ROS-responsive drug delivery systems for biomedical applications
2018-03-28
Wuya College of Innovation,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang 110016,China
1. Introduction
Recently,the design of drug delivery systems(DDSs)has attracted more and more attentions,because DDSs are able to effectively deliver many kinds of therapeutic drugs to the disease sites[1].Nevertheless,treatment outcome is usually restricted by non-specific drug release and quick plasma elimination,which not only needs the increase in drug dose but also brings undesirable side effects.Consequently,stimuli-response DDSs are of crucial importance due to their site-specific release ability in the existence of a stimulus that has relation with certain disease symptom[2–4].Typical physiological stimuli applied to DDSs include endogenous signals such as ROS,redox potential,acidity,hypoxia and enzyme,or exogenous signals such as temperature,light,ultra sound and magnetic field[5–7].One of the most popular biological stimuli is ROS because their overproduction have been happening in several types of diseases and the emerging ROS-sensitive materials have immense potential in the aspect of biomedicine development[8,9].
Typical ROS consist of the superoxide anion(O2−),hydrogen peroxide(H2O2),and hydroxyl radicals(OH),and one of the ROS species can convert into another through a series of reaction processes[10,11].ROS can be produced endogenously from NADPH enzyme or mitochondrial metabolism as well as exogenously via photodynamic or non-photodynamic actions.Intracellular superoxide(O2−)is mainly generated from the NADPH oxidation with the action of NAPH oxidase enzymes(NOXs)or by electron leak from the aerobic metabolism of mitochondria.Meantime,O2−can be transformed into H2O2rapidly by superoxide dismutases(SODs).When H2O2reaches a certain high concentration,OH·forms via reactions with metal cations(Fe2+/Cu+),which results in irreversible damage to cellular DNA,lipids or proteins.In addition,H2O2can also be converted to H2O by peroxiredoxins(PRx),glutathione peroxidase(GPx)and catalase(CAT)(Fig.1A)[12].ROS function as a double-edged sword in cells.Low levels of ROS play important roles in supporting cellular life cycles,acting as cellular signaling molecules by reversibly oxidizing protein thiol groups,thereby modifying the protein structure and function.Cells have a variety of mechanisms to regulate the balance between the formation and elimination of ROS to control them at a moderate level.However,the imbalance could generate oxidative stress with high levels of ROS,resulting in oxidative damage to the cellular constituents(e.g.,DNA,lipids and proteins),apoptosis or necrosis,and probably the promotion of cancer-causing mutations[13–15].Plenty of evidences suggest that many kinds of cancer cells have higher levels of ROS compared with the normal body cells.It was reported that the ROS concentration in cancer cells reaches 100µM,approximately 100 times higher than that in normal cells[16,17].Many cancer cells are well adapted to oxidative stress because of their inherently flexible redox status and this abnormal biochemical change in the disease sites has inspired researchers to exploit the high level of ROS for developing target-specific DDSs.By utilizing ROS-sensitive materials,numerous ROS-responsive DDSs have been developed and investigated for the treatment purposes.Besides the endogenous ROS,photosensitizers(PSs)or other chemical agents can also be utilized as the representative exogenous ROS sources to trigger drug release[18,19].
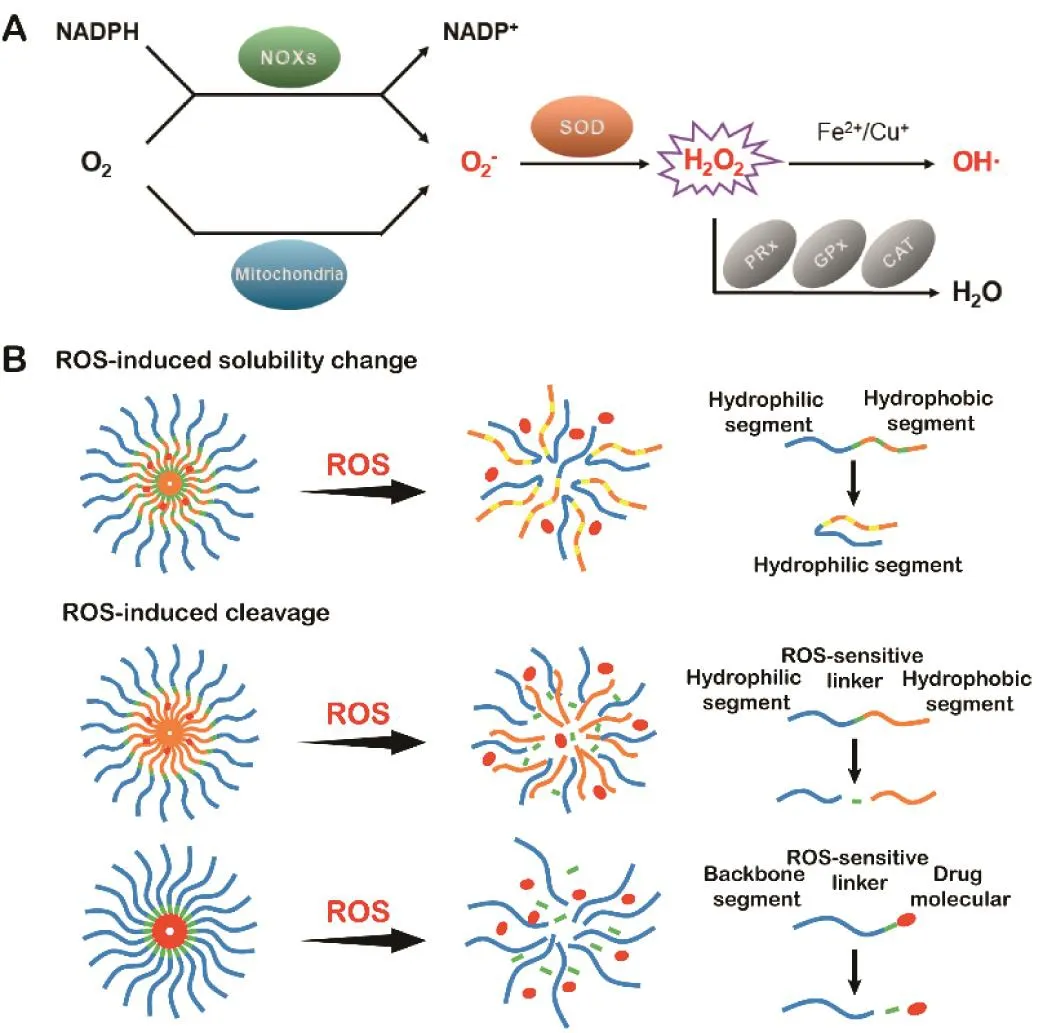
Fig.1–(A)Illustration of the intracellular production process of ROS.(B)Illustration of ROS-sensitive drug delivery systems.
There are many kinds of ROS-sensitive carriers or prodrugs investigated in the applications of smart DDSs,including those structures that contain thioether,selenide/telluride,diselenide,thioketal,arylboronic ester,aminoacrylate,oligoproline,peroxalate ester and mesoporous silicon[20,21].The chemical structures and reaction mechanisms of these linkers are summarized in Table 1 and discussed in respective sections.According to the design of these linkers,the main drug release mechanism can be ascribed to ROS-induced carrier solubility change,ROS-induced carrier cleavage or ROS-induced prodrug linker cleavage(Fig.1B)[8].The aim of this article is to provide an overview of current knowledge that center on ROS-responsive DDSs and its applications in biomedical field.
2. DDSs with ROS-induced solubility change
2.1. Thioether-containing polymers
Thioether-containing polymers are one type of the most popular ROS-sensitive materials investigated in biomedical research[47,48].In the presence of oxidative conditions,thioether-containing polymers undergo phase transition from hydrophobic sul fide to more hydrophilic sulfoxide or sulfone(Table 1).Early in 2004,Hubbell’s group developed the ABA block polymers consisting of poly(ethylene glycol)(PEG)and poly-(propylene sul fide)(PPS)for the design of ROS-responsive polymeric vesicles[22].After treating with H2O2,the DDSs released its payloads due to the destabilization caused by phase transition.
Utilizing this property,Cheng et al.designed and fabricated ROS-sensitive free-blockage controlled mesoporous silica nanocarriers modified with hydrophobic phenyl sulfide groups(MSNs-PhS)[23].As illustrated in Fig.2A,the PhS groups could be oxidized to electron withdrawing hydrophilic phenyl sulfoxide or phenyl sulfone under the stimulation of ROS.Due to phase transition of PhS groups,the nanopores could be gradually wetted and lead to the release of doxorubicin(DOX)from the nanopores.Furthermore,this smart system could selectively release the entrapped DOX inhuman breast adenocarcinoma cells(MCF-7)triggered by intracellular ROS but not in normal human umbilical vein endothelial cells(HUVECs)containing low levels of ROS.Based on ROS-responsive wetting behavior of nanopores,MSNs-PhS indicated a potential possibility for thein vivosite-specific delivery of anticancer drug.
In addition,the natural amino acid methionine is also a promising ROS-responsive candidate due to its thioether residue.Utilizing dual responsiveness to both ROS and enzymes,Yoo et al.designed protease-activatable cell-penetrating peptide,poly(L-methionine-block-L-lysine)-PLGLAG-PEG(MLMP),possessing ROS-triggered phase transition for enhanced therapeutic effects[24].It was found that both release of the PEG blocks and exposure of the poly-L-lysine chains by excess MMP-2 enzyme around the tumor tissues could assist the cellular penetration.Then,the thioether groups in the methionine chains could be oxidized by the excess ROS in the cytosol of cancer cells,and the thioether was converted to highly water-soluble sulfoxide moiety,releasing the encapsulated DOX for sitespeci fic chemotherapy.
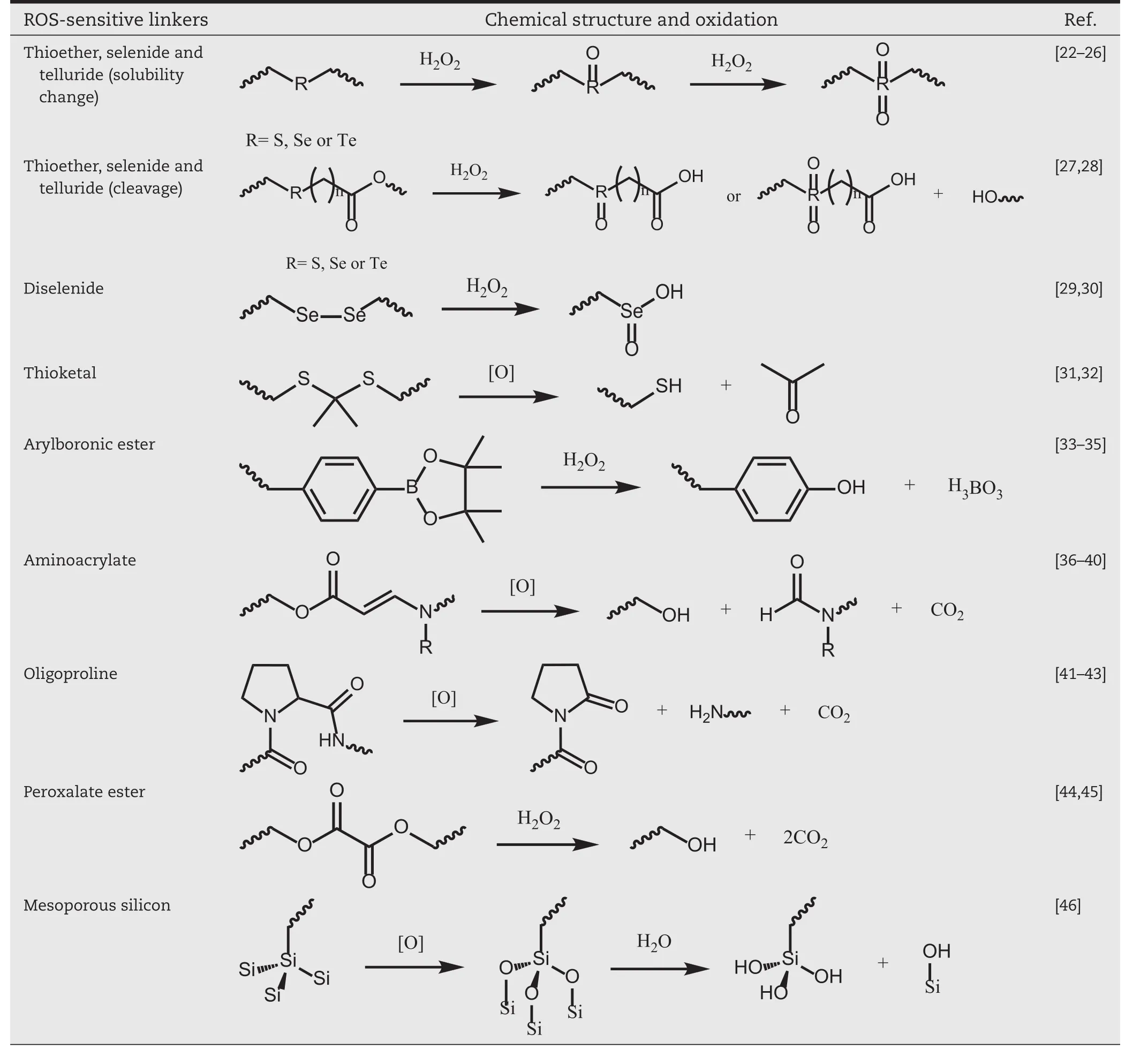
Table 1–Representative ROS-sensitive linkers and their oxidation products.
2.2. Selenide-or telluride-containing polymers
In addition,recently,the elements of selenium(Se)and tellurium(Te)have gained increasing attention[49,50].Se and Te resemble the sulfur(S)in many ways especially in the oxidation-sensitive ability because they are of the same chalcogen family on the periodic table[51].Similar to the oxidation mechanism of thioether group,the ROS-induced hydrophobic-to-hydrophilic transition can also happen after the oxidation of monoselenium-or monotellurium-containing polymers(Table 1),which is usually applied to the design of ROS-responsive DDSs[52].For instance,Liu and coworkers developed a ROS-sensitive carrier by using selenidecontaining amphiphilic hyperbranched copolymer micelle for the efficient delivery of DOX(Fig.2B)[25].Under intracellular oxidation conditions in target cancer cells,the nanocarrier disassembled rapidly to release the encapsulated DOX because the hydrophobic selenide could be readily oxidized into hydrophilic selenone resulting in the transformation of amphiphilic polymer into the completely hydrophilic one.Furthermore,the selenium compounds could also serve as anticancer agents and enhance the immune response,which endowed the selenide-containing nanocarrier excellent intrinsic antitumor efficiency.Particularly,it is necessary to note that the polymers are well biocompatible and can be eliminated by enzyme digestion after the drug release,which have raised the expectations of ROS-responsive DDSs in the antitumor therapy applications.
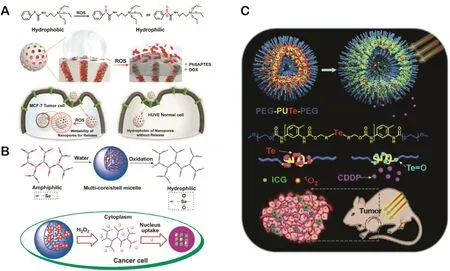
Fig.2–(A)Schematic diagram of the free-blockage on–off characteristics of nanopores on MSNs functionalized with PhSAPTES due to ROS-responsive wettability and its selective intracellular release of DOX.Redrawn based on Cheng et al.[23].Copyright 2017,John Wiley&Sons,Inc.(B)Multicore/shell structure of the self-assembled micelles and the H2O2-triggered intracellular drug release.Redrawn based on Liu et al.[25].Copyright 2013,American Chemical Society.(C)Schematic diagram of near-infrared triggered ROS-stimuli CDDP release from amphiphilic polymer PEG-PUTe-PEG based nanoparticles.Redrawn based on Yoo et al.[26].Copyright 2017,Elsevier.
Telluride-containing polymers possess ultra ROS responsiveness compared with S or Se due to its lower electronegativity[53–56].Taking advantage of this property,Li et al.developed a novel drug delivery system by simultaneously encapsulating cisplatin(CDDP)and indocyanine green(ICG)in the novel amphiphilic tellurium-containing polymer(PEG-PUTe-PEG)based nanoparticles(Fig.2C)[26].Under the stimuli of a nearinfrared laser,the telluride atoms in the nanocarriers could be easily oxidized by1O2produced by ICG,which made the hydrophobic block of the polymer more hydrophilic,thus promoting selective drug release at target tissues and reducing side effects compared with the CDDP alone.In addition,the photothermal effect of ICG played a synergistic antitumor effect with photodynamic therapy(PDT)and chemotherapy.
2.3. Arylboronic ester-containing polymers
The arylboronic acid/ester groups have been designed as ROS-sensitive groups for various applications,one of which is based on a solubility switch strategy via H2O2oxidation(Table 1)[57–59].In 2011,Broaders et al.modified the hydroxyls of watersoluble polymer dextran with arylboronic ester groups to obtain the water insoluble oxidation-sensitive dextran(Oxi-DEX)[33].Oxi-DEX microparticles were then prepared by using double emulsion method.Oxi-DEX particles were stable in pH 7.4 phosphate buffer,but quickly became completely transparent within 2 h in the presence of H2O2(1 mM).After the oxidation of boronic esters in the presence of H2O2,the hydroxyl groups of dextran were exposed and the polymer changed back to the hydrophilic parent form,which lead to concurrent behavior of polymer degradation and the payloads release.
3. DDSs with ROS-induced cleavage
3.1. Thioether,selenide and telluride
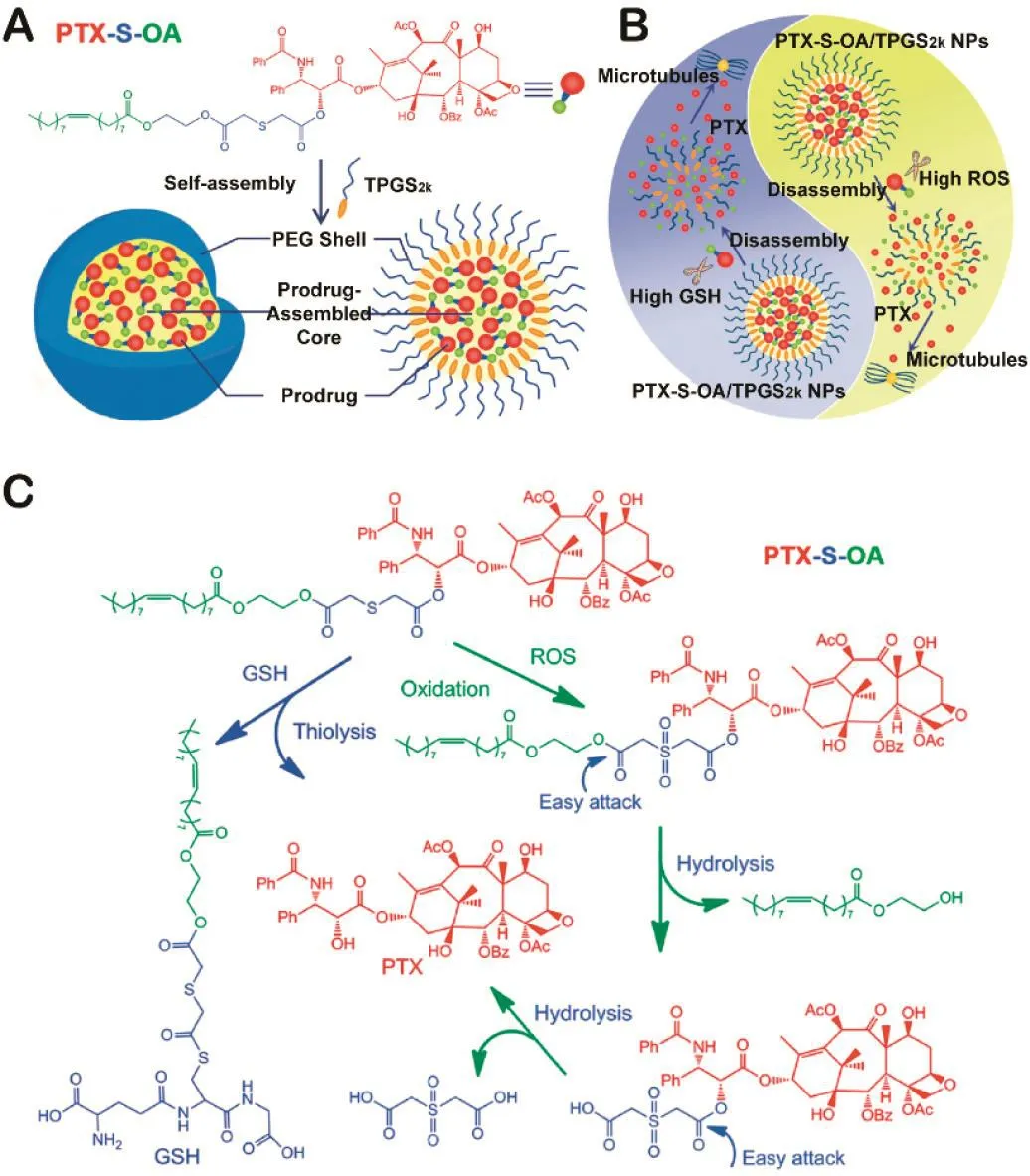
Fig.3–(A)Illustration of the preparation of PEGylated prodrug NPs of PTX-S-OA.(B)Illustration of redox dualresponsive drug release of prodrug NPs in the presence of two opposite stimuli within tumor cells.(C)Redoxsensitive drug release mechanism of PTX-S-OA triggered by GSH/ROS.Redrawn based on Luo et al.[28].Copyright 2016,American Chemical Society.
As mentioned above,thioether-,selenide-and telluridecontaining polymers possess the ROS-induced solubility switch ability for site-speci fic drug release.Meanwhile,after the thioether,selenide and telluride are oxidized to hydrophilic groups,the proximal ester bond will be more easily hydrolyzed(Table 1).Therefore,this type of ROS-induced cleavage could be applied to the design of novel DDSs such as prodrug.As we know,there are abnormal levels of ROS and GSH existing in the tumor microenvironment[60].Therefore,redox dualresponsive prodrug-nanosystem presented notable advantages[61–64].Early in 2013,Wang et al.developed an amphiphilic prodrug(OEG-2S-SN38)nanoassemblies which could be degraded under either ROS or GSH environment to release the coupled parent drug[27].They proposed that the single thioether close to the ester bond conjugated to SN38 played the key role in controlling the redox-sensitivity,rather than the sulfur atom that is further away from the ester bond.In addition to the long distance,dithioether would consume twice as much ROS or GSH as single thioether does.Inspired by the dual redox-responsive ability of thioethers,single thioether may have better efficacy than dithioether as a redox dual-sensitive linkage.As shown in Fig.3,Luo et al.successfully developed a novel self-assembled redox dual-responsive prodrugnanosystem(PTX-S-OA/TPGS2kNPs)and first investigated the stimuli-responsive hydrophobic small molecule prodrugs in detail[28,65].As a result,the single thioether linked prodrug(PTX-S-OA)was distinctly superior to the dithioether-inserted conjugate(PTX-2S-OA)in terms of redox dual-sensitive drug release andin vivoantitumor efficacy.
3.2. Diselenide
Selenium-containing materials have been popularly used as antioxidants[66].In particular,diselenide can exert excellent activity in response to either oxidants or reductants,which makes it a promising candidate for dual redox response.Generally,diselenide can be oxidized and cleaved to seleninic acid under the action of oxidants and reduced to selenol in the presence of reductants(Table 1)[67].Taking advantage of this property,Ma and coworkers designed the block copolymer consisting of a hydrophobic diselenide-containing segment and two hydrophilic polyethylene glycol(PEG)segments(Fig.4A).Meanwhile,the self-assembly ability in water and the disassembly behavior with the action of oxidants and reductants were both investigated[29].As shown in Fig.4B,Se–Se bond owned the unique dual redox sensitivities,and it could be cleaved to seleninic acid by oxidants(0.01%H2O2)or reduced to selenol in a reductive condition(0.01%GSH).Therefore,this type of dual redox-responsive block copolymer micelles can be applied to the design of smart DDSs for small molecules delivery.
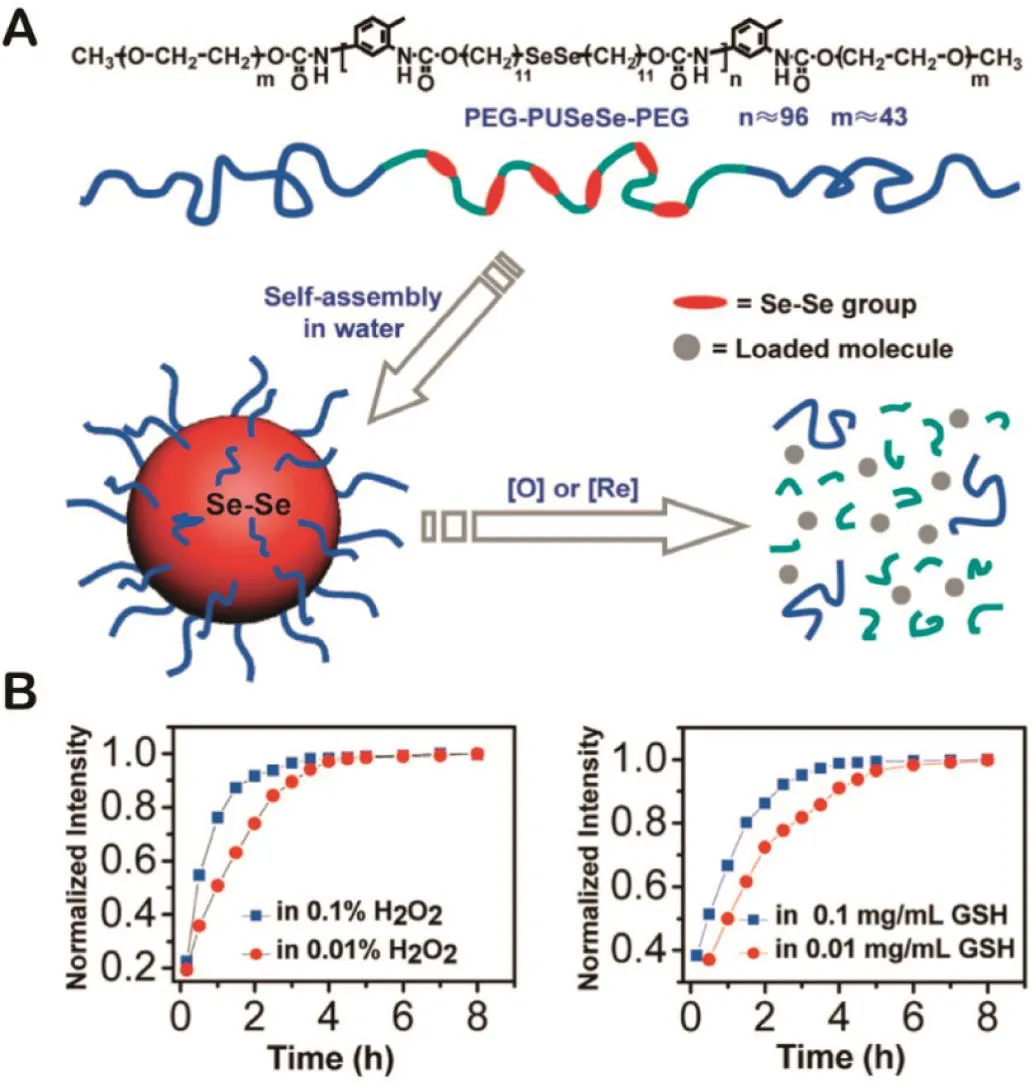
Fig.4–(A)Structure of PEG-PUSeSe-PEG and illustration of the redox responsive disassembly of PEG-PUSeSe-PEG micelles.(B)Release behavior of Rhodamine B in H2O2and GSH.Redrawn based on Ma et al.(2010)[29].Copyright 2009,American Chemical Society.
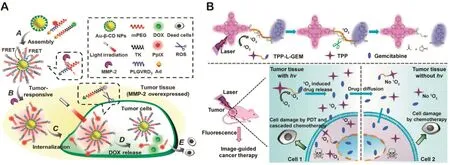
Fig.5–(A)Schematic diagram of multifunctional AuNPs with MMP-2 responsibility for programmed FRET-based tumor imaging and light trigger ROS-responsive on-demand drug release.Redrawn based on Han et al.[31].Copyright 2015,The Royal Society of Chemistry.(B)Schematic diagram of ROS mediated thioketal linker cleavage and cascaded gemcitabine release for combination of photodynamic therapy and subsequent chemotherapy in tumor tissue.Redrawn based on Liu et al.[32].Copyright 2016,John Wiley&Sons,Inc.
In another example,Deepagan et al.developed a novel anticancer drug carrier by employing thein situdiselenidecrosslinked micelles(DCMs),which could respond to the high levels of ROS in tumor tissues[30].The selenol-containing triblock copolymers DCMs consist of polyethylene glycol(PEG)and polypeptide derivatives.In the micelle preparation process,the hydrophobic core was used to encapsulate the chemotherapy drug DOX,and the shell was formed due to the crosslink of diselenide.The DCMs were able to keep their structural integrity in normal physiological conditions,while the ROS-rich circumstances could lead to the transformation of hydrophobic diselenide into hydrophilic selenic acid resulting in the quick release of DOX from the DOX-DCMs.In addition,compared with the non-crosslinked counterparts,DOXDCMs could deliver more drugs to tumor tissues in tumorbearing mice after intravenous injection.Therefore,DCMs hold tremendous potential as excellent drug carriers for the delivery of antitumor agents.
3.3. Thioketal
In recent years,thioketal linker has been generally applied to the biomedical research because it can be rapidly cleaved by ROS species and degraded into acetone and thiols as byproducts(Table 1)[68–71].For instance,the PEGylated PS(protoporphyrin IX,PpIX)was conjugated to AuNPs through an MMP-2 sensitive peptide linker(PLGVR)by Han and coworkers[31].In addition,the functional AuNPs was further conjugated with DOX through a thioketal linker(Fig.5A).The PpIX exhibited enhanced fluorescence in SCC-7 cancer cells compared with that in COS7 fibroblast cells,indicating that Au/PpIX/DOX NPs could specifically target tumor tissue and liberate quenched PpIX with the action of overexpressed MMP-2.Meanwhile,DOX exhibited stronger fluorescence in SCC-7 cells with light irradiation for 30 minutes compared with the control group.Upon cell internalization and subsequent light irradiation,the thioketal linkers could be cleaved to release the coupled DOX for an enhanced combination therapy.
The ROS-sensitive thioketal linker is also used in the design of the prodrugs[72].Liu et al.developed a novel multifunctional photosensitive prodrug named TPP-L-GEM[32].As shown in Fig.5B,gemcitabine was conjugated with mesotetraphenylporphyrin(TPP)through a ROS-sensitive thioketal linker.Under light irradiation,gemcitabine could be released from the prodrug triggered by the cleavage of thioketal linker in the presence of1O2generated from TPP.As a result,TPP-LGEM exhibited excellent tumor growth inhibition efficacy with the combination of PDT and chemotherapy.
3.4. Arylboronic ester
Recently,several arylboronic ester derivatives have been popularly studied due to their superior degradation kinetics[73,74].Besides the method of ROS-induced solubility change mentioned above,carrier or prodrug containing arylboronic ester linker can also be cleaved in ROS-rich conditions(Table 1).As shown in Fig.6A,Lux et al.designed a self-degradable carrier that contained a boronic ester used as the H2O2-sensitive group[34].The nanoparticles were highly sensitive,which could release 50%of the reporter dye after exposure to 100µM H2O2for 10 h.On account of the rapid oxidization ability,the polymer was degraded to produce phenol and borate by quinone methide rearrangement,which releases their cargo selectively in low biologically relevant levels of H2O2.
Taking advantage of this property,Kim et al.designed an activatable prodrug for the treatment of metastatic tumors(Fig.6B)[35].The dissociation of the conjugates was induced by boronate ester cleavage triggered by physiological levels of H2O2,resulting in activation of the fluorophore(coumarin)for detection and delivery of chemotherapeutic agent SN-38.Furthermore,intratracheal administration showed effective antitumor activity as well as prolonged survival time in a mouse metastatic lung disease model.While lung tissues of a salinetreated control group showed numerous black colonies of B16F10 cells,the group of prodrug inhibited tumor growth and likely preserved more normal lung tissue.This novel prodrug platform represents the potential for efficient tumor-targeted drug delivery system with the specifically activated by ROS in the tumor environment.
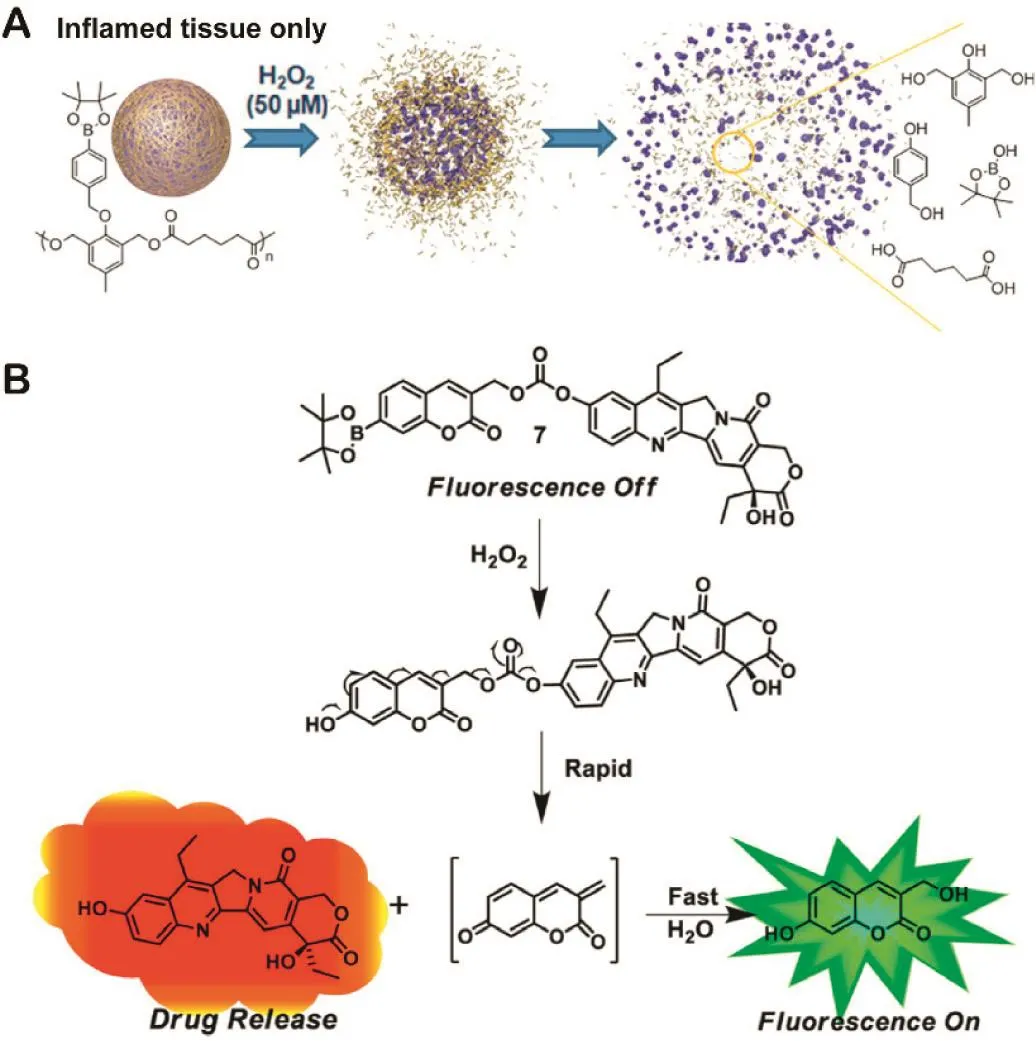
Fig.6–(A)Mechanism of polymeric particle 2 degradation upon exposure to H2O2.Redrawn based on Lux et al.[34].Copyright 2012,American Chemical Society.(B)Proposed reaction mechanism of prodrug 7 with H2O2under physiological conditions.Redrawn based on Kim et al.[35].Copyright 2014,American Chemical Society.
3.5. Aminoacrylate
An aminoacrylate linker was also found to be cleaved by ROS oxidation(Table 1)[8].Yuan et al.demonstrated this application via designing a ROS-responsive polymer which consists of a PS and oligoethylenimine(OEI)conjugated via a ROS-sensitive aminoacrylate linker for light-controlled gene delivery(Fig.7A)[36].By taking advantage of aggregation-induced emission(AIE)PS,the efficient ROS generation upon light irradiation could disrupt the membranes and break the polymer into smaller components for simultaneous endo/lysosomal escape and DNA discharge,which are essential steps for efficient gene delivery.
Based on a similar design concept,Hossion’s group proposed a ROS and light dual-responsive prodrug consisting of both an aminoacrylate linker and a deactivated PS(dPS)[37].Since dPS had no fluorescence and could not generate ROS by irradiation,only after the activation of dPS by cellular esterase,parent drugs could release under the control of visible light.The effective drug delivery(99%efficiency)of coumarin prodrug was successfully demonstrated after incubation with MCF-7 cells by irradiation with low intensity visible light for 30 minutes.
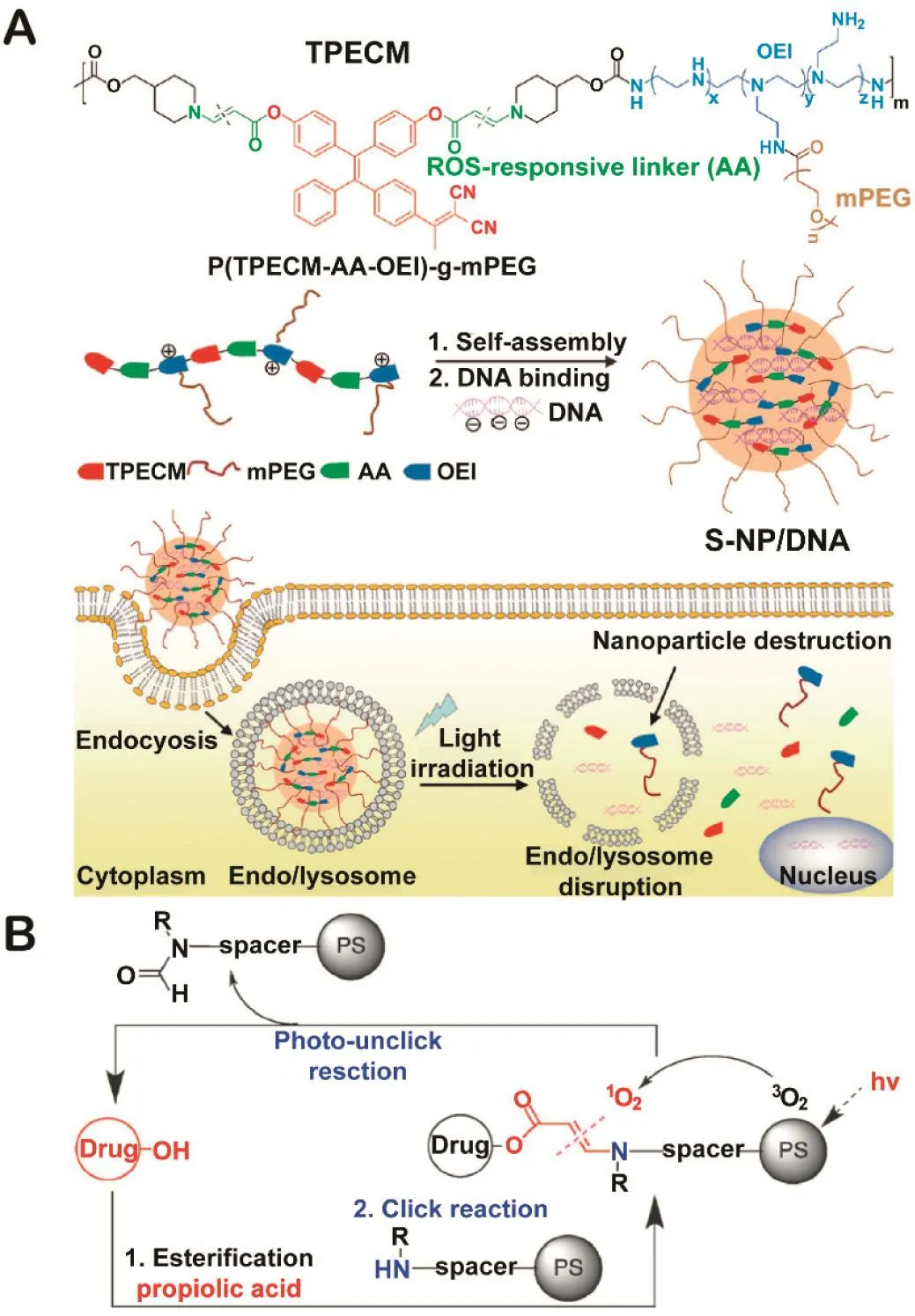
Fig.7–(A)Schematic diagram of ROS-sensitive nanoparticles(S-NPs)self-assembled from P(TPECM-AAOEI)-g-mPEG and their complexation with DNA to form S-NPs/DNA for transgene expression.Redrawn based on Yuan et al.[36].Copyright 2015,John Wiley&Sons,Inc.(B)Facile synthesis and cleavage of aminoacrylate and release of a parent drug after its oxidative cleavage.Redrawn based on Bio et al.[38].Copyright 2012,The Royal Society of Chemistry.
In another study of You’s group,the degradation of aminoacrylate by1O2was named“photo-unclick chemistry”for the first time(Fig.7B)[38].Conjugates were obtained in two synthesis steps and could be cleaved rapidly under light irradiation.Using this photo-unclick chemistry,the same group reported low energy activated prodrugs CMP−L–CA4 and Pc-(L-CA4)2,composed of a core-modified porphyrin(CMP)or a phthalocyanine(Pc),an1O2-labile aminoacrylate linker(L)and a cytotoxic drug combretastatin A-4(CA4)[39,40].Due to the fast cleavage of aminoacrylate linker under the irradiation of far-infrared light,the prodrug system was outstanding with enhanced antitumor efficacy compared with its noncleavable control in mouse model.This is the first application of sitespecific DDSs of anticancer drug via photo-unclick chemistry in an animal model.In addition,the photo-unclickable prodrug Pc-(L-CA4)2was more multifunctional and outstanding with visualized optical imaging and a combination of PDT and chemotherapy.
3.6. Oligoproline
Among stimuli-responsive materials,polypeptides have great advantages due to their biocompatibility and biodegradability[75,76].It was reported that peptides that contain amino acid such as glutamic acid,aspartic acid and proline particularly tend to undergo peptide backbone cleavage and degradation by oxidants[77,78].Among these three amino acid residues,proline was further demonstrated to result in the peptide bond cleavage upon the oxidation of proline residues to the 2-pyrrolidone derivative(Table 1).
In 2011,Yu’s group designed the polymeric scaffold crosslinked with proline oligomers as a promising ROS-responsive drug delivery carrier[41].The other unique physiological environments of intracellular space such as the acidic pH could also be served as a certain trigger for the smart DDSs.The same group later reported a type of PEGylated oligoproline peptidederived nanocarriers for dual stimuli-responsive gene delivery by utilizing the overproduced ROS environment induced by inflammatory activation[42].It was only when the nanoparticles were exposed to extracellular ROS as well as intracellular low pH environment that the polymers could be degraded to realize site-speci fic drug delivery.The polymers demonstrated excellent pH and ROS dual responsiveness for both endosomal escape and improved targeting ability in anin vitromodel of pathogenic vascular smooth muscle cells.
Except for various kinds of ROS-responsive nanosized intelligent drug or gene delivery system,the macroscopic ROS-responsive polymeric scaffolds for tissue engineering have also received increasingly interest.Recently,a novel ROS-responsive scaffold was successful fabricated with efficient crosslinking poly(ε-caprolactone)(PCL)and ROS-responsive proline peptide oligomer by the same group[43].On account of scaffold degradation caused by oligoproline peptide backbone cleavage upon the chronically increased levels of ROS environment,this scaffold enabled site-specific delivery and security degradation for long-term tissue engineering applications.
3.7. Peroxalate ester
It has been reported that peroxalate ester can be oxidized rapidly by H2O2to generate the intermediate dioxetanedione that spontaneously degrades into CO2due to its high energy(Table 1).Hence,the polymer with peroxalate ester linkages serve as a new family of biocompatible and biodegradable materials,which is applied to deliver therapeutic drug to inflammation sites or tumor tissues that overproduce ROS species[79,80].For instance,vanillin is served as a suitable food flavoring agent by the Food and Drug Administration[81].In addition,vanillin also has anti-inflammatory activity to inhibit the expression level of various proinflammatory cytokines,demonstrating great potential for the treatment of inflammation related diseases[44,82].Kwon et al.designed polyvanillin oxalate(PVO)as a polymeric prodrug of vanillin,which incorporated drugs in the biodegradable polymer backbone and released drugs during their degradation(Fig.8A)[44].Meanwhile,PVO was molecularly designed to contain both H2O2-sensitive peroxalate ester bonds and acid-cleavable acetal linkages in its backbone because in flammation is characterized by acidic pH and overproduction of ROS.Results showed that PVO released vanillin during the degradation of its backbone in the existence of H2O2and acid pH,and the vanillin was able to serve as therapeutic agent in the treatment of ROS-related inflammatory diseases.
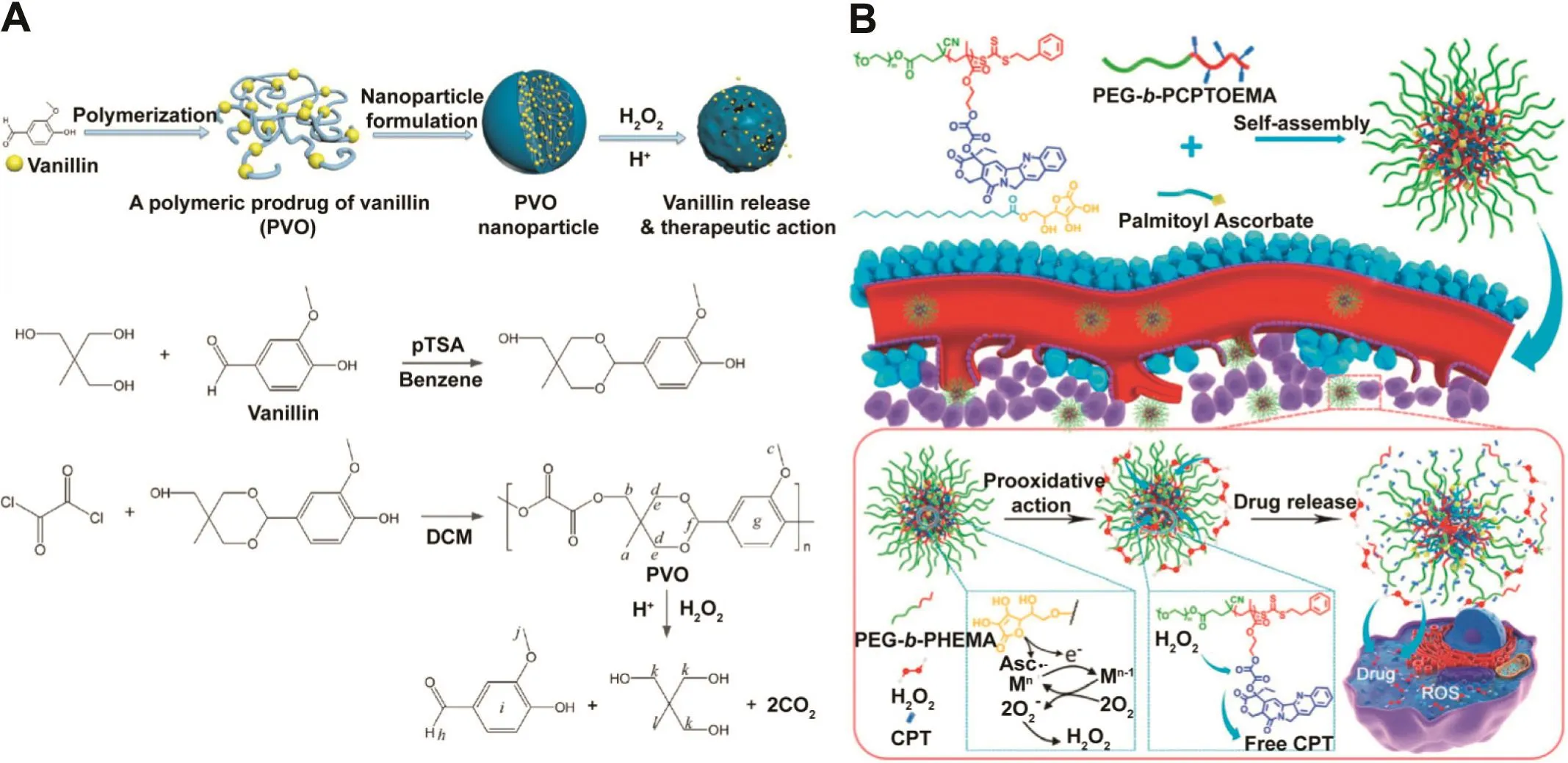
Fig.8–(A)Schematic diagram of fully biodegradable and dual stimuli-responsive PVO nanoparticles.Redrawn based on Kwon et al.[44].Copyright 2013,American Chemical Society.(B)Schematic diagram of formation of self-sufficing H2O2-responsive polymer prodrug-PA hybridmicelles via self-assembly in aqueous solution and their utility in upregulating H2O2 level in tumor tissues and triggering subsequent CPT release to exert synergistic oxidation-chemotherapy.Redrawn based on Li et al.[45].Copyright 2016,Elsevier.
Fig.9–(A)Loading of molecular payloads in porous Si microparticles by covalent coupling of either the fluorescent molecule Alexa Fluor 488 or the anticancer drug doxorubicin.(B)Schematic diagram of oxidationtriggered hydrolysis reaction.Redrawn based on Wu et al.[46].Copyright 2008,American Chemical Society.
Li et al.constructed a H2O2-responsive camptothecin(CPT)delivery nanocarrier via formation of polymer prodrugpalmitoyl ascorbate(PA)hybrid micelles(HPMs)(Fig.8B)[45].The incorporation of PA was hypothesized to endow the nanocarriers with H2O2self-sufficing ability,which not only improved oxidative stress to the tumor via H2O2production but also triggered CPT release from the nanocarriers via peroxalate ester cleavage.Excessive H2O2and released CPT were demonstrated to penetrate into cells efficiently,which showed synergisticin vitrocytotoxicity toward cancer cells.Therefore,efficientin vivosynergistic oxidation-chemotherapy could be obtained by systemic administration of the self-sufficing H2O2-responsive nanocarriers.
3.8. Mesoporous silicon
According to reports in the literature,the Si–Si back bonds of mesoporous silicon also possess the ROS-sensitive ability(Table 1)[83].As known to all,the mere adsorption of therapeutic agents to Si surface generally leads to unsatisfactory results due to the non-specific,rapid burst drug release.In one research of Wu’s group,two model drug doxorubicin and Fluor 488 were linked to an intermediate chain and coupled to the porous Si surface via Si–C bonds[46].The Si–C bonds were stable while the Si–Si back bonds could be oxidized resulting in the insertion of oxygen to form the Si–O–Si linkages.Compared with the Si–C bonds,the S–O bonds were susceptible to hydrolysis,thus leading to the oxidation-triggered release of the linkermolecule conjugates(Fig.9A and B).Meanwhile,oxidation of porous Si eliminated the quenching pathway,which provides an effective way to monitor the progress of drug release.Results showed that the release of payloads was accelerated in the presence of high levels of ROS.
4. Conclusion
In recent years,ROS-responsive DDSs have shown great potential in a series of biomedical applications,such as cancer or inflammation related disease targeted therapy.Numerous examples of ROS-responsive platforms have been illustrated in the main article,including the delivery of therapeutic drugs,nucleic acids,proteins and fluorescence dyes used for cancer therapy,anti-inflammation,diabetes therapy and disease diagnosis[84–87].Depending on the different types of payloads,the smart DDSs can be applied to load drugs through hydrophobic,electrostatic and covalent interaction,which allow cargos to be released via ROS-induced carrier phase change or ROS-induced cleavage[88].ROS-responsive DDSs with carriers containing thioether,selenide or telluride usually release cargos by means of ROS-induced carrier solubility change,and the ROS sensitivity intensify with the increase in electronegativity.Carriers containing diselenide,thioketal,arylboronic ester,aminoacrylate,oligoproline,peroxalate ester and mesoporous silicon usually release the cargo via ROS-induced carrier cleavage.In addition,these structures are also frequently used in ROS-activatable prodrugs which directly liberate the linked drugs under ROS stimuli.It is generally recognized that DDSs with relatively high ROS sensitivity or ROS-linker conjugated drug usually possess better release efficiency.
Except for the endogenous ROS,we can also combine ROS-responsive DDSs with other strategies such as PDT or non-PDT which are able to generate ROS in target locations[89].When synergizing with PDT,the PS not only lead to light triggered ROS-induced payload release but also eliminate disease via the toxic effects of excessive ROS,resulting in improved therapeutic efficiency.Such multiple-stimuli responsive DDSs can also be designed in response to other endogenic stimuli such as temperature,acidity,hypoxia and enzyme for enhanced ability to target disease sites[90–94].In summary,despite the existence of great challenges,the design of ROS-responsive DDSs demonstrated a promising way to effectively deliver therapeutic agents to disease sites and it will become more and more popular in the biomedical field.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was financially supported by Program for Liaoning Innovative Research Team in University(LT2014022).
[1]Gaurav T,Ruchi T,Birendra S,et al.Drug delivery systems:an updated review.Int J Pharm Investig 2012;2:2–11.
[2]Ding C,Tong L,Feng J,et al.Recent advances in stimuliresponsive release function drug delivery systems for tumor treatment.Molecules 2016;21:1715.
[3]Yan X,Wang F,Zheng B,et al.Stimuli-responsive supramolecular polymeric materials.Chem Soc Rev 2012;41:6042–65.
[4]Chen X,Cheng X,Soeriyadi A,et al.Stimuli-responsive functionalized mesoporous silica nanoparticles for drug release in response to various biological stimuli.Biomater Sci 2013;2:121–30.
[5]Chen H,Liu D,Guo Z.Endogenous stimuli-responsive nanocarriers for drug delivery.Chem Lett 2016;12:991–1003.
[6]Mo R,Gu Z.Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery.Mater Today 2016;19:274–83.
[7]Mura S,Nicolas J,Couvreur P.Stimuli-responsive nanocarriers for drug delivery.Nat Mater 2013;12:991–1003.
[8]Lee SH,Gupta MK,Bang JB,et al.Current progress in reactive oxygen species(ROS)-responsive materials for biomedical applications.Adv Healthc Mater 2013;2:908–15.
[9]Xu Q,He C,Xiao C,et al.Reactive oxygen species(ROS)responsive polymers for biomedical applications.Macromol Biosci 2016;16:635–46.
[10]Brown DI,Griendling KK.Regulation of signal transduction by reactive oxygen species in the cardiovascular system.Circ Res 2015;116:531–49.
[11]Finkel T.Signal transduction by reactive oxygen species.J Cell Biol 2001;194:7–15.
[12]Schieber M,Chandel N.ROS function in redox signaling and oxidative stress.Curr Biol 2014;24:453–62.
[13]Kay NE.ROS:double-edged sword for leukemic cells.Blood 2006;107:2212–3.
[14]Martin KR,Barrett JC.Reactive oxygen species as double-edged swords in cellular processes:low-dose cell signaling versus high-dose toxicity.Hum Exp Toxicol 2002;21:71–5.
[15]Pietraforte D,Malorni W.Focusing at the double-edged sword of redox imbalance:signals for cell survival or for cell death?Antioxid Redox Signal 2014;21:52–5.
[16]Giorgio M,Trinei M,Migliaccio E,et al.Hydrogen peroxide:a metabolic by-product or a common mediator of ageing signals?Nat Rev Mol Cell Biol 2007;8:722–8.
[17]Pelicano H,Carney D,Huang P.ROS stress in cancer cells and therapeutic implications.Drug Resist Updat 2004;7:97–110.
[18]Issa MC,Manelaazulay M.Photodynamic therapy:a review of the literature and image documentation.An Bras Dermatol 2010;85:501–11.
[19]Zhou Z,Song J,Nie L,et al.Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy.Chem Soc Rev 2016;45:6597–626.
[20]Lallana E,Tirelli N.Oxidation-responsive polymers:which groups to use,how to make them,what to expect from them(biomedical applications).Macromol Chem Phys 2013;214:143–58.
[21]Song CC,Du FS,Li ZC.Oxidation-responsive polymers for biomedical applications.J Mater Chem B Mater Biol Med 2014;2:3413–26.
[22]Napoli A,Valentini M,Tirelli N,et al.Oxidation-responsive polymeric vesicles.Nat Mater 2004;3:183–9.
[23]Cheng Y,Jiao X,Xu T,et al.Free-blockage mesoporous anticancer nanoparticles based on ROS-responsive wetting behavior of nanopores.Small 2017;13:1701942.
[24]Yoo J,Sanoj NR,Lee D,et al.Protease-activatable cellpenetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy.J Control Release 2017;264:89–101.
[25]Liu J,Pang Y,Zhu Z,et al.Therapeutic nanocarriers with hydrogen peroxide-triggered drug release for cancer treatment.Biomacromolecules 2013;14:1627–36.
[26]Li F,Li T,Wei C,et al.Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment.Biomaterials 2017;133:208–18.
[27]Wang J,Sun X,Mao W,et al.Tumor redox heterogeneityresponsive prodrug nanocapsules for cancer chemotherapy.Adv Mater 2013;25:3670–6.
[28]Luo C,Jin S,Dan L,et al.Self-assembled redox dualresponsive prodrug-nanosystem formed by single thioetherbridged paclitaxel-fatty acid conjugate for cancer chemotherapy.Nano Lett 2016;16:5401–8.
[29]Ma N,Li Y,Xu H,et al.Dual redox responsive assemblies formed from diselenide block copolymers.J Am Chem Soc 2010;132:442–3.
[30]Deepagan VG,Kwon S,You DG,et al.In situ diselenidecrosslinked polymeric micelles for ROS-mediated anticancer drug delivery.Biomaterials 2016;103:56–66.
[31]Han K,Zhu JY,Wang SB,et al.Tumor targeted gold nanoparticles for FRET-based tumor imaging and light responsive on-demand drug release.J Mater Chem B Mater Biol Med 2015;3:8065–9.
[32]Liu LH,Qiu WX,Li B,et al.A red light activatable multifunctional prodrug for image-guided photodynamic therapy and cascaded chemotherapy.Adv Funct Mater 2016;26:6257–69.
[33]Broaders KE,Grandhe S,Fréchet JM.A biocompatible oxidation-triggered carrier polymer with potential in therapeutics.J Am Chem Soc 2011;133:756–8.
[34]Lux DGC,Joshi-Barr S,Nguyen T,et al.Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide.J Am Chem Soc 2012;134:15758–64.
[35]Kim EJ,Bhuniya S,Lee H,et al.An activatable prodrug for the treatment of metastatic tumors.J Am Chem Soc 2014;136:13888–94.
[36]Yuan Y,Zhang CJ,Liu B.A photoactivatable AIE polymer for light-controlled gene delivery:concurrent endo/lysosomal escape and DNA unpacking.Angew Chem Int Ed Engl 2015;54:11419–23.
[37]Hossion AL,Bio M,Nkepang G,et al.Visible light controlled release of anticancer drug through double activation of prodrug.ACS Med Chem Lett 2012;4:124–7.
[38]Bio M,Nkepang G,You Y.Click and photo-unclick chemistry of aminoacrylate for visible light-triggered drug release.Chem Commun(Camb)2012;48:6517–9.
[39]Bio M,Rajaputra P,Nkepang G,et al.Site-specific and farred-light-activatable prodrug of combretastatin A-4 using photo-unclick chemistry.J Med Chem 2013;56:3936–42.
[40]Bio M,Rajaputra P,Nkepang G,et al.Far-red light activatable,multifunctional prodrug for fluorescence optical imaging and combinational treatment.J Med Chem 2014;57:3401–9.
[41]Yu SS,Koblin RL,Zachman AL,et al.Physiologically-relevant oxidative degradation of oligo(proline)-crosslinked polymeric scaffolds.Biomacromolecules 2011;12:4357–66.
[42]Gupta MK,Lee SH,Crowder SW,et al.Oligoproline-derived nanocarrier for dual stimuli-responsive gene delivery.J Mater Chem B Mater Biol Med 2015;3:7271–80.
[43]Lee SH,Boire TC,Lee JB,et al.ROS-cleavable proline oligomer crosslinking of polycaprolactone for proangiogenic host response.J Mater Chem B Mater Biol Med 2014;2:7109–13.
[44]Kwon J,Kim J,Park S,et al.Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin.Biomacromolecules 2013;14:1618–26.
[45]Li J,Ke W,Wang L,et al.Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2production for synergistic oxidation-chemotherapy.J Control Release 2016;225:64–74.
[46]Wu EC,Park JH,Park J,et al.Oxidation-triggered release of fluorescent molecules or drugs from mesoporous Si microparticles.ACS Nano 2008;2:2401–9.
[47]Hu P,Tirelli N.Scavenging ROS:superoxide dismutase/catalase mimetics by the use of an oxidation-sensitive nanocarrier/enzyme conjugate.Bioconjug Chem 2012;23:438–49.
[48]Sokolovskaya E,Rahmani S,Misra A,et al.Dual-stimuli responsive microparticles.ACS Appl Mater Interfaces 2015;7:9744–51.
[49]Ma N,Li Y,Ren H,et al.Selenium-containing block copolymers and their oxidation-responsive aggregates.Polym Chem 2010;1:1609–14.
[50]Tan X,Yu Y,Liu K,et al.Single-molecule force spectroscopy of selenium-containing amphiphilic block copolymer:toward disassembling the polymer micelles.Langmuir 2012;28:9601–5.
[51]Liang J,Liu B.ROS-responsive drug delivery systems.Bioeng Transl Med 2016;1:239–51.
[52]Fredga A.Organic selenium chemistry.Ann N Y Acad Sci 1972;192:1–9.
[53]Cao W,Gu Y,Li T,et al.Ultra-sensitive ROS-responsive tellurium-containing polymers.Chem Commun(Camb)2015;51:7069–71.
[54]Fan F,Wang L,Li F,et al.Stimuli-responsive layer-by-layer tellurium-containing polymer films for the combination of chemotherapy and photodynamic therapy.ACS Appl Mater Interfaces 2016;8:17004–10.
[55]Fang R,Xu H,Cao W,et al.Reactive oxygen species(ROS)-responsive tellurium-containing hyperbranched polymer.Polym Chem 2015;6:2817–21.
[56]Wang L,Fan F,Cao W,et al.Ultrasensitive ROS-responsive coassemblies of tellurium-containing molecules and phospholipids.ACS Appl Mater Interfaces 2015;7:16054–60.
[57]Liu X,Xiang J,Zhu D,et al.Fusogenic reactive oxygen species triggered charge-reversal vector for effective gene delivery.Adv Mater 2016;28:1743–52.
[58]Zhang D,Wei Y,Chen K,et al.Biocompatible reactive oxygen species(ROS)-responsive nanoparticles as superior drug delivery vehicles.Adv Healthc Mater 2015;4:69–76.
[59]Wang J,Zhang Y,Archibong E,et al.Leveraging H2O2levels for biomedical applications.Adv Biosys 2017;1:1700084.
[60]Grek CL,Tew KD.Redox metabolism and malignancy.Curr Opin Pharmacol 2010;10:362–8.
[61]Fang J,Seki T,Maeda H.Therapeutic strategies by modulating oxygen stress in cancer and inflammation.Adv Drug Deliv Rev 2009;61:290–302.
[62]Karimi M,Ghasemi A,Zangabad PS,et al.Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems.Chem Soc Rev 2016;45:1457–501.
[63]Lee FY,Vessey A,Rofstad E,et al.Heterogeneity of glutathione content in human ovarian cancer.Cancer Res 1989;49:5244–8.
[64]Guo J,Wang Y,Wang J,et al.A novel nanogel delivery of poly-α,β-polyasparthydrazide by reverse microemulsion and its redox-responsive release of 5-Fluorouridine.Asian J Pharm Sci 2016;11:735–43.
[65]Sun B,Luo C,Cui W,et al.Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy.J Control Release 2017;264:145–59.
[66]Xu H,Cao W,Zhang X.Selenium-containing polymers:promising biomaterials for controlled release and enzyme mimics.Acc Chem Res 2013;46:1647–58.
[67]Saravanakumar G,Kim J,Kim WJ.Reactive-oxygen-speciesresponsive drug delivery systems:promises and challenges.Adv Sci 2016;4:1600124.
[68]Kim JS,Jo SD,Seah GL,et al.ROS-induced biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics.J Ind Eng Chem 2015;21:1137–42.
[69]Shim MS,Xia Y.A reactive oxygen species(ROS)-responsive polymer for safe,efficient,and targeted gene delivery in cancer cells.Angew Chem Int Ed Engl 2013;52:6926–9.
[70]Wilson DS,Dalmasso G,Wang L,et al.Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines.Nat Mater 2010;9:923–8.
[71]Yue C,Zhang C,Gabriel A,et al.Near-infrared light triggered ROS-activated theranostic platform based on Ce6-CPTUCNPs for simultaneous fluorescence imaging and chemophotodynamic combined therapy.Theranostics 2016;6:456–69.
[72]Yuan Y,Liu J,Liu B.Conjugated-polyelectrolyte-based polyprodrug:targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source.Angew Chem Int Ed Engl 2014;53:7163–8.
[73]Song CC,Ji R,Du FS,et al.Oxidation-accelerated hydrolysis of the ortho ester-containing acid-labile polymers.ACS Macro Lett 2013;2:273–7.
[74]Su Z,Chen M,Xiao Y,et al.ROS-triggered and regenerating anticancer nanosystem:an effective strategy to subdue tumor’s multidrug resistance.J Control Release 2014;196:370–83.
[75]Kramer JR,Deming TJ.Glycopolypeptides via living polymerization of glycosylated-L-lysine N-carboxyanhydrides.J Am Chem Soc 2010;132:15068–71.
[76]Kramer JR,Deming TJ.Glycopolypeptides with a redoxtriggered helix-to-coil transition.J Am Chem Soc 2012;134:4112–5.
[77]Requena JR,Levine RL,Stadtman ER.Recent advances in the analysis of oxidized proteins.Amino Acids 2003;25:221–6.
[78]Amici A,Levine RL,Tsai L,et al.Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions.J Biol Chem 1989;264:3341–6.
[79]Kang C,Cho W,Park M,et al.H2O2-triggered bubble generating antioxidant polymeric nanoparticles as ischemia/reperfusion targeted nanotheranostics.Biomaterials 2016;85:195–203.
[80]Kim S,Park H,Song Y,et al.Reduction of oxidative stress by p-hydroxybenzyl alcohol-containing biodegradable polyoxalate nanoparticulate antioxidant.Biomaterials 2011;32:3021–9.
[81]Lirdprapamongkol K,Sakurai H,Kawasaki N,et al.Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells.Eur J Pharm Sci 2005;25:57–65.
[82]Liang JA,Wu SL,Lo HY,et al.Vanillin inhibits matrix metalloproteinase-9 expression through down-regulation of nuclear factor-kappaB signaling pathway in human hepatocellular carcinoma cells.Mol Pharmacol 2009;75:151–7.
[83]Buriak JM.Organometallic chemistry on silicon and germanium surfaces.Chem Rev 2002;102:1271–308.
[84]Dickinson BC,Huynh C,Chang CJ.A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells.J Am Chem Soc 2010;132:5906–15.
[85]Nagata M.Inflammatory cells and oxygen radicals.Curr Drug Targets Inflamm Allergy 2005;4:503–4.
[86]Sun Q,Zhou Z,Qiu N,et al.Rational design of cancer nanomedicine:nanoproperty integration and synchronization.Adv Mater 2017;29:1606628.
[87]Yu J,Qian C,Zhang Y,et al.Hypoxia and H2O2dual-sensitive vesicles for enhanced glucose-responsive insulin delivery.Nano Lett 2017;17:733–9.
[88]Liechty WB,Kryscio DR,Slaughter BV,et al.Polymers for drug delivery systems.Annu Rev Chem Biomol Eng 2010;1:149–73.
[89]Yao J,Feng J,Chen J.External-stimuli responsive systems for cancer theranostic.Asian J Pharm Sci 2016;11:585–95.
[90]Daniel KB,Callmann CE,Gianneschi NC,et al.Dual-responsive nanoparticles release cargo upon exposure to matrix metalloproteinase and reactive oxygen species.Chem Commun(Camb)2016;52:2126–8.
[91]Gupta MK,Martin JR,Werfel TA,et al.Cell protective,ABC triblock polymer-based thermoresponsive hydrogels with ROS-triggered degradation and drug release.J Am Chem Soc 2014;136:14896–902.
[92]Han P,Li S,Cao W,et al.Red light responsive diselenidecontaining block copolymer micelles.J Mater Chem B Mater Biol Med 2013;1:740–3.
[93]Pu HL,Chiang WL,Maiti B,et al.Nanoparticles with dual responses to oxidative stress and reduced ph for drug release and anti-inflammatory applications.ACS Nano 2014;8:1213–21.
[94]Wang L,Cao W,Yi Y,et al.Dual redox responsive coassemblies of diselenide-containing block copolymers and polymer lipids.Langmuir 2014;30:5628–36.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Formulation design of granules prepared by wet granulation method using a multi-functional single-punch tablet press to avoid tableting failures
- Two kinds of ketoprofen enteric gel beads(CA and CS-SA)using biopolymer alginate
- Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel
- Tablets of paliperidone using compression-coated technology for controlled ascending release
- Quantification and spatial distribution of salicylic acid in film tablets using FT-Raman mapping with multivariate curve resolution
- Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery
