus,Stable Micro Systems,UK)in compression mode.The sample was filled into 1 ml syringe with 27-guage needle and then this needle was clamped with a stand.The administration using a small needle size is known to cause less pain at the injection site,thereby improving patient comfort and compliance[24,25].The upper probe of the texture analyzer moved downward until it came into contact with the syringe barrel base.A constant speed of 1.0 mm/sec and constant force of 0.1 N were applied.The distance required to expel the contents for a barrel length of 20 mm was measured and recorded.The procedure was carried out at room temperature and all the measurements were performed in triplicate.Force displacement pro files were determined and the force at a distance of 10 mm was selected for analysis.The area under the resulting curve was used to determine the work of expulsion.
2.3.5.Rate of matrix formation
The water diffusion rate into the prepared systems was performed by measuring the distance of the change from sol into the turbid gel phase in a transparent tube.This test was carried out by filling the samples into a transparent tube with 6 mm diameter and then the tube was immersed into PBS pH 6.8.The distance of water front diffusion was observed,according to the apparent system that would change from being transparent to opaque when the water diffused into the gels,at 4 and 24 h.Subsequently,the diffusion rate was calculated(n=3)using the following formula;

2.3.6.Solvent diffusion study
For checking the characteristic of solvent diffusion,an experiment was set up to simulate an artificial periodontal pocket by using agarose and observed under stereo microscope(Motic SMZ-171 Series).Brie fly,agarose was dissolved in boiling water(0.6%w/v),and the solutions were poured into a Petri dish(diameter 4.5 cm).At the center of the gels,cylindrical holes(diameter 6 mm)were made and filled with 150µl liquid formulation using a standard syringe.To simply analyze,all samples were dyed with amaranth(0.1 g/10 ml).During the solvent exchange,the change was captured with stereo microscope every 5 min within 30 min.Solvent fronts were measured for 5 zones in triplicate.The rate of solvent diffusion into agarose gels was correlated as follows:

2.3.7.Determination of surface morphology
The prepared ISMs were investigated with an environmental scanning electron microscopy(ESEM)(FEI,Quanta 450,Netherlands)at −10 °C with moisture of 50%RH by allowing for a gaseous environment in the specimen chamber.
2.3.8.In vitro drug release study
The dialysis membrane method is used for the evaluation ofin vitrodrug release studies.About 1 g sample was weighed and added into a dialysis tube(Spectrapor,MW cutoff:6000–8000).The medium used was 100 ml phosphate buffer pH 6.8.The temperature was maintained at 37°C with the rotational speed at 50 rpm using shaking incubator Model SI4(Shel Lab,Cornelius,USA).Aliquot,10 mL each,was withdrawn from the release medium at time intervals and the withdrawal amount of aliquot was replaced with fresh medium.The amount of samples was determined by UV–vis spectrophotometer at 320 nm.All of the experiments were triplicated,and the mean cumulative drug release±S.D.was calculated.
2.3.9.Antimicrobial study
For antimicrobial activities test,standard microbes(S.aureusATCC 6538P,E.coliATCC 25922 andC.albicansATCC 17110)and anaerobic microbes(S.mutansATCC 27175 andP.gingivalisATCC 33277)were used.The agar-cup diffusion method was used and the broth culture was prepared with the turbidity approximately 108 cells/ml.Then,the swab was spread onto the agar plate and allowed to dry.Upon the surface of the seabed agar,the initially sterilized cylinder cups were carefully placed.After filling of the prepared gels into the cylinder cup(8 mm diameter and 10 mm height),it was allowed to be incubated at 37°C for 24–48 h.For anaerobic bacteria,the test was conducted in an anaerobic incubator(Forma Anaerobic System,Thermo Scientific,Ohio,USA).The antimicrobial susceptibility test disc containing ampicillin 10µg/disc was used as positive control for an antibacterial test, and clotrimazole of 10 μg/cupwas used as positive control for an antifungal test.The antimicrobial activities were measured as the diameter(mm)of inhibition zone(n=3).
2.3.10.Statistical analysis
Statistical significance of the measurements was examined using the one-way analysis of variance(ANOVA)followed by the least significant difference(LSD)post hoc test.The significance level was set atP<0.05.The analysis was performed using SPSS for Windows(version 11.5).
3. Results and discussion
NMP is miscible with CO and both of them could dissolve ERS;therefore,DH-loaded solvent exchanged induced-ERS ISG could be prepared in a solution form.All DH-loaded formula showed the clear bright yellow solution.There was no drug precipitation for formula in which NMP was used as a solvent whereas there was DH powder dispersed homogeneously in formula in which only CO was used as solvent due to the insolubility of DH in CO.
3.1. Viscosity and rheological behavior
The effect of polymer loading and the ratio of NMP and CO on the viscosity were investigated.The formula without an addition of ERS was less viscous and not measured for their viscosities whereas those containing ERS were viscous solutions.40%ERS+1:1 CO:NMP showed the highest viscosity and followed by 35%ERS+1:1 CO:NMP and 40%ERS+1:10 CO:NMP,respectively,and 30%ERS+CO>30%ERS+1:1 CO:NMP>30%ERS+NMP as shown in Fig.1.Therefore,ERS solution using higher NMP amount exhibited rather lower viscosity.The NMP is the ideal solvent used in ISG because of its solubility power and low toxicity[26,27].However,with the incorporation of CO into the ERS solution,the viscosity of the solution was significantly increased.40%ERS+1:1 CO:NMP demonstrated the highest viscosity as a result of CO which was a poor solvent for highest amount ERS,thus the polymer–polymer interactions were favored,leading to the formation of aggregates and an increased viscosity of polymer solution.In a good solvent,polymer–solvent interactions are dominant resulting in polymer extension and stretching,while polymer–polymer interactions are promoted in a poor solvent causing polymer coiling up and a minimized environmental viscosity[28,29].The low viscosity was evident in a high amount of NMP systems with lower ERS concentration such as 30%ERS+NMP.Moreover,the apparent viscosity of all ISGs was increased when the concentration of ERS was increased.Therefore,in comparison,40%ERS+1:1 CO:NMP comprising higher amount of CO and polymer displayed the notably and significantly higher viscosity than other formula(P<0.05).
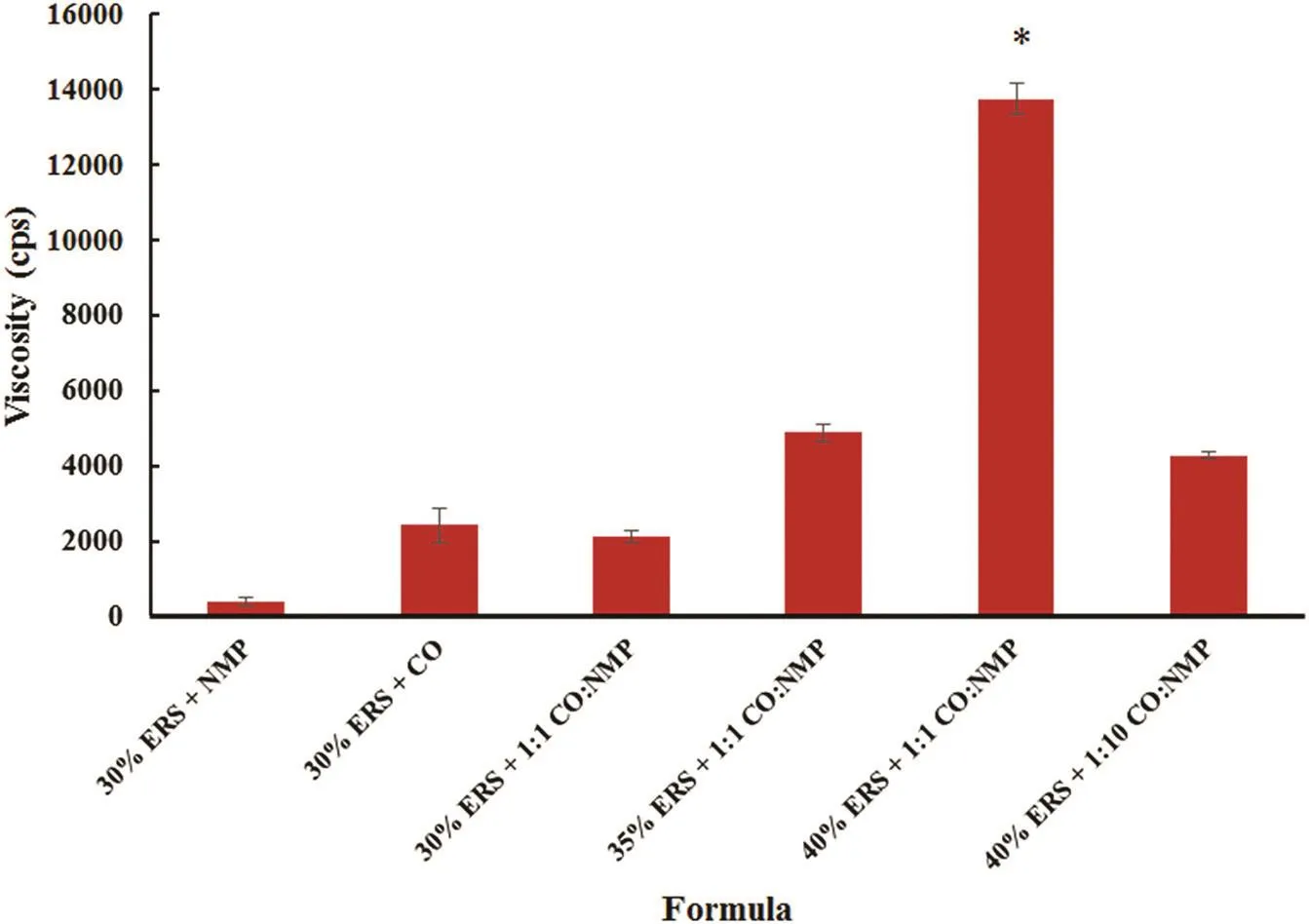
Fig.1–Viscosity of DH-loaded solvent exchange induced-ERS ISGs(n=3).The asterisk(*)represents a significant difference(P<0.05).

Fig.2–Flow curve of DH-loaded solvent exchange induced-ERS ISGs at(A)25 °C and(B)37 °C.Trianglerepresent the up-curves,and the diamondrepresents the down-curve.
The rheological behaviors of DH-loaded formula were investigated as a function of polymer amount and temperature.The shear stress against shear rate flow curves are shown in Fig.2.All formulations exhibited the Newtonian behavior,indicating the up curve did coincide with the down curve.In this study,the shear stress of all formula increased owing to the increasing of a polymer amount in the gel.The curve moved to a higher shear stress value was due to the compact structure of the formulations.On the other hand,the effect of temperature on the rheological behavior of the solution was also investigated.Temperature did not change the flow behavior of the systems but only decreased the shear stress.The viscosity measurement was determined at 25 °C and 37 °C to understand its viscosity and flow behaviors during the administration by injection at room temperature and after injection into gingival crevicular fluid of the periodontal pocket in a human body,respectively[30].Typically,the viscosity of solution is decreased as the temperature is higher;therefore,the viscosity of systems at 37 °C was lower than that at 25 °C.The shear stress of the formulations also decreased at 37°C.Moreover,the sharp decrease in the shear stress at 37°C indicates the loose structure at a higher temperature[30].The shear stress of formulation containing 40%of ERS was relatively higher than the formula comprising 35%and 30%ERS,respectively.This rheological behavior supports the result of the viscosity test.
3.2. In vitro gel formation
Typically the ISG should remain as liquid formulation but on injection it may undergo a gelation because an exchange of NMP and gingival fluid happens.When the solvent exchange occurred,the gel would be changed as an opaque mass or solidlike.Formula comprising 30%ERS and NMP transformed slowly from fluid into turbid matrix at the bottom of the test tubes within 30 sec after being injected into the PBS solution(Fig.3).Typically,the higher the polymer concentration the faster the precipitation rate[31].Formula containing a higher amount of ERS(35%and 40%)transformed into gel immediately when contacted with PBS solution and it floated near the surface of the PBS owing to its rapid transformation.The gelation rate therefore depended on the concentration of polymer in ISG.30%ERS+CO did not transform from liquid into gel because the hydrophobicity of CO prevented the solvent exchange.The ratio of CO to NMP also affected the onset of the gel formation.As the amount of ERS was increased,the resultant gel became opaque and more solid-like.The buoyancy of gel was evident in formula containing 35%and 40%ERS because the high amount of ERS.30%ERS+1:1 CO:NMP did not change into gel owing to the discontinuation of the polymer network[31],while formula containing 35%and 40%ERS showed the faster separation rate into matrix with lower density than the medium owing to lower density of CO.Therefore,DH-loaded formula comprising a higher amount of ERS demonstrated the rapid and complete transformation.According to thein vitroinjection experiments,it was suggested that the DH-loaded ISG comprising high amount of ERS or lower amount of CO could be easily injected and formedin situgel immediately.
3.3. Syringeability
In the development of ISG,an important issue also lies in the ease of injection[25,32].Syringeability is the force required to expel the product through the needle.This used injection speed in this experiment was similar to the previous report[25].The force required to expel was nearly negligible when NMP alone was tested(Table 2).All the formulas displayed the significantly higher work of syringeability when the amount of polymer was increased as previously reported[33].Syringeability force was dependent on the viscosity,which could be attributed to linear relationship between force required to inject and viscosity of formula[34].40%ERS+1:1 CO:NMP showed the highest force of syringeability that was significantly higher than 40%ERS+1:10 CO:NMP and other formula(P<0.05)due to the high polymer loading and also the inclusion of CO.Nevertheless,all the prepared systems could expel through the needle for administration by injection.ERS has been reported for its ability of mucous adhesion[35,36];therefore,the obtained depot after solvent exchange could localize at the periodontal pocket for control of DH release.

Fig.3–In vitro matrix formation of DH-loaded solvent exchange induced-ERS ISGs at different times.

Table 2–Syringeability of DH-loaded solvent exchange induced-ERS ISGs and DH-loaded NMP(n=3).
3.4. Matrix formation and solvent diffusion
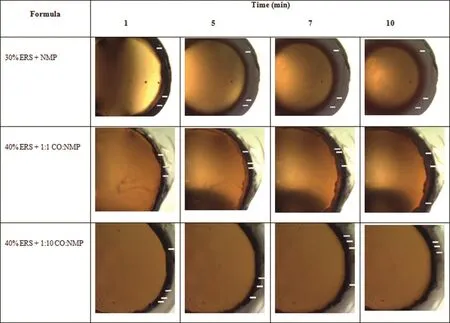
Fig.4–Transformation of DH-loaded solvent exchange induced-ERS ISGs after response to PBS pH 6.8 in agarose gel at different time intervals.
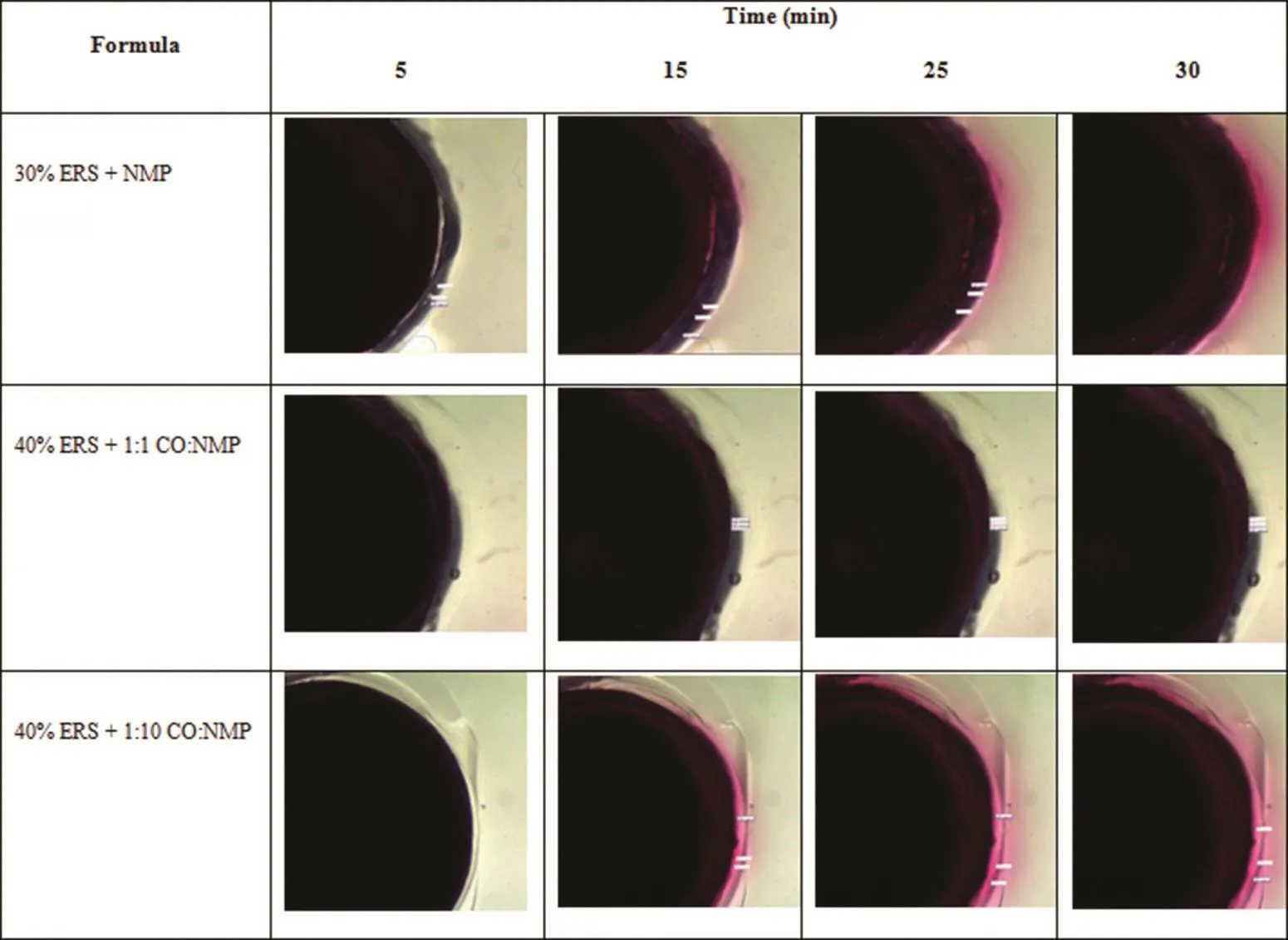
Fig.5–Diffusion of amaranth with NMP from DH-loaded solvent exchange induced-ERS ISGs into agarose gel prepared with PBS pH 6.8.
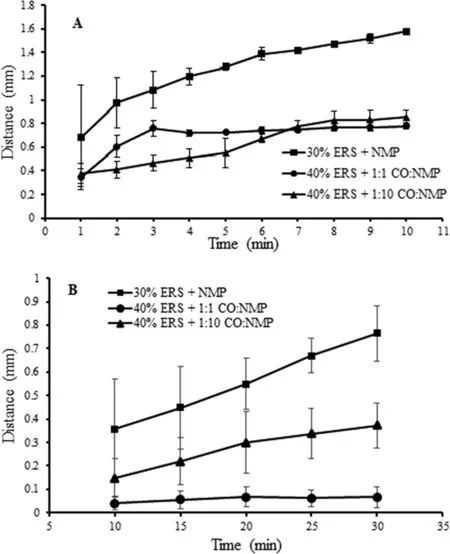
Fig.6–Relationship between distance of PBS diffusion into system(A)and NMP diffusion into agarose of DH-loadedsolvent exchange induced-ERS ISGs(B)(n=3).
The phase separation of ERS into polymeric matrix after solvent exchange of NMP from IGS with PBS from agarose gel was observed(Fig.4).The solvent diffusion is one of the major criteria of solvent exchange process which is important for the controlled drug release[37].This study was used to elucidate the transport mechanism using a simple image analysis technique.The amaranth font movement was used to indicate the NMP diffusion(Fig.5).For the diffusion of water into the formulations,the opaque region surrounding the yellow region represented the ERS matrix from the outer agarose medium into the inner formulations.The rate of matrix formation was observed in 30%ERS+NMP,40%ERS+1:1 CO:NMP and 40%ERS+1:10 CO:NMP.The increasing polymer amount made an increase of the viscosity of ISG which contributed to the decrease in water diffusion into the system[21,38].Owing to the lower amount of ERS in 30%ERS+NMP,the rapid exchange of NMP with water was observed.Therefore,this formulation showed the highest matrix formation rate with the smaller radius of inner solution well than the others(Fig.4 and Fig.6A).On the other hand,the dense area of the latter two formulations created the low water penetration coefficient and this made a lower diffusion rate[39].Moreover,the inclusion of a higher amount of CO in the prepared gel system resulted in an increased viscosity which could lead to the penetration of water into the system difficulty.Typically a rapid solvent extraction made the fast drug release rate[30,38].First,the rate of water diffusion of 40%ERS+1:1 CO:NMP seemed to be higher than that of 40%ERS+1:10 CO:NMP.However,the water penetration rate of 40%ERS+1:1 CO:NMP was decreased later and constant with time.This may be due to the fact that when the water penetrated into the solution,the ERS gradually transformed into the solid-like depot.This situation creates a thicker barrier with time for penetration of water into inner system.Although the aqueous penetration rate of 40%ERS+1:10 CO:NMP was slower at the beginning,it was not significantly different from 40%ERS+1:1 CO:NMP after 7 min.Nevertheless,it is apparently seen that all formulations containing CO created the barrier which made the water penetration difficult.Typically,low penetration rate is associated with the high retention rate with prolongation in drug release[40].
In this study,the diffusion of NMP was also investigated with the movement of the amaranth dyed ISG into the agarose gel under stereomicroscope.30%ERS+NMP showed the highest solvent front distance and diffusion rate(Fig.5 and Fig.6B).The aqueous diffusion and solvent exchange are the critical parameters on the drug release.The formula containing 35%and 40%ERS showed the slower diffusion of NMP into the surrounding agarose medium.Moreover,formulation comprising a higher amount of CO showed significantly slower solvent front diffusion region because the increased viscosity decreased the diffusion rate[41,42].It has been reported that the added hydrophobic plasticizer in the systems slowed down the water and solvent exchange rate[8,43].The theophylline release from pellets coated with latex aqueous dispersions of ethylcellulose(EC)or acrylic polymers was more prolonged with increasing hydrophobic plasticizer content[44].Therefore,the lowest solvent front distance occurred in formulation comprising a higher CO amount.
3.5. Surface morphology
ESEM has been used for characterizing the morphology of hydrogel in cooling condition[45,46].The moisture in the ESEM chamber could induce the transformation of system;therefore,40%ERS+1:10 CO:NMP exhibited the apparently more phase separation like the opaque spots on sample surface than 40%ERS+1:1 CO:NMP(Fig.7).CO incorporated formula had lower this opaque spots.The 35%ERS dissolved in NMP without CO addition was easily exposed to moisture thus the more huge transformation was found after an exposure to moisture.The experimentalin situwetting of water-sensitive mudrocks[47]and authigenic smectite[48]for water-clay reactions has been reported using an environmental scanning electron microscope.
3.6. In vitro drug release
Dialysis membrane method has been typically employed for testing drug release from ISGs[31,38,49].DH release pro file evaluated from this method is shown in Fig.8A.The drug release from ISGs was slower than DH solution.The DH solution using NMP as solvent without polymer addition showed the fastestburst like drug release.On the other hand,DH release from formula comprising 40%ERS showed slower drug release.The drug release rate from ISG comprising 40%ERS was lower owing to the physical entanglements between polymers to control the drug diffusion[30].Moreover,an addition of CO in 40%ERS notably prolonged the DH release as shown in Fig.8A.Carvedilol-loaded nanocapsules using ERS as a capsule wall exhibited a mucoadhesive retention time properties with controlled drug permeability across the sublingual mucosa in the presence of simulated saliva flux[50].Schematic of solvent exchange and formation of antibiotic agent-loaded ERS ISG is presented in Fig.8B.The type of solvent and the amount of polymer concentration are critical factors in determining the drug release of ISG[51].NMP promoted a rapid phase inversion associated with a high drug burst due to the formation of a porous rubbery gel structure.The high initial burst can be explained by the lag time between the immersion of ISG system in the release medium and the complete gelation,while the latter sustainable release can be attributed to the diffusion of the compounds through the polymeric matrix.However,the increased amount ERS could minimize the burst-release effect and could give the more sustainable drug release pro file.In the present study,the system comprising 40%ERS could minimize the burst release behavior and the more sustainable drug release was attained.Moreover,the specific properties of the network of polymer chains,e.g.,the chain length,their flexibility and mobility,their water uptake and swelling behavior,extent of plasticization,or potential interactions between polymer and drug,would all potentially affect the diffusion rates in the polymer matrix and the drug release rate[52].In contrast,without gelling agent,the complete release of the DH was evident in less than 3 h.When the prepared gel was injected into the buffer solution,the solvent exchange of NMP happened to form thein situgel almost immediately,whereas the hydrophobic substance limited the water entry into the matrix,delayed phase inversion and consequently the burst release was reduced by slowing down the solvent liberation[43].In the present study,the burst DH release was also controlled by the inclusion of CO.CO slowed down the entry of water and the drug diffusion,and it therefore minimized the burst release and delayed the drug release(Fig.8A).This study revealed that the ISG system significantly reduced a burst effect because of the retardation both of solvent and drug diffusions by an addition of CO.The obtained phase separated ERS/CO matrix as presented in Fig.8(B)could entrap DH inside and modulate the DH release.The increased CO amount in 40%ERS+1:1 CO:NMP promoted the more hydrophobic and viscous environment to retard the drug release.Therefore the lowering polarity of system with hydrophobic substance could prolong the drug release from the ISGs system.
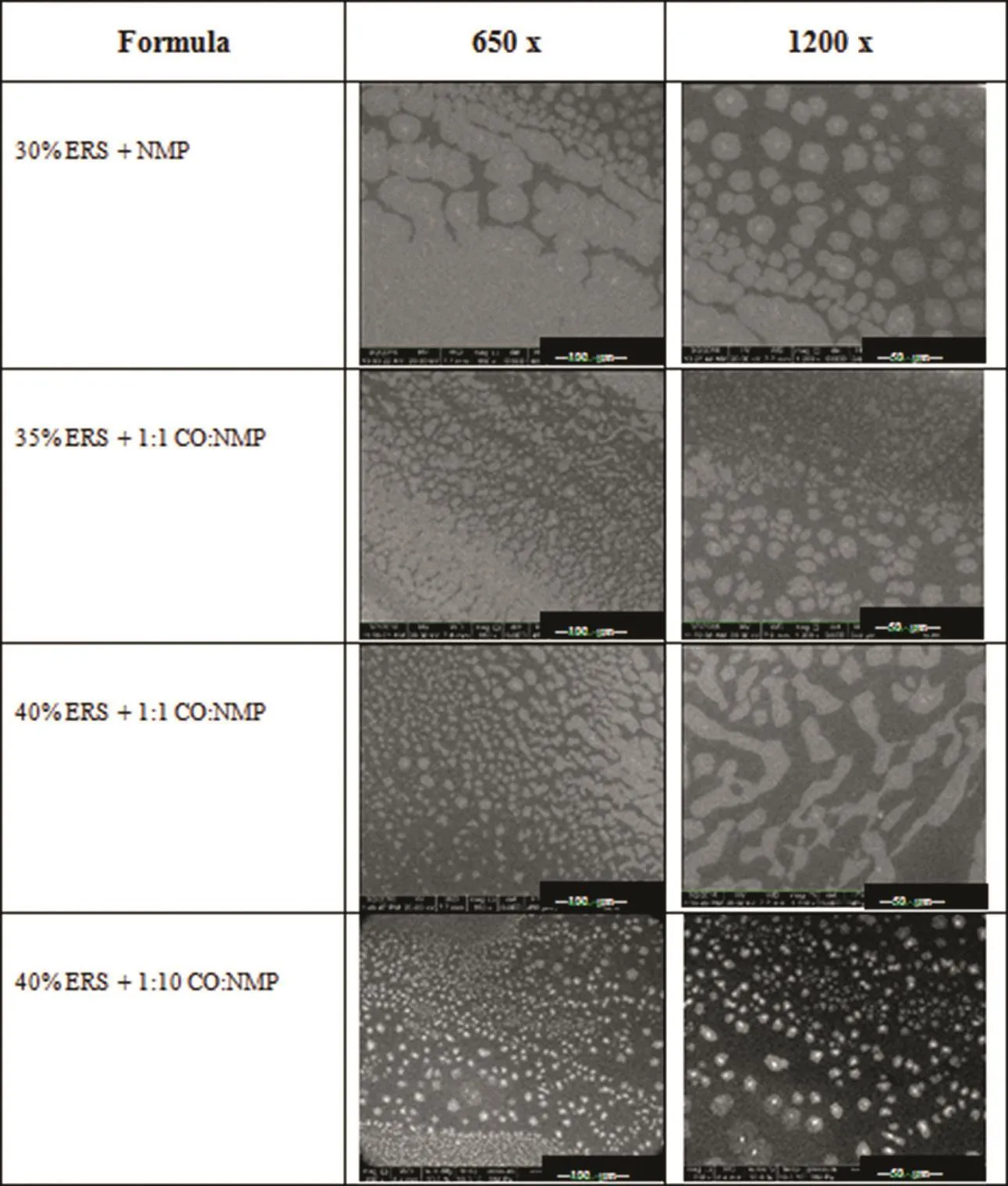
Fig.7–ESEM photomicrographs of DH-loaded solvent exchange induced-ERS ISGs at different magnifications.
3.7. Antimicrobial activities
The inhibition zone diameter against all bacteria of 40%ERS+1:10 CO:NMP was significantly higher than that of 40%ERS+1:1 CO:NMP as exhibited in Table 3(P<0.05)except for antifungal activity againstC.albicans.This larger bacterial inhibition zone of the first formulation was owed to the easier DH diffusion in this less viscous formulation and also due to the antimicrobial activity of higher amount of NMP[53,54].In the previous study,NMP-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel possessed the antimicrobial activities againstS.aureus,E.coliandC.albicanswith dose-dependent manner[53,54].NMP structure contains the five-membered lactam.Since the solubility parameter of NMP is similar to that of ethanol and DMSO[55],NMP could solubilize the lipid in cell membrane and promote the leakage of microbial cell membranes.Among all microbes,the inhibition zone diameter ofC.albicanswas the lowest because this organism was not susceptible to DH[30].Moreover,according to the previous study CO possessed antimicrobial activity againstC.albicans[12].Therefore comparing the two formulations,the inhibition zone diameter forC.albicanswas higher in formulation containing higher amount of CO.Owing to its hydrophobicity,CO rendered to partition the lipids of the bacterial cell membrane and mitochondria,disturbing the cell structure and rendering them more permeable[56,57].Consequently,the extensive leakage from bacterial cells or the exit of critical molecules and ions leads to the death of bacteria[58].CO has been used as a natural antibacterial agent against cariogenic and periodontopathogenic bacteria[10].Herbal preparations such as oil and toothpaste containing clove have shown an antifungal activity against a number of fungi includingC.albicansand act as a promising agent in the treatment of oral diseases and other infections[59,60].Thus,CO addition into DH-loaded ERS ISG will increasingly inhibit broad pathogen microorganism in periodontal pocket with a controlled release manner of DH from ERS matrix.
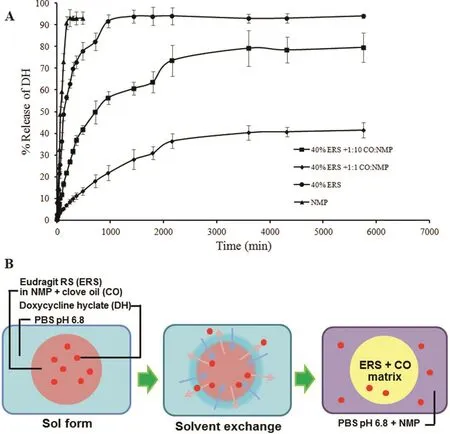
Fig.8–Release pro file of DH with different systems using dialysis method(n=6)(A)and schematic of solvent exchange and formation of DH-loaded solvent exchange induced-ERS ISGs comprising CO(B).
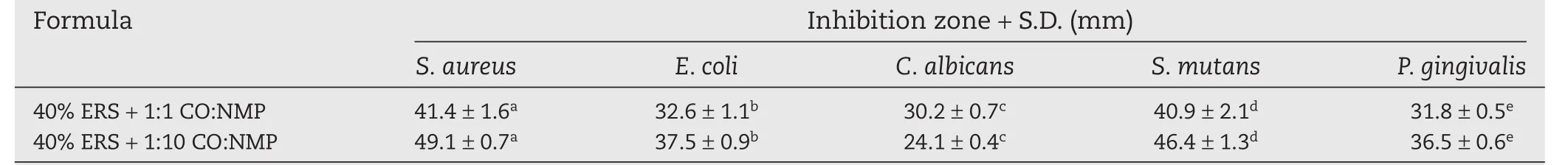
Table 3–Inhibition zone diameter of DH-loaded solvent exchange induced-ERS ISGs against different microorganisms(n=3).
4. Conclusions
Both NMP and CO could dissolve ERS;therefore,DH-loaded solvent exchanged induced-ERS ISG could be prepared in a solution form.The CO addition retarded the solvent exchange and prolonged the DH release from ERS ISG with minimization of burst release.Anin vitrotransformation from solution to matrix-like was more rapid when the ERS amount was increased while the diffusion rate of NMP and PBS was slower.The apparent sustainable drug release was obtained by using higher amount of ERS and CO.The developed injectable DH-loaded solvent exchange induced-ERS ISG comprising CO exhibited Newtonian flow and acceptable for expelling through the 27-gauge needle for administration by injection to periodontal pocket.In addition,they exhibited the effective inhibition ofS.aureus,E.coli,S.mutans,and P.gingivalis;therefore,DH-loaded ERS ISG comprising 1:10 CO:NMP was the potentially localized delivery system for periodontitis.
Conflicts of interest
The authors report no conflicts of interest in this work.
The researchers are grateful to the Research and Development Institute,Silpakorn University(Grant No.SURDI 58/01/38).This research work was also facilitated by the Faculty of Pharmacy,Silpakorn University,Thailand.We thank Dr.Kamonpan Boonkit for her English proof-reading and also Kalvalin Worrawethanakul and Thitirat Fuangwattana for their valuable comments and help.
[1]Pascale D,Gordon J,Lamster I,et al.Concentration of doxycycline in human gingival fluid.J Clin Periodontol 1986;13:841–4.
[2]Jigar V.A review on novel in situ polymeric drug delivery system.Int J Pharma Res Dev 2011;3(5):53–9.
[3]Medlicott NJ.Delivery systems for the administration of the drugs to the periodontal pocket.Adv Drug Deliv Rev 1994;13:181–203.
[4]Srichan T,Phaechamud T.Designing solvent exchangeinducedin situforming gel from aqueous insoluble polymers as matrix base for periodonttitis treatment.AAPS PharmSciTech 2017;18(1):194–201.doi:10.1208/s12249-016-0507-1.
[5]Seymour RA,Heasman PA.Tetracyclines in the management of periodontal diseases.A review.J Clin Periodontol 1995;22:22–35.
[6]Polson AM,Garrett S,Stoller NH,et al.Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis.II.Clinical results.J Periodontol 1997;68:119–26.
[7]Kranz H,Bodmeier R.Structure formation and characterization of injectable drug loaded biodegradable devices:in situimplants versusin situmicroparticles.Eur J Pharm Sci 2008;34:164–72.
[8]Snejdrova E,Dittrich M.Pharmaceutical applications of plasticized polymers.In:Luqman M,editor.Recent advances in plasticizers.InTech;2012 ISBN:978-953-51-0363-9.Available from:http://www.intechopen.com/books/recent-advances-in-plasticizers/pharmaceutical-applications-of-plasticizedpolymers.[Accessed 16 October 2016].
[9]Blaszy M,Holley RA.Interaction of monolaurin,eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms.Int J Food Microbiol 1998;39:175–83.
[10]Moon S-E,Kim H-Y,Cha J-D.Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria.Arch Oral Biol 2011;56(9):907–16.
[11]Labib GS,Aldawsari H.Innovation of natural essential oilloaded Orabase for local treatment of oral candidiasis.Drug Des Devel Ther 2015;9:3349–59.
[12]Mahadlek J,Charoenteeraboon J,Phaechamud T.Zinc oxide gels for periodontitis treatment.J Metal Mater Mineral 2010;20(3):159–63.
[13]Rowe RC,Sheskey PJ,Quinn EM.Handbook of pharmaceutical excipients.6th ed.Washington,DC:Pharmaceutical Press and American Pharmaceutical Association;2009.
[14]Patel RR,Patel JK.Development and evaluation of in situ novel intragastric controlled-release formulation of hydrochlorothiazide.Acta Pharm 2011;61:73–82.
[15]Ibric´S,Jovanovic´M,Djuric´Z,et al.The application of generalized regression neural network in the modeling and optimization of aspirin extended release tablets with Eudragit®RS PO as matrix substance.J Control Release 2002;82(2):213–22.
[16]Wu C,McGinity JW.Influence of methylparaben as a solidstate plasticizer on the physicochemical properties of Eudragit®RS PO hot-melt extrudates.Eur J Pharm Biopharm 2003;56:95–100.
[17]Park J-B,Lee B-J,Kang C-Y,et al.Process analytical quality control of tailored drug release formulation prepared via hot-melt extrusion technology.J Drug Deliv Sci Technol 2017;38:51–8.
[18]Hales D,Vlase L,Porav SA,et al.A quality by design(QbD)study on enoxaparin sodium loaded polymeric microspheres for colon-specific delivery.Eur J Pharm Sci 2017;100:249–61.
[19]Balzus B,Sahle FF,Hönzke S,et al.Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium.Eur J Pharm Biopharm 2017;115:122–30.
[20]Mahadlek J,Charoenteeraboon J,Phaechamud T.Benzoyl peroxidein situforming antimicrobial gel for periodontitis treatment.Key Eng Mater 2013;545:63–8.
[21]Phaechamud T,Jantadee T,Mahadlek J,et al.Characterization of antimicrobial agent loaded Eudragit RS solvent exchange-inducedin situforming gels for periodontitis treatment.AAPS PharmSciTech 2017;18:494–508.doi:10.1208/s12249-016-0534-y.
[22]Esposito E,Carotta V,Scabbia A,et al.Comparative analysis of tetracycline-containing dental gels:poloxamer and monoglyceride-based formulation.Int J Pharm 1996;142:9–23.
[23]Popa L,Ghica MV,Dinu-Pirvu C.Periodontal chitosan-gels designed for improved local intra-pocket drug delivery.Farmacia 2013;61:240–9.
[24]Arendt-Nielsen L,Egekvist H,Bjerring P.Pain following controlled cutaneous insertion of needles with different diameters.Somatosens Mot Res 2006;23:37–43.
[25]Supper S,Anton N,Boisclair J,et al.Chitosan/glucose 1-phosphate as new stable in situ forming depot system for controlled drug delivery.Eur J Pharm Biopharm 2014;88:361–73.
[26]Engelhardt G,Fleig H.Methyl-2-pyrrolidinone(NMP)does not induce structural and numerical chromosomal aberrationsin vivo.Mutat Res 1993;298:149–55.
[27]Jouyban A,Fakhree MA,Shayanfar A.Review of pharmaceutical applications ofN-methyl-2-pyrrolidone.J Pharm Pharm Sci 2010;13(4):524–35.
[28]Camargo JA,Sapin A,Nouvel C,et al.Injectable PLA-based in situ forming implants for controlled release of ivermectin a BCS Class II drug:solvent selection based on physicochemical characterization.Drug Dev Ind Pharm 2013;39:146–55.
[29]Kaufman HS,Falcetta JJ.Introduction to polymer science and technology.In:SPE textbook.New York:John Wiley&Sons;1977.p.1–268.
[30]Phaechamud T,Mahadlek J,Chuenbarn T.In situforming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment.Mater Des 2016;89:294–303.
[31]Abashzadeh S,Dinarvand R,Sharifzadeh M,et al.Formulation and evaluation of anin situgel forming system for controlled delivery of triptorelin acetat.Eur J Pharm Sci 2011;44:514–21.
[32]Xiong W,Gao X,Zhao Y,et al.The dual temperature/pH-sensitive multiphase behavior of poly(N-isopropylacrylamide-co-acrylic acid)microgels for potential application inin situgelling system.Colloids Surf B Biointerfaces 2011;84:103–10.
[33]Chang JY,Oh YK,Kong HS,et al.Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gel on vaginitis.J Control Release 2002;82:39–50.
[34]Rungseevijitprapa W,Bodmeier R.Injectability of biodegradablein situforming microparticle systems(ISM).Eur J Pharm Sci 2009;36:524–31.
[35]Pignatello R,Bucolo C,Ferrara P,et al.Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen.Eur J Pharm Sci 2002;16:53–61.
[36]Sudhakar Y,Kuotsu K,Bandyopadhyay AK.Buccal bioadhesive drug delivery–a promising option for orally less efficient drugs.J Control Release 2006;114:15–40.
[37]Samprovalaki K,Robbins PT,Fryer PJ.A study of diffusion of dyes in model foods using a visual method.J Food Eng 2012;110:441–7.
[38]Phaechamud T,Mahadlek J.Solvent exchange-induced in situ forming gel comprising ethyl cellulose-antimicrobial drugs.Int J Pharm 2015;494:381–92.
[39]Yamamoto S,Saeki T,Inoshita T.Drying of gelled sugar solutions–water diffusion behavior.Chem Eng J 2002;86:179–84.
[40]Wang L,Wang A,Zhao X,et al.Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism.Int J Pharm 2012;427:284–92.
[41]Levy G,Jusko WJ.Effect of viscosity on drug absorption.J Pharm Sci 1965;54:219–25.
[42]Patel VF,Patel NM.Statistical evaluation of influence of viscosity and content of polymer on dipyridamole release from floating matrix tablets:a technical note.AAPS PharmSciTech 2012;8(3):Article 69.Available from:http://www.aapspharmscitech.org.[Accessed 28 October 2016].
[43]Liu H,Venkatraman SS.Cosolvent effects on the drug release and depot swelling in injectable in situ depotforming systems.J Pharm Sci 2012;101(5):1783–93.
[44]Saettone MF,Perini G,Rijli P,et al.Effect of different polymer-plasticizer combinations on ‘in vitro’release of theophylline from coated pellets.Int J Pharm 1995;126:83–8.
[45]Constantin M,Bucatariu S,Harabagiu V,et al.Poly(N-isopropylacrylamide-co-methacrylic acid)pH/thermoresponsive porous hydrogels as self-regulated drug delivery system.Eur J Pharm Sci 2014;2:86–95.
[46]Hariyadi DM,Ma Y,Wang Y,et al.The potential for production of freeze-dried oral vaccines using alginate hydrogel microspheres as protein carriers.J Drug Deliv Sci Technol 2014;24:178–84.
[47]Huggett JM,Uwins PJR.Observations of water-clay reactions in water-sensitive sandstone and mudrocks using an environmental scanning electron microscope.J Pet Sci Eng 1994;10:211–22.
[48]Baker JC,Uwins PJR,Mackinnon IDR.ESEM study of illite/smectite freshwater sensitivity in sandstone reservoirs.J Pet Sci Eng 1993;9:83–94.
[49]Venkatesh MP,Anis S,Kumarv TM.Design and development of an injectable in situ forming drug delivery system of methotrexate for the treatment of rheumatoid arthritis.J Drug Deliv Sci Technol 2013;23:445–53.
[50]Chaves PS,Ourique AF,Frank LA,et al.Carvedilol-loaded nanocapsules:mucoadhesive properties and permeability across the sublingual mucosa.Eur J Pharm Biopharm 2017;114:88–95.
[51]Brodbeck KJ,DesNoyer JR,McHugh AJ.Phase inversion dynamics of PLGA solutions related to drug delivery:part II.The role of solution thermodynamics and bath-side mass transfer.J Control Release 1999;62(3):333–44.
[52]Wischke C,Schwendeman SP.Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles.Int J Pharm 2008;364:298–327.
[53]Phaechamud T,Mahadlek J.Analysis for texture and topography of doxycycline hyclate thermosensitive systems comprising zinc oxide.Indian J Pharm Sci 2013;75:385–92.
[54]Phaechamud T,Mahadlek J,Cgariebteerabiibm J,et al.Characterization and antimicrobial activity ofN-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel.Indian J Pharm Sci 2013;74:498–504.
[55]Hansen CM,Just L.Prediction of environmental stress cracking in plastics with Hansen solubility.Ind Eng Chem Res 2001;40:21–5.
[56]Jeyakumar E,Lawrence R,Pal T.Comparative evaluation in the efficacy of peppermint(Mentha piperita)oil with standards antibiotics against selected bacterial pathogens.Asian Pac J Trop Biomed 2011;S253–S257.
[57]Sikkema J,de Bont JAM,Poolman B.Interaction of cyclic hydrocarbons with biological membranes.J Biol Chem 1994;69:8022–8.
[58]Denyer SP,Hugo WB.Biocide-induced damage to the bacterial cytoplasmic membranes.In:Mechanisms of action of chemical biocides.Oxford:Blackwell Scientific Publications;1991.
[59]Yigit N,Aktas E,Ayyildiz A.Antifungal activity of toothpastes against oral Candida isolates.J Mycol Med 2008;18:141–6.
[60]Tyagi AK,Malik A.Liquid and vapour-phase antifungal activities of selected essential oils againstCandida albicans:microscopic observations and chemical characterization ofCymbopogon citratus.BMC Complement Altern Med 2010;10:65.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Combretastatin A4/poly(L-glutamic acid)-graft-PEG conjugates self-assembled to nanoparticles
- Development of lamellar gel phase emulsion containing baru oil(Dipteryx alata Vog.)as a prospective delivery system for cutaneous application
- Preparation and toxicity evaluation of a novel nattokinase-tauroursodeoxycholate complex
- Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery
- Quantification and spatial distribution of salicylic acid in film tablets using FT-Raman mapping with multivariate curve resolution
- Tablets of paliperidone using compression-coated technology for controlled ascending release
