Formulation design of granules prepared by wet granulation method using a multi-functional single-punch tablet press to avoid tableting failures
2018-03-28TkshiOsmurYoshikoTkeuchiRiskoOnoderMshiroKitmurYoshiteruTkhshiKoheiThrHirofumiTkeuchi
Tkshi Osmur,Yoshiko Tkeuchi,Risko Onoder,Mshiro Kitmur,Yoshiteru Tkhshi,Kohei Thr,Hirofumi Tkeuchi,*
aLaboratory of Pharmaceutical Engineering,Gifu Pharmaceutical University,1-25-4 Daigaku-Nishi,Gifu 501-1196,Japan
bPharmaceutical Technology Department,Sawai Pharmaceutical Co.Ltd,12-34,Hiroshibacho,Suita-Shi,Osaka 564-0052,Japan
1. Introduction
In our previous paper[15],we tried to evaluate all three properties by using the GamlenTablet Press (GTP-1; Gamlen Tableting Ltd.,Nottingham,UK),a benchtop single-punch tablet press,and demonstrated that the strength of the tablet(TFS)and the friction between die and tablet during ejection(ejection stress)can be used as an indicator of“Compactability”and“Manufacturability”,respectively.Weevaluated“Compactability”and“Manufacturability”by plottingTFS(i.e.,“Compactability”)on the x-axis against ejection stress(i.e.,“Manufacturability”)on the y-axis.We have empirically known that the critical tablet properties for commercial products,TFS and ejection force,are 2 MPa and 5 MPa,when compressed at a compaction pressure of 200 MPa.The tablets having these properties such as a TFS of 2 MPa or higher and an ejection stress of 5 MPa or lower,are suitable to manufacture stably and withstand the transportation and the use of end-user.Thus,we centered the intersection point of the two lines,where TFS(on the X-axis)equals 2 MPa and ejection stress(on the Y-axis)equals 5 MPa in the plot.As shown in Fig.1,this plotting makes it possible to visualize the quantitative characterization of“Tableting properties”,and thus to reach an optimum tablet formulation quickly.
We successfully predicted the effects of the amount of lubricant on “Tableting properties”(“Manufacturability”)and determined the appropriate amount and mixing time of lubricant in a formulation design by this plot [15,16].This evaluation method also proved that it is able to predict the results of commercial-scale tablet production regardless of punch shape[16].It could also detect subtle differences in the amount of lubricant,and predict sticking problems on a rotary tableting machine.Therefore,we assessed the utility of our method in the formulation design of tablets to prevent tableting failures.
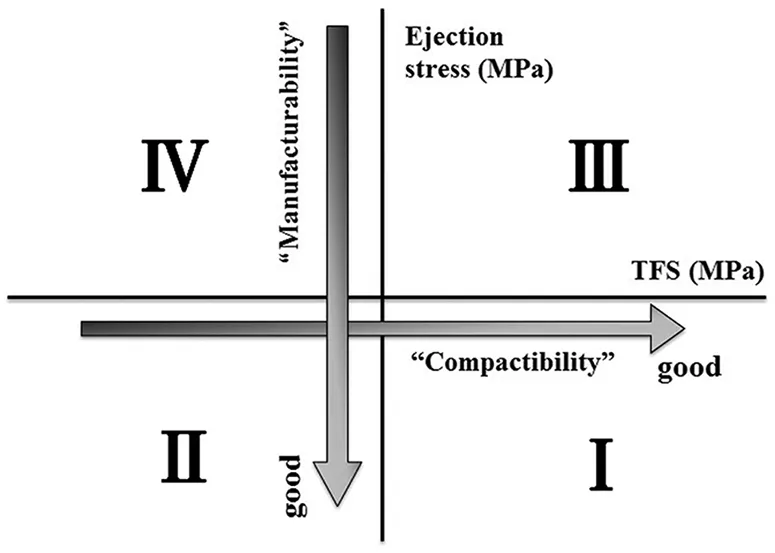
Fig.1 – Plot of“Tableting properties”.
In this study,we tried to apply this method considering the three factors, “Compressibility”, “Compactability”,and“Manufacturability”,with the single-punch tablet press(GTP-1)to set up the final formulation for a commercial tablet.The drug is called “active pharmaceutical ingredient A”(API-A),which is used as a treatment for osteoporosis.We designed a 240-mg tablet containing 60 mg API-A.As the primary component of API-A tablets is a fine powder with an average diameter of about 10µm,which is highly adhesive and has poor flowability,we used a wet granulation method for tablet production,in contrast to the direct compression method in our previous reports[15,16].In the early stage of formulation design,we formulated several tablets on a small scale and then reached the best formulations at the large scale.We also examined the usefulness of our evaluation method to improve “Tableting properties”during the scaling up of production.
2. Materials and methods
2.1. Materials
API-A has a melting point of about 259°C and a molecular weight of 510.04,and is prepared with an average particle diameter of about 10µm.As formulation additives,we bought anhydrous lactose(DCL21,DMV,The Netherlands),granulated lactose(Dilactose S,Freund Corporation,Japan),crospovidone(CPD:Polyplasdone XL-10,ISP Technologies,USA),povidone(PVP:K-30,Dai-ichi Kogyo Seiyaku,Japan),polysorbate 80(NikkolTO-10M,Nikko Chemical,Japan),and magnesium stearate(MgSt;Taihei Chemical,Japan).
2.2. Methods
2.2.1.Preparation of sample granules
Each 240 mg tablet contained 60 mg of API-A,along with anhydrous lactose and granulated lactose as vehicles,CPD as a disintegrant,PVP as a binder,polysorbate 80 as a solubilizing agent,and MgSt as a lubricant in the quantities shown in Table 1.First,API-A,anhydrous lactose,granulated lactose,and CPD(1)were mixed in a fluidized bed granulator.The flowing powder mixture was sprayed with a solution of PVP and polysorbate 80 and granulated in the fluidized bed granulator.The granules were dried and passed through a 22-mesh screen.They were then mixed with CPD(2)and MgSt in a rotary mixer to prepare the sample granules.
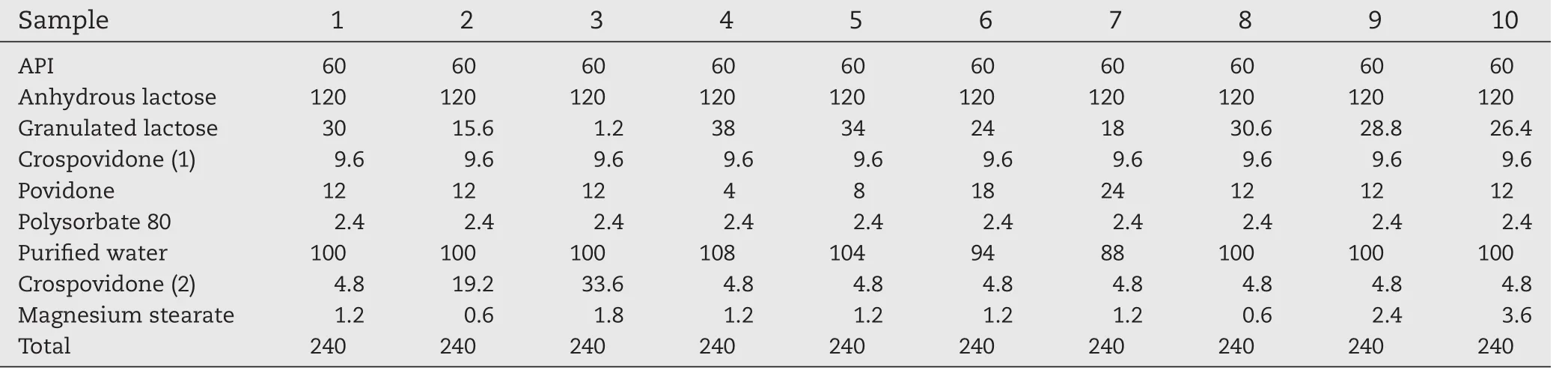
Table 1–Formulations of tablets(mg).
Sample granules were prepared at two manufacturing scales.At the small scale,720 g(3000 tablets’worth)was prepared in a small fluidized bed granulator(MP-01,Powrex,Japan)and a 5-L rotary mixer(VM-5 V-shaped blender,Tokuju,Japan).At the large scale,4800 g(20,000 tablets’worth)was prepared in a large fluidized bed granulator(FLO-5,Freund Sangyo,Japan)and a 30-L rotary mixer(VM-30 V-shaped blender,Tokuju).
徐浡君的风景油画的创作思想,深度建立在荒野哲学的基础之上。所谓荒野哲学,就是以其对生命和自然的深刻体悟、对美丽荒野的细致描绘、对家园毁损和生存危机的忧患意识、对现代生活观念的历史性反思,也被誉为“绿色哲学”。正是荒野哲学在一定意义上改变着整个人类的思想观念和生活方式,它在伦理学与自然、自然中的价值、实践中的环境哲学,以及体验中的自然等诸多方面,渗透出人与自然、人与环境、人与生态的全方位思考,对极端的人类中心主义无限掠夺自然的错误行径,予以深刻的叩问与批判,这就是徐浡君风景油画的哲学意蕴与文化学内涵。
2.2.2.Evaluation of“Tableting properties”using the GTP-1
The GTP-1 measures the upper punch pressure and displacement during compression,the ejection force(the friction between the die wall and the tablet during ejection),and the strength of the tablet(TFS)after ejection.To make a tablet,100 mg of powder is placed in the die of the GTP-1 and compressed at 4.9 kN by the upper punch(a flat punch 6 mm in diameter)at a fixed 30 mm/min.All formulations were pressed and measured three times.The methods of calculation and plotting are described in our previous report[15].
2.2.3.Evaluation of formulations on the rotary tableting machine
Samples were compressed on a rotary tableting machine(Virgo,Kikusui Seisakusho,Japan)in the formulations shown in Table 1.Each 240-mg tablet was compressed at 11 kN(in some cases at 16 or 20 kN)and 30 rpm,in an oval shape with a major axis diameter of 12 mm and a minor axis diameter of 6.5 mm.We set the target physical properties of tablets as a hardness of at least 60 N,a thickness of 4.40 mm,and a disintegration time in water of within 7 min.Hardness of 5 tablets was measured in the direction of the minor axis with a tablet hardness tester(PC-30,Okada Seiko,Japan).Thickness of 5 tablets was measured with a dial thickness gauge(MFG,Ozaki,Japan).Disintegration time of 6 tablets was tested with a disintegration tester(HM-61E,Toyama Sangyo,Japan)without the support disk according to the method described in the Japanese Pharmacopoeia.We also tested friability of 20 tablets in a tablet friability tester(Friabilator TFT-120,Toyama Sangyo,Japan),looking for cracking or capping after 1000 to 4000 rotations.
3. Results and discussion
3.1. Evaluation of“Tableting properties”using the GTP-1
Using our method for evaluating “Tableting properties”,we plotted TFS on the x-axis against ejection stress on the y-axis(Fig.1).When tablet hardness is sufficient,the point will be plotted on the positive side of the x-axis.When friction is negligible,the point will be plotted on the negative side of the y-axis.Therefore,range(I)indicates superior“Compactability”and “Manufacturability”.In contrast,range(IV)indicates tablet weakness and high friction on the die wall,meaning poor“Compactability”and “Manufacturability”.
3.1.1.Amount of disintegrant
We examined the effect of disintegrant on TFS.Each tablet contained CPD at 14.4 mg(6%)in Sample 1,28.8 mg(12%)in Sample 2,or 43.2 mg(32%)in Sample 3(Table 1).TFS was>2 MPa in Sample 1,and the point was plotted in range(III)(Table 2;Fig.2).TFS was<2 MPa in Sample 2 and <1 MPa in Sample 3,and the points were plotted in range (IV),indicating poor“Compactability”and,in Sample 3,insufficient hardness.
3.1.2.Amount of binder
We examined the effect of binder on TFS in formulations with 14.4 mg of disintegrant per tablet.Each tablet contained PVP at 4 mg in Sample 4,8 mg in Sample 5,12 mg in Sample 1,18 mg in Sample 6,or 24 mg in Sample 7(Table 1).TFS was<2 MPa in Samples 4 and 5,and the points were plotted in range(IV),indicating poor “Compactability”(Table 3;Fig.3).Therefore,≥12 mg of binder is needed to give sufficient hardness(Table 3;Fig.3).
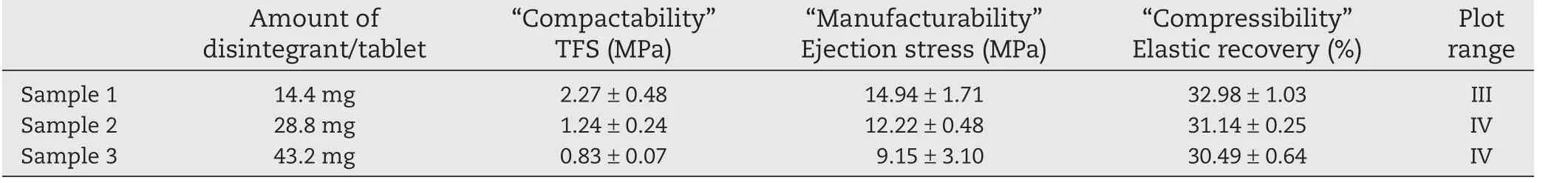
Table 2 –“Tableting properties”of formulations with different amounts of disintegrant,evaluated using benchtop singlepunch tablet press.
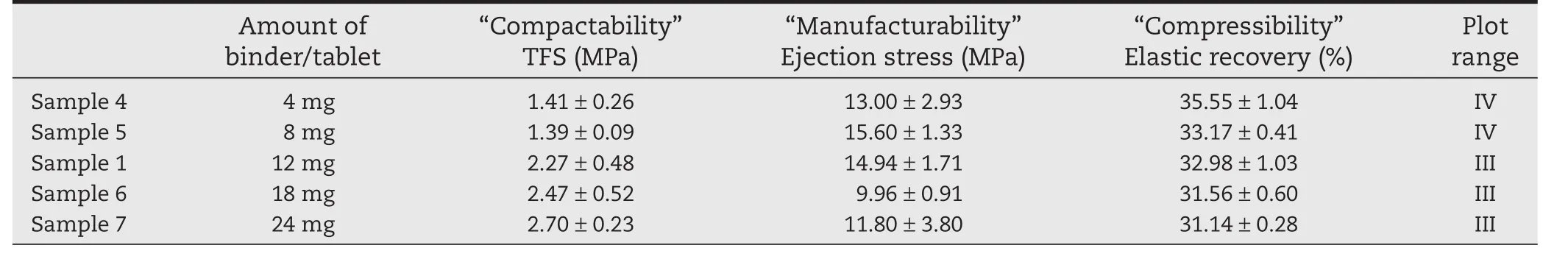
Table 3 –“Tableting properties”of formulations with different amounts of binder,evaluated using benchtop singlepunch tablet press.
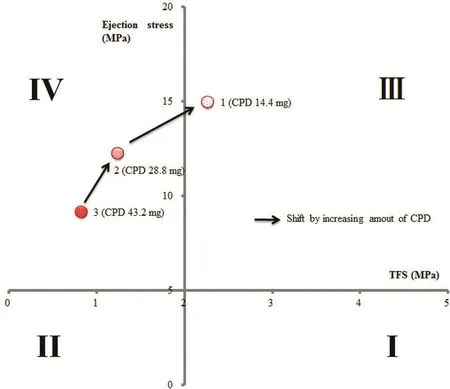
Fig.2 –“Tableting properties”of formulations with different amounts of disintegrant,evaluated using benchtop single-punch tablet press.
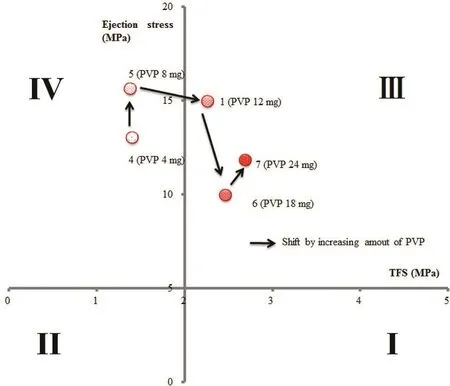
Fig.3 –“Tableting properties”of formulations with different amounts of binder,evaluated using benchtop single-punch tablet press.
3.1.3.Amount of lubricant
Formulations giving sufficient tablet hardness were Sample 1(12 mg PVP+14.4 mg CPD),Sample 6(18 mg PVP+14.4 mg CPD),and Sample 7(24 mg PVP+14.4 mg CPD).However,because Samples 6 and 7 contained a lot of binder,delayed disintegration time could be expected.We therefore further examined Sample 1-based formulations.
We have previously shown that it is advisable to design formulations to optimize both “Compactability”and“Manufacturability”,but the results of Samples1,6,and7showed high ejection stress(≥5 MPa),and the points were plotted in range(III),indicating poor“Manufacturability”.Insufficient MgSt causes tableting failures such as sticking and binding[16].To improve “Manufacturability”,we added different amounts of MgSt to Sample 1-based formulations.Each tablet contained 0.6 mg MgSt in Sample 8,1.2 mg MgSt in Sample 1,2.4 mg MgSt in Sample 9,and 3.2 mg MgSt in Sample 10.The ejection stress decreased as the amount of lubricant increased(Table 4;Fig.4).That of Sample 8was extremely high,and the sides of the tablets were deeply damaged during ejection.For this reason,TFS of Sample 8 was low.Samples9(2.4 mgMgSt)and10(3.6 mgMgSt)were plotted in range(I),indicating good“Compactability”and“Manufacturability”(Table 4;Fig.4).
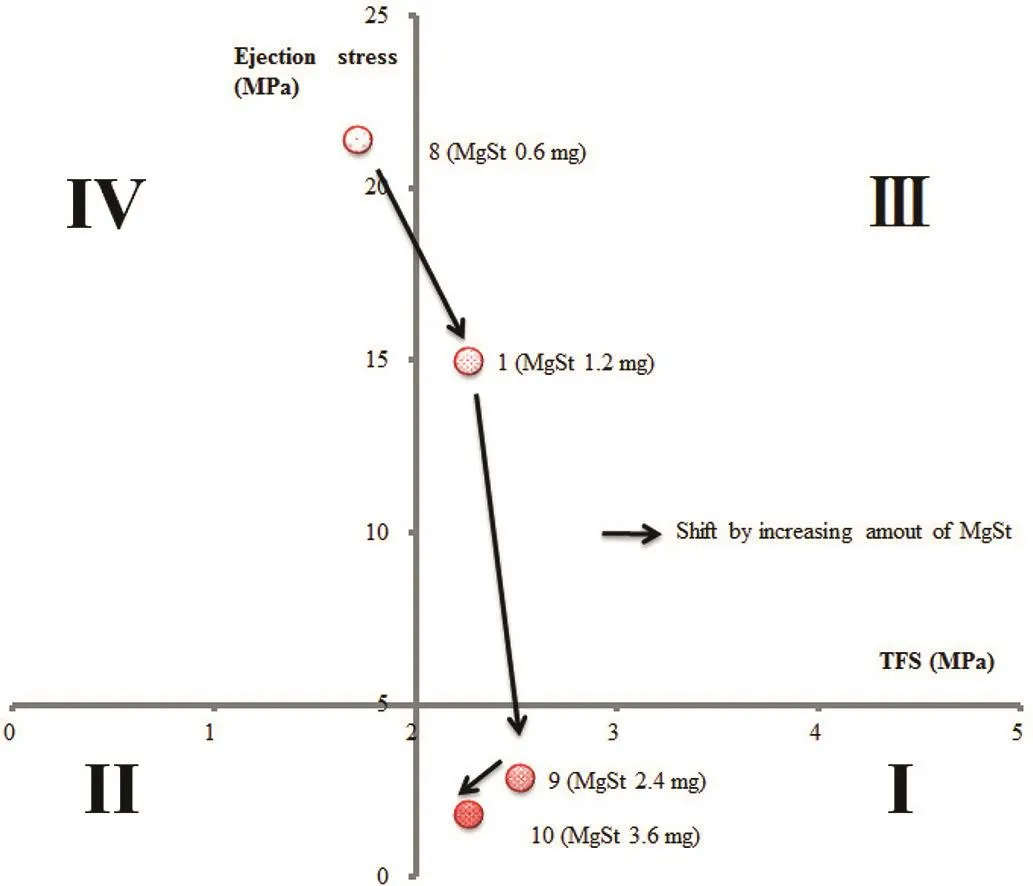
Fig.4 –“Tableting properties”of formulations with different amounts of lubricant,evaluated using benchtop single-punch tablet press.
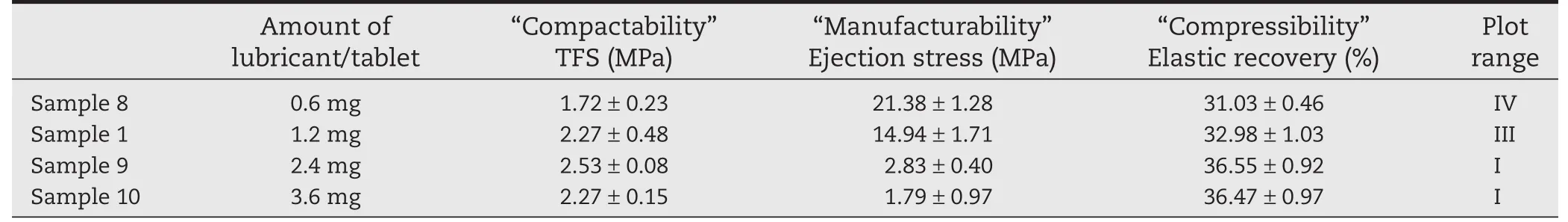
Table 4 –“Tableting properties”of formulations with different amounts of lubricant,evaluated using benchtop singlepunch tablet press.
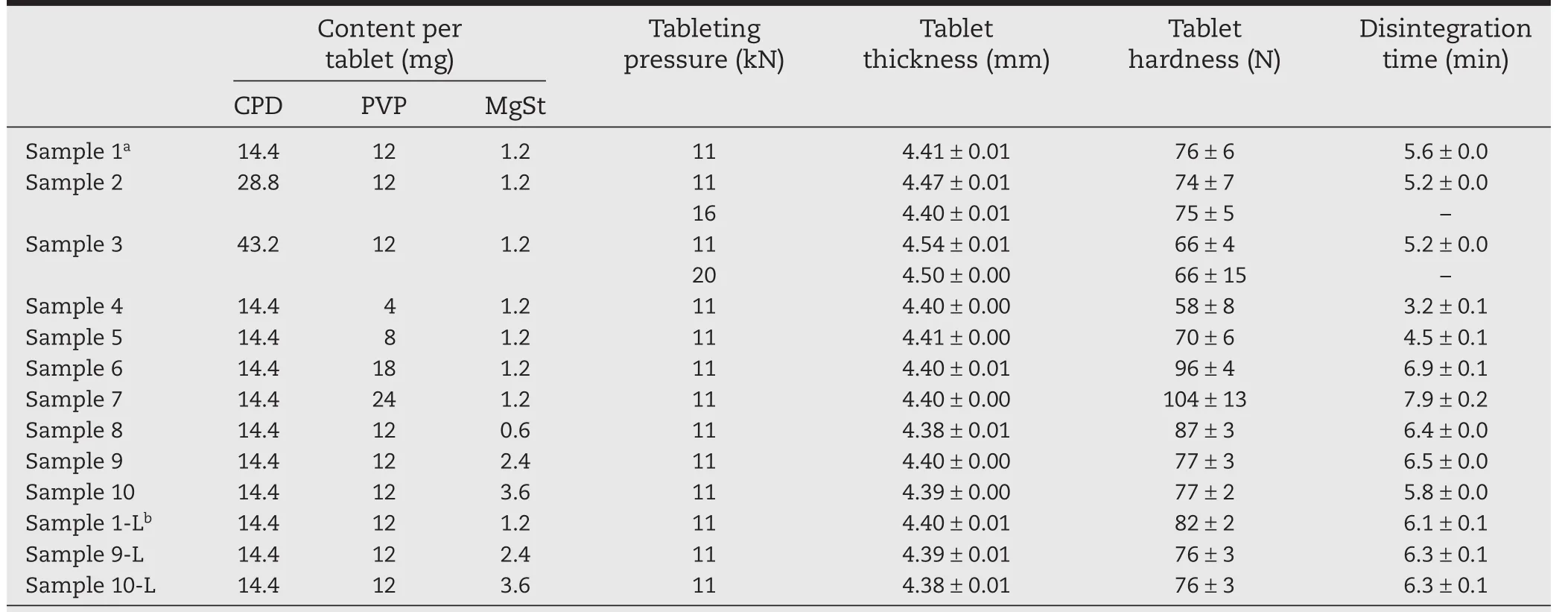
Table 5–Physical properties of tablets compressed by rotary tableting machine.
3.2. Evaluation of formulations on the rotary tableting machine
To validate the results described in Section 3.1,we prepared samples on a rotary tableting machine and tested the“Manufacturability”and physical properties of the tablets(Table 5).
3.2.1.Amount of disintegrant
Samples 2(28.8 mg CPD)and 3(43.2 mg CPD)showed poor“Compactability”in Section 3.1.On the rotary tableting machine,sample 1(14.4 mg CPD)reached the target tablet thickness(4.40 mm)when compressed at 11 kN(Table 5).However,Sample 2 exceeded the target thickness at 4.47 mm.To reduce the thickness to 4.40 mm,we had to increase the pressure to 16 kN.Sample 3 exceeded the target thickness even more at 4.54 mm,and was still 4.50 mm thick at 20 kN.All three samples disintegrated within the target of 7 min.All also reached the target hardness of 60 N,but Samples 2 and 3 tended to crack in capping layers(laminar separation)during hardness testing(Fig.5(A)).Tablets that crack in this way during transportation will split,potentially leading to capping.For this reason,we tested the friability of these samples(Table 6).Sample 1 did not crack in capping layers even after 3000 rotations.Sample 2 tablets compressed to 4.47 mm did not crack even after 3000 rotations,but among the tablets reduced to 4.40 mm,2 tablets cracked after 2000 rotations and 4 cracked after 3000 rotations.Furthermore,among the tablets of Sample 3,10 tablets 4.54 mm thick and all 20 tablets 4.50 mm thick cracked.Thus,Samples 2 and 3(TFS≤2 MPa by GTP-1)were likely to experience capping-like breakage when made on a rotary tableting machine.Tablets with>14.4 mg of disintegrant were at high risk of capping failure.Therefore,the appropriate amount of disintegrant per tablet was 14.4 mg.The poor predicted“Compactability”of Samples 2 and 3 was reflected in the actual tableting results.

Fig.5–Tableting failures:(A)Capping and(B)Binding.
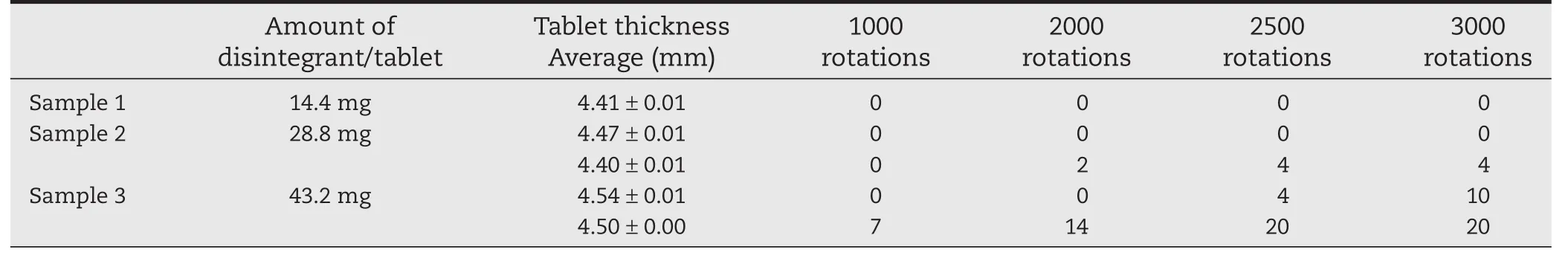
Table 6–Friability test of formulations with different amounts of disintegrant(to confirm capping-like breakage):data show number of breakages among 20 tablets.
3.2.2.Amount of binder
Evaluation using the GTP-1 showed that≥12 mg of binder is needed for sufficient hardness.We compressed Samples 4 (4 mg PVP),5(8 mg PVP),1(12 mg PVP),6(18 mg PVP),and 7(24 mg PVP)on the rotary tableting machine(11 kN,30 rpm)and tested their“Manufacturability”and physical properties(Table5).Every sample reached the target thickness of 4.40 mm and the target hardness of 60 N.In particular,the formulations that had good“Compactability”in the GTP-1 results(Samples 7,6,and 1)had high tablet hardness.The target disintegration time was within 7 min,but,as predicted(Section 3.1.3),that of Sample 6 was near the upper limit,and that of Sample 7 exceeded the target.Tableting failures did not occur,but capping-like breakage tended to occur in Samples 4 and 5,which had relatively low TFS in the GTP-1 evaluation.In the friability tests,Sample 4 showed a capping-like breakage after 3500 rotations,and Sample 5 after 4000 rotations(Table 7).The poor predicted “Compactability”of Samples 4 and 5 was also reflected in the actual tableting results.
3.2.3.Amount of lubricant
Evaluation using the GTP-1 showed that ejection stress decreased as the amount of lubricant increased. Samples 9 (2.4 mg MgSt)and 10(3.6 mg MgSt)were plotted in range(I),indicating good “Compactability”and “Manufacturability”.We compressed Samples 8(0.6 mg MgSt),1(1.2 mg MgSt),9,and 10 on the rotary tableting machine(11 kN,30 rpm)and tested their“Manufacturability”andphysical properties(Table5).Every formulation reached the target thickness of 4.40 mm.As shown in Fig.5(B),binding of Sample 8 occurred soon after the start of compression;granule adhesion to the inner wall of the die was severe,and damage to the sides of the tablets occurred.The other formulations were compressed without manufacturing failures.Sample 8 had the worst“Manufacturability”in the GTP-1 evaluation and on the rotary tableting machine.When the amount of lubricant was changed in samples 1,9 and 10,every formulation reached the target hardness of 60 N.It is well known that excessive amount of lubricant in the tablet formulation decreases hardness and prolongs disintegration time of resultant tablets,because hydrophobic lubricant covers the surface of granules too much.However,as on the GTP-1,tablet hardness did not decrease as the amount of lubricant was increased.As the range of lubricant amount used in the present study was not so much compared with usual tablet formulations,lubricantmay not completely cover the surface of granules to weaken the binding between granules,while it can work as lubricant at the surface of die wall.Every formulation also reached the target disintegration time of within 7 min.Therefore,each tablet needs≥1.2 mg of lubricant to maximize“Manufacturability”.
3.3. Scaling up
Because Samples 1(1.2 mg MgSt),9(2.4 mg MgSt),and 10(3.2 mg MgSt)did not cause problems in “Manufacturability”or quality at the small manufacturing scale(720 g),we scaled up production(4800 g).Evaluation using the GTP-1 showed no change in “Tableting properties”(Tables 4,8).We compressed samples prepared at the large scale on the rotary tableting machine(11 kN,30 rpm)and tested their“Manufacturability”and physical properties(Table 5).No tableting failure was observed,and 20,000 tablets were compressed in each sample.We conclude,therefore,that at whatever manufacturing scale,the results are reliably predicted by the GTP-1.Therefore,scalingup will be simplified if done according to the target“Tableting properties”determined experimentally by using our evaluation method.
4. Conclusion
It is important to design tablet formulations so as to avoid potential manufacturing failures.Previously,we assessed ourevaluation method in the design of formulations prepared by direct compression.Here,we assessed it in the design of formulations prepared by wet granulation.We optimized the amounts of binder,disintegrant,and lubricant and prepared samples by fluid bed granulation,and evaluated the“Tableting properties”of the samples using the GTP-1.Tableting failures(capping and binding in particular)occurred when samples that the GTP-1 had evaluated as having poor“Compactability”or“Manufacturability”were compressed on an actual rotary tableting machine.In particular,the tablets were at risk of capping when TFS measured by the GTP-1 was≲1.5 MPa,and of binding when ejection stress was≳20 MPa.Thus,problems predicted by the GTP-1 were con firmed in actual tableting.We would therefore be able to design tablet formulations that avoid tableting failures at the commercial scale by optimizing the composition through evaluation on the GTP-1.We would also be able to scale up production on the same basis.
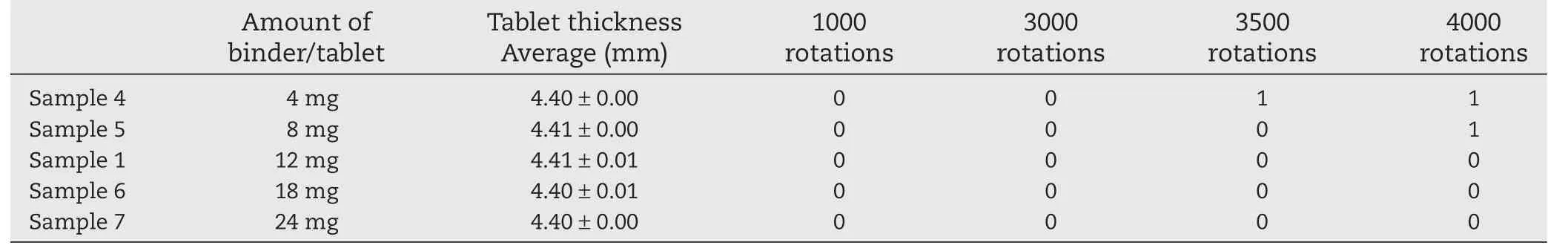
Table 7–Friability test of formulations with different amounts of binder(to con firm capping-like breakage):data show number of breakages among 20 tablets.

Table 8 –“Tableting properties”of formulations prepared at 20,000-tablet scale,evaluated using benchtop single-punch tablet press.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
[1]Kawakita K,Ludde K-H.Some considerations on powder compression equations.Powder Technol 1969;11:61–8.
[2]Heckel RW.Density-pressure relationships in powder compaction.Trans Metall Soc AIME 1961;221:671–5.
[3]Heckel RW.An analysis of powder compaction phenomena.Trans Metall Soc AIME 1961;221:1001–8.
[4]Klevan I,Nordstrom J,Tho I,et al.A statistical approach to evaluate the potential compression parameters for classification of pharmaceutical powder materials.Eur J Pharm Biopharm 2010;75:425–35.
[5]David ST,Augsburger LL.Plastic flow during compression of directly compressible fillers and its effect on tablet strength.J Pharm Sci 1977;66:155–9.
[6]Tesfai S,Goran A.Relationships between the effective interparticulate contact range and the tensile strength of tablets of amorphous and crystalline lactose of varying particle size.Eur J Pharm Sci 1999;8:235–42.
[7]Sugimori K,Mori S,Kawashima Y.Introduction of a new index for the prediction of capping tendency of tablets.Chem Pharm Bull 1989;37:458–62.
[8]Urabe M,Ito S,Itai S,et al.Assessment of tableting properties using in finitesimal quantities of powdered medicine.Int J Pharm 2003;263:183–7.
[9]Urabe M,Ito S,Itai S,et al.Assessment of tableting properties using in finitesimal quantities of powdered medicine II.J Drug Deliv Sci Technol 2006;16:357–61.
[10]Kikuta J,Kitamori N.Frictional properties of tablet lubricants.Drug Dev Ind Pharm 1985;11:845–54.
[11]Delacourte A,Guyot JC,Colombo P,et al.Effectiveness of lubricants and lubrication mechanism in tablet technology.Drug Dev Ind Pharm 1995;21:2187–99.
[12]Shah AC,Mlodozeniec AR.Mechanism of surface lubrication:influence of duration of lubricant-excipient mixing on processing characteristics of powders and properties of compressed tablets.J Pharm Sci 1977;10:1377–82.
[13]Eiliazadeh B,Pitt K,Briscoe B.Effects of punch geometry on powder movement during pharmaceutical tableting processes.Int J Solids Struct 2004;41:5967–77.
[14]Roberts M,Ford JM,Macleod GS,et al.Effect of punch tip geometry and embossment on the punch tip adherence of a model ibuprofen formulation.J Pharm Pharmacol 2004;56:947–50.
[15]Osamura T,Takeuchi Y,Onodera R,et al.Characterization of tableting properties measured with a multi-functional compaction instrument for several pharmaceutical excipients and actual tablet formulations.Int J Pharm 2016;510:195–202.
[16]Osamura T,Takeuchi Y,Onodera R,et al.Prediction of effects of punch shapes on tableting failure by using a multi-functional single-punch tablet press.Asian J Pharm Sci 2017;https://doi.org/10.1016/j.ajps.2017.05.001.In press.
猜你喜欢
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Combretastatin A4/poly(L-glutamic acid)-graft-PEG conjugates self-assembled to nanoparticles
- Development of lamellar gel phase emulsion containing baru oil(Dipteryx alata Vog.)as a prospective delivery system for cutaneous application
- Preparation and toxicity evaluation of a novel nattokinase-tauroursodeoxycholate complex
- Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery
- Quantification and spatial distribution of salicylic acid in film tablets using FT-Raman mapping with multivariate curve resolution
- Tablets of paliperidone using compression-coated technology for controlled ascending release
