植物种子油脂合成代谢及其关键酶的研究进展
2018-03-10柳延涛孔宪辉王旭文李卫华
蔡 曼 柳延涛 王 娟 孔宪辉 王旭文 李卫华 余 渝 刘 丽
(新疆农垦科学院棉花研究所;农业部西北内陆区棉花生物学与遗传育种重点实验室;新疆兵团棉花遗传改良与高产栽培重点实验室1,石河子 832000)(石河子大学;新疆兵团绿洲生态农业重点实验室2,石河子 832003)(新疆农垦科学院作物研究所3,石河子 832000)
植物体的脂肪酸代谢是维系其生命活动的基本代谢之一,也为人类提供了重要的能量来源。由于人体不能自身合成一些必需的脂肪酸,从植物种子中提取植物油食用便成为必需脂肪酸摄入的主要方式之一。植物种子中储存的脂肪酸常以三酰甘油酯(Triglyceride acyl groups,TAGs)(即在甘油骨架上附连3个脂肪酸)的形式存在。油脂是植物种子储存能量的主要形式,为种子萌发和幼苗前期生长提供必不可少的能量来源。除食用外,脂肪酸在工业生产中也有着重要的价值,如可作为生产油漆、表面活性剂、润滑剂以及尼龙、医药等原料。因此,改良植物种子中脂肪酸含量及组分以满足食用及工业需要的研究工作,越来越得到人们的重视。培育含油量更高、不饱和脂肪酸比例更健康的油料作物新品种是作物育种的任务之一。
植物脂类代谢途径非常复杂。首先在种子的发育过程中,蔗糖作为合成脂肪酸的主要碳源,从光合作用的器官(如叶片)转运到种子细胞中,通过植物糖酵解产生大量的三酰甘油合成前体,如磷酸二羟丙酮、丙酮酸。丙酮酸经过氧化脱羧形成乙酰-CoA,它运送到质体中进行脂肪酸的从头合成,合成的脂肪酸再运送到内质网与3-磷酸甘油组装形成三酰甘油,合成的三酰甘油最后运输到油体中进行储存。这个复杂的生理生化过程受各种功能酶和转录因子的调控,这些功能酶和转录因子在拟南芥[1]、油菜[2-4]、大豆[5]、玉米[6]、麻疯树[7]、棉花[8]等作物中均有报道,其中ACCase、DGAT、FAS等关键酶基因及转录因子LEC、WRI、Dof等都是研究的热点。本文综述了植物种子油脂合成代谢中参与调控该途径的关键酶和转录因子的研究进展,为开展植物油脂改良提供借鉴。
1 油脂合成途径
1.1 脂肪酸的生物合成
脂肪酸是油脂的主要组成成分,其合成主要在质体中进行,首先由乙酰CoA羧化酶(Acetyl CoA carboxylase,ACCase)催化乙酰CoA形成脂肪酸链的二碳单位的直接供体—丙二酸单酰CoA(Malonyl CoA,Mal-CoA)。再由脂肪酸合酶(Fatty acid synthase,FAS)系统,经过缩合、还原、脱水、再还原的过程进行碳链的延伸(图1)。FAS系统是一个多酶复合体,包括酰基载体蛋白(Acyl carrier protein,ACP)和6种酶,所有的催化反应均在ACP上进行。FAS催化连续循环的聚合反应,每次循环增加2个碳的酰基碳链,直至合成含有ACP的饱和脂肪酸棕榈酸(16:0-ACP)和硬脂酸(18:0-ACP),在去饱和酶的作用下形成不饱和脂肪酸,其中包括棕榈油酸和油酸等单不饱和脂肪酸及亚油酸和亚麻酸等长链多聚不饱和脂肪酸。然后,在酰基-ACP硫酯酶(acyl-ACP thioesterase,FAT)的催化下,将脂肪酸从ACP上释放出来。依据作用的底物不同,可将FAT分为FATA和FATB,它们有具有碳链长度特异性[12-13],并且其活性影响着脂肪酸的组成。
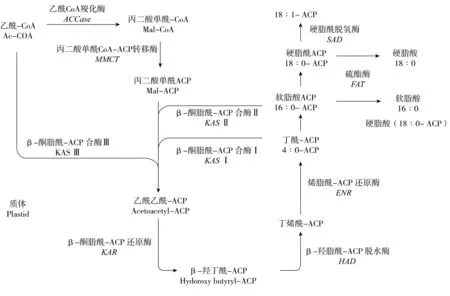
图1 脂肪酸从头合成
1.2 三酰甘油的合成
从ACP上释放的游离脂肪酸在长链脂酰辅酶A合成酶(Long-chain acyl-CoA synthetase,LACS)的作用下形成脂酰-CoA,LACS位于质体外膜,它催化形成的脂酰CoA是三酰甘油合成的底物。将三酰甘油的合成前体脂酰CoA和3-磷酸甘油运输到内质网中进行组装[14-15]。参与脂酰CoA从质体运输到内质网的转运蛋白研究的还不是很清楚,目前对其运输的机制有几个推测。其一,通过磷脂酰胆碱(Phosphatidyl cholines,PC)将合成的脂肪酸从质体运输到内质网,其作用机制可能是在质体膜上,由LPCAT催化将新合成的脂肪酸合成到PC上,然后通过PC实现从质体到内质网的运输[16]。其二,有报道ABC(ATPbinding cassette)转运蛋白也可以实现该转运过程[17]。拟南芥有12个ABCA家族(ABC家族的一个亚家族)成员,它们的功能和作用机制都还不清楚,有待进一步研究。
2 油脂合成代谢中的关键酶
2.1 乙酰CoA羧化酶
乙酰-CoA羧化酶(Acetyl CoA carboxylase,ACCase)是脂肪酸生物合成的关键酶之一。植物种子中ACCase催化乙酰CoA羧化形成丙二酸单酰CoA,是脂肪酸合成以及油脂形成的关键调控步骤,而且ACCase也是影响植物整个生命过程的重要基因[18-19]。
ACCase有异质型和同质型2种类型。在双子叶植物和非禾本科单子叶植物的质体中,ACCase为异质型,由4个亚基构成[20]:生物素羧基载体蛋白(BCCP)、生物素羧化酶(BC)、羧基转移酶的2个亚基α-CT和β-CT。同质型ACCase存在于植物细胞质中,其每个亚基兼具ACCase的所有催化功能,但只有当它们聚合成完整的酶后才有活性[21];还存在于藻类、酵母、动物及部分植物的胞质中[22]。在大多数高等植物中,同质型ACCase催化产生的丙二酸单酰CoA用于脂肪酸链的延伸和类黄酮等许多次生代谢产物的合成,而异质型则用于脂肪酸的从头生物合成[23]。
研究表明,植物种子脂肪酸的合成速率和油脂的积累同ACCase的活性密切相关性。如在大豆油脂形成的种子发育早期到中期,高油大豆ACCase的活性是低油大豆的2倍[24];拟南芥低含油量突变体wrinkled1ACCase的表达量明显小于野生型[25]。在油菜中,将油菜种子特异表达启动子与拟南芥同质型ACCase基因融合,然后在大豆转移肽的转运下,将ACCase导入油菜叶绿体,获得的转基因油菜成熟种子的ACCase活性比对照提高10~20倍,并且含油量增加5%[26]。由于异质型比同质型的结构更为复杂,所以对异质型的研究也相对较少。研究发现,蓖麻种子发育过程中,ACCase反应活性及BC和BCCP的表达量与油脂积累存在着一定的对应关系[27]。将羧基转移酶β亚基(accD)在各种组织的质体中过量表达导致转基因植株叶片脂肪酸含量增加,植株叶片明显增长,虽然转基因后代种子的脂肪酸含量与野生型没有显著变化,但种子产量提高近2倍,从而提高单株种子的含油量[28]。
2.2 脂肪酸合成酶
脂肪酸合成酶(Fatty acid synthesis,FAS)是一个多酶复合体。FAS主要由6种酶构成:乙酰CoA-ACP转移酶、丙二酸单酰CoA-ACP转移酶(MCAT)、β-酮脂酰-ACP合酶(KAS)、β-酮脂酰-ACP还原酶(KAR)、β-羟脂酰-ACP脱水酶(HAD)、烯脂酰-ACP还原酶(ENR)。其中KAS、KAR、HAD和ENR分别调控催化、缩合、还原、脱水和再还原过程,形成了一个脂肪酸延长酶系统,其功能与FAS类似,只是将乙酰-CoA用中链或长链酰基-CoA代替[29],反应过程中各种酰基均以酰基-CoA的形式参与反应,而不是以酰基-ACP形式参与反应[30]。
目前,研究最为广泛的脂肪酸合成酶是植物质体FASII的β-酮脂酰-ACP合酶(KAS),它由3种酶构成,分别为KASI、KAS II和KASIII。KASIII催化乙酰CoA结合丙二酰CoA生成4:0-ACP,KASI催化4:0-ACP到16:0-ACP的碳链延长,而KAS II则催化16:0-ACP到18:0-ACP的生成[31-32]。前人研究表明通过控制KASIII基因表达可以改善油料作物的脂肪酸组成,如基因敲除突变油菜的KASIII和FATB基因,使得双突变体的中链脂肪酸各成分含量提高[33]。KASI在脂肪酸形成及积累过程具有很重要的作用[34],拟南芥kasI突变型含油量及育性明显降低,通过互补表达实验恢复了突变体的育性、含油量和脂肪酸组成;而KASII突变会影响植株的表型,拟南芥kasII突变型互补表达麻疯树的JcKASII恢复了野生型的表型[35]。
KAR、HAD和ENR是脂肪酸从头合成的相关基因,在碳链延伸循环中起着重要的作用。早前研究发现,大肠杆菌ENR基因在脂肪酸的碳链延伸中起决定性作用[36]。拟南芥ENR蛋白缺失的一个突变体表现出脂肪酸合成体系的损坏以及脂类含量的下降[37]。KAR和ENR可能是脂肪酸合成所需要的,HAD则可能发挥着不同的生物化学功能[38]。Bourgis等[39]分析了油棕(高油植物)和椰枣(低油植物)果皮的油脂合成相关各基因的表达量,结果表明油棕的KAR、HAD和ENR基因表达水平明显高于椰枣,其中KAR和HAD的表达量是椰枣中的8倍。在棉花中,通过同源克隆的方法已经得到陆地棉的GhKAR、GhHAD和GhENR基因的cDNA全长[40],研究发现,KAR、HAD和ENR基因在脂肪酸的合成中有重要作用[41]。在棉花中过表达GhKAR和GhENR基因可以提高棉籽含油量,棉籽中不饱和脂肪酸相对含量较对照增加10%左右[42]。
植物体内饱和脂肪酸可在去饱和酶的作用下形成不饱和脂肪酸,其中包括棕榈油酸和油酸等单不饱和脂肪酸及亚油酸和亚麻酸等长链多聚不饱和脂肪酸。植物体去饱和酶存在于质体,以脂酰-ACP为底物,从双键向脂肪酸甲基端通过脂酰脱氢酶继续去饱和[43]。在内质网上,单不饱和脂肪酸以磷脂或甘油糖脂的形式继续去饱和,如磷脂酰胆碱上的油酸,在内质网上去饱和成为亚油酸或亚麻酸链[44]。
2.3 脂肪酸去饱和酶
饱和脂肪酸由脂酰-ACP去饱和酶催化去饱和,形成单不饱和或多不饱和脂肪酸。脂酰-ACP去饱和酶中研究最多的是硬脂酸脱氢酶(Stearoyl-ACP desaturase,SAD)和脂肪酸脱氢酶(Fatty acid desaturase,FAD)。SAD催化硬脂酸去饱和产生油酸,然后以脂形式存在的油酸被运转到类囊体膜中或进入细胞质中进一步去饱和;若要进一步去饱和形成多不饱和脂肪酸则需FAD[45]。SAD和FAD是决定脂肪酸不同组分含量的2个关键酶,可以通过调节它们编码基因的表达来改善油脂品质。有相关研究证明,FAD2沉默会明显的提高植物油酸的含量,具体为使甘蓝型油菜和芥菜型油菜的油酸质量分数分别升高至89%和75%[46]。在棉花中已成功构建了种子特异性启动子NAPIN调控的ihpRNA干扰表达载体和针对GhFAD2-1基因的人工miRNA表达载体[47]。通过抑制SAD基因的表达可以提高硬脂酸含量,在棉花相关研究中曾有报道,利用干涉技术来抑制SAD1的表达使得棉籽的硬脂酸质量分数从2%增加到40%,油酸质量分数从15%提高到77%[48]。SAD可以影响FA组成,还可以增强植物抗逆性,如烟草质体中过表达2种野生型马铃薯的Δ9脱氢酶基因后,改变了其脂肪酸组成,增加叶片和种子的不饱和脂肪酸含量,提高了植株耐寒性[49]。在棉花中,通过同源克隆的方法已经得到陆地棉的GhSAD2基因的cDNA全长,但它们对脂肪酸累积及抗寒性方面的作用及有待进一步研究[50]。
2.4 二酰甘油脂酰转移酶
二酰甘油脂酰转移酶(Diacylglycerol acyltransferase,DGAT)是三酰甘油合成的限速酶,其作用是催化二酰甘油加上脂肪酸酰基形成三酰甘油。1998年首次在小鼠中发现DGAT的cDNA[51],随后在拟南芥中克隆到了DGAT1的cDNA[52],以后在土壤真菌拉曼被孢霉中也克隆到DGAT2的2个同源基因DGAT2A和DGAT2B[53]。
植物主要发现有3种类型的DGAT:DGAT1、DGAT2和DGAT3[54]。DGAT1是植物种子油脂合成的关键酶,过量表达AtDGAT1,使得转基因拟南芥种子中的DGAT活性比野生型高10%~70%,种子的千粒重和含油量也比野生型高[55]。DGAT2则被认为是大量累积特殊脂肪酸(如蓖麻油酸)的主控基因,但DGAT2也在橄榄[56]和油棕[57]中参与常规TAG的累积。在玉米中表达真菌粗糙脉孢霉的短链S-NcDGAT2,小幅度提高了玉米的含油量,改变了脂肪酸的组成,提高了油酸含量降低了亚油酸含量,并对产量没有显著影响[58]。DGAT3研究的比较少,花生DGAT3在花后8~24 d的种子中特异表达。然后在种子成熟过程中逐渐降低直至表达消失,而在叶和根中却没有表达[59]。
对于DGAT在调控油脂合成方面的研究,主要集中于DGAT1与DGAT2基因家族,尽管这2种DGAT的蛋白序列存在差异,但它们都具备催化二酰甘油结合脂酰-CoA形成三酰甘油的功能。一般来说,在大部分植物中DGAT1在三酰甘油合成代谢中的作用更加广泛,而DGAT2更侧重于特殊脂肪酸的积累,两者的作用并不相互排斥[60]。Wurie等[61]研究发现DGAT2作用于DGAT1基因的上游,影响TAG合成与储藏。研究表明,DGAT的表达影响植物种子发育,并影响种子含油量,脂肪酸组成与种子粒重等[62]。
3 三酰甘油生物合成过程中的转录因子
3.1 LEC
LEC(Leafy cotyledon)是胚胎发生发育过程中关键的调控因子,它控制胚胎发育过程中的多个方面,同时它也对脂肪酸的合成起着重要的调控作用。LEC2、FUS3和ABI3属于植物特异性转录因子B3超级家族成员,三者共同构成AFL(ABI3/FUS3/LEC2)家族[63]。这些转录因子可以调控发育成熟期种子的性状,如油脂积累、子叶特性等。其中LEC2和LEC1可互相上调对方表达[64]同时也可影响其他转录因子的表达。已有研究证明LEC1和LEC2是影响WRI表达的上游调控基因[65-66]。拟南芥LEC的突变体lec1、lec2和fus3存在显著的胚胎成熟缺陷[67]。
Li等[68]克隆了花生LEC1两个成员的cDNA,研究发现,LEC1在花生种子发育的不同时期表达有差异,种子的表达水平最高。过表达AtLEC1和BnLEC1基因,使得转基因拟南芥植株脂肪酸的种类和脂质的含量大幅度提高。有研究表明LEC可能是通过增加流向脂肪酸合成的碳流,从而增加脂肪酸含量[69],具体可能是通过调控脂肪酸合成相关基因来影响油份含量,包括编码乙酰辅酶A羧化酶、控制脂肪酸合成的关键酶以及参与糖酵解和脂质积累的基因[70]。
3.2 WRI
WRI(WRINKLED1)是种子油脂合成及累积调控的关键转录因子,它能直接调控糖酵解和脂肪酸代谢过程,进而提高脂肪酸合成相关基因的整体表达水平。该基因编码蛋白含有2个AP2/EREBP结构域,控制种子蔗糖转化成油脂[71]。
1998年,首次发现了具有种子皱缩表型的拟南芥突变体,命名为wrinkled1(wri1),该突变体含油量下降了80%,蔗糖含量增加,且糖酵解相关的酶活性均普遍下降[72]。通过cDNA芯片等方法分析发现,在种子油形成中WRI主要在转录水平上调控质体糖酵解途径的关键酶[73]、脂肪酸合成相关的酶来调控油脂的积累。与种子油类似,在非种子油油棕的中果皮中,WRI通过调控其下游基因丙酮酸激酶(PK)、丙酮酸脱氢酶(PDH)、生物素羧基载体蛋白1(BCCP1)、ENR、己糖激酶(HXK)的表达来影响油脂的含量。
近年来,有不少有关WRI用于作物育种的研究报道。如在拟南芥中过表达油菜BnWRI1基因,其种子含油量增加了10%~40%[74]。玉米中过表达ZmWRI1基因,转基因后代株系中含油量的明显增加并且没有造成淀粉含量的下降[75],并对其他农艺性状也没有明显影响[76]。Li等[77]在油菜中过表达BnWRI1使花期提前,并且种子含油量提高18%~38%。另外,在单子叶植物二穗短柄草(Brachypodiumdistachyon)的叶片中异位表达WRI1后,会使叶片中的TAG含量提高32.5倍,游离的FA含量提高2倍[78]。
3.3 Dof
Dof(DNA binding with one finger)是植物特有的一类转录因子,参与高等植物的复杂生理活动的调控,如植物对激素、光的响应,同时它还参与脂肪酸的合成调控。在Dof蛋白的N末端有一个独特的由52个氨基酸组成的高度保守的Dof结构域,而在这个保守的结构域中CX2CX21CX2C基序形成一个单锌指结构,单锌指结构中1个Zn2+与4个Cys残基共价结合[79]。
首先在玉米中鉴定Dof转录因子[80]。大豆中鉴定到28个Dof转录因子,其中GmDof4和GmDof11参与油脂合成。有研究表明,GmDof4和GmDof11能分别与ACCase和长链乙酰CoA合成酶基因的启动子区域结合,激活该基因的表达,从而增加种子油脂的含量[81]。
4 展望
植物种子是植物油的主要来源,改良植物种子脂肪酸组成和提高含油量是油料作物研究的永恒课题。油脂合成是一个复杂的生理生化过程,有关油分改良的研究主要集中在植物脂肪酸合成途径和三酰甘油合成途径上,目前,可以通过分子标记辅助育种以及转基因手段来提高植物油份含量以及改变油分的组成,达到作物改造的目的。研究表明,增强三酰甘油途径中酰基转移酶的表达比增强脂肪酸合成能更有效地提高种子含油量[82]。目前油脂合成相关基因在植物抗逆方面也有大量的研究,如脂肪酸合酶复合体的关键基因KAR、ENR在碳链延伸循环中起着重要的作用,通过测定抗寒指标,研究其转基因株系维持细胞渗透调节能力有所增强,抗寒性有所提高[41]。研究最多的去饱和酶SAD基因,在银杏和棉花中低温胁迫诱导结果表明,SAD基因在不同程度低温处理下均有上调表达[83]。油脂生物合成的相关基因在植物抗逆方面也有大量的研究,如乙酰辅酶A羧化酶ACCase在除草剂方面的研究[84]。油脂生物合成涉及大量的基因,筛选出对种子油份改良作用贡献大并更能适应逆境胁迫的相关基因是下一步的工作重点。近年来,随着人们生活水平的不断提高,对植物油的品质需求也越来越高。在科研工作者的不断努力下,脂肪酸合成、TAG组装过程与油脂代谢调控网络越来越清晰,通过现代基因工程技术来改良植物油脂含量和品质、提高植物抗逆性将会是一条有效途径。
[1]WANG H Y,GUO J H,LAMBERT K N,et al.Developmental control ofArabidopsisseed oil biosynthesis[J].Planta,2007,226:773-783
[2]KANG F,RAWSTHORNE S.Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape(BrassicanapusL.)[J].Plant Journal,1994,6:795-805
[3]ZHAO J,BECKER H C,ZHANG D,et al.Conditional QTL mapping of oil content in rapeseed with respect to protein content and traits related to plant developmentand grain yield[J].Theoretical and Applied Genetics,2006,113:33-38
[4]WU J G,SHI C H,ZHANG H Z.Partitioning genetic effects due to embryo,cytoplasm and maternal parent for oil content in oilseed rape(BrassicanapusL.)[J].Genetics and Molecular Biology,2006,29:533-538
[5]TEICHERT S A,AKOH C C.Stearidonic acid soybean oil enriched with palmitic acid at the sn-2 position by enzymatic interesterification for Use as human milk fat analogues[J].Journal of Agricultural Food and Chemistry,2011,59:5692-5701
[6]LI H,PENG Z Y,YANG X H,et al.Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels[J].Nature Genetics,2013,45:43-50
[7]QU J,MAO H Z,CHEN W,et al.Development of marker-free transgenicJatrophaplants with increased levels of seed oleic acid[J].Biotechnology for Biofuels,2012(5):10
[8]LIU Z J,GUO B S,ZHANG Y,et al.Research and improvement on the oil content and composition of cottonseed[J].Molecular Plant Breeding,2012,10(6):1038-1048
[9]SIMCOX P D,REID E E,CANVIN D T,et al.Enzymes of the glycolytic and pentose phosphate pathways in proplastids from the developing endosperm ofRicinuscommunisL[J].Plant Physiology,1977,59:1128-1132
[10]STITT M,REES T A.Estimation of the activity of the oxidative pentose phosphate pathway in pea chloroplasts[J].Phytochemistry,1980,19:1583-1585
[11]HUANG Y,WANG J F,ZHANG H S.Advances on plant pentose phosphate pathway and its key enzymes[J].Chinese Bulletin Botany,2004,21(2):139-145
[12]JONES A,DAVIES H M,VOELKER T A.Palmitoyl-Acyl carrier protein(ACP)thioesterase and the evolutionary origin of plant acyl-ACP thioesterases[J].Plant Cell,1995,7(3):359-371
[13]SALAS J J,OHLROGGE J B.Characterization of substrate specicity of plantFatAandFatBacyl-ACP thioesterases[J].Archives Biochemistry Biophysics,2002,403:25-34
[14]BAUD S,LEPINIEC L.Physiological and developmental regulation of seed oil production[J].Progress in Lipid Research,2010,49:235-249
[15]MANUEL A,TRONCOSO P,ARUNA K,et al.Comparative deep transcriptional proling of four developing oilseeds[J].Plant Journal,2011,68:1014-1027
[16]TJELLSTROM H,YANG Z,ALLEN D K,et al.Rapid kineticlabeling of Arabidopsis cell suspension cultures:implicationsfor models of lipid export from plastids[J].Plant Physiology,2012,158:601-611
[17]KIM S,YAMAOKA Y,ONO H,et al.AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum[J].Proceedings of the National Academy of Sciences of USA,2013,110:773-778
[18]BAUD S,GUYON V,KRONENBERGER J,et al.Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development inArabidopsis[J].Plant Journal,2003,33:75-86
[19]WANG F L,WU G T,LANG C X,et al.Acetyl-CoA Carboxylase in Plant[J].Plant Physiology Communications,2006,42(1):10-14
[20]ALBAN C,JOB D,DOUCE R.Biotin metabolism in plants[J].Annual Review of Plant Physiology and Plant Molecular Biology,2000,51:17-47
[21]GUO G G.Basic Biochemistry[M].Beijing:Higher Education Press,2001:211-233
[22]ROESSLER P G,OHLROGGE J B.Cloning and characterization of the gene that encodes in the algaCyclotellacryptica[J].Journal Biological Chemistry,1993,268:19254-19259
[23]NIKOLAU B J,OHLROGGE J B,WURTELE E S.Plant biotin-containing carboxylases[J].Archives Biochemistry Biophysics,2003,414:211-222
[24]GENGENBACH B G,SOMERS D A,WYSE D L,et al.Methods and an acetyl-CoA carboxylase gene for conferring herbicide tolerance and an alteration in oil content of plants:United States, Us6069298[P].2000
[25]SASAKI Y,NAGANO Y.Plant acetyl-CoA carboxylase:structure,biosynthesis,regulation,and gene manipulation for plant breeding[J].Bioscience Biotechnology Biochemistry,2004,68:1175-1184
[26]ROESLER K,SHINTANI D,SAVAGE L,et al.Targeting of theArabidopsishomomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds[J].Plant Physiology,1997,113:75-81
[27]ROESLER K R,SAVAGE L J,SHINTANI D K,et al.Co-purification,co-immunoprecipitation,and coordinate expression of acetyl-coenzyme A carboxylase activity,biotin carboxylase,and biotin carboxyl carrier protein of higher plants[J].Planta,1996,198:517-525
[28]MADOKA Y,TOMIZAWA K I,MIZOI J,et al.Chloroplast transformation with modifiedaccDoperon increased acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco[J].Plant Cell Physiology,2002,43:1518-1525
[29]JAMES D W,LIM E,KELLER J,et al.Direcled tagging of theArabidopsisFATTY ACID ELONGA TIONI(EAE1)gene with maize transposoa aclivator[J].Plant Cell,1995(7):309-319
[30]DITTRICH F,ZAJONC D,HUHNE K,et al.Fatty acid elongation in yeast biochemical charateristics of the enzyme system and isolation of elongation-defective mutants[J].European Journal of Biochemistry,1998,252:477-485
[31]PIDKOWITCH M S,NGUYEN H T,HEILMANN I,et al.Modulating seed ß-ketoacyl-acyl carrier protein synthase II level converts the composition of a temperate seed oil to that of a palm-like tropical oil[J].Proceedings of the National Academy of Science USA,2007,104:4742-4747
[32]LI X F.Cloning and analysis of genes of fatty acid biosynthesis-related enzymes(KASI、FatB)and genetic transformation ofahFAD2BinArachishypogaeaL.[D].Beijing:China Agricultural University,2010
[33]WU G Z,XUE H W.Arabidopsisβ-ketoacyl-[Acyl Carrier Protein]synthase is crucial for fatty acid synthesis and plays a role in chloroplast division and embryo development[J].Plant Cell,2010,22:3726-3744
[34]WEI Q,LI J,ZHANG L,et al.Cloning and characterization of a β-ketoacyl-acyl carrier protein synthaseⅡ fromJatrophacurcas[J].Plant Physiology,2012,169:816-824
[35]STOLL C,LÜHS W,ZARHLOUL M K,et al.Knockout of KASIII regulation changes fatty acid composition in canola(BrassicanapusL.)[J].European Journal of Lipid Science and Technology,2010,108:277-286
[36]RICHARD J H,CHARLES O R.Enoyl-acyl carrier protein reductase(fabI)plays a determinant role in completing cycles of fatty acid elongation inescherichiacoli[J].Journal of Biological Chemistry,1995,270:26538-26542
[37]MOU Z,HE Y K,DAI Y,et al.Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology[J].Plant Cell,2000,12:405-417
[38]CHI X Y,YANG Q L,PAN L J,et al.Cloning and expression analysis of β-ketoacyl-ACP reductase,β-hydroxyacyl-ACP dehydrase and enoyl-ACP reductase fromArachishypogaeaL.[J].Genomics and Applied Biology,2010(2):1-9
[39]BOURGIS F,KILARU A,CAO X,et al.Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning[J].Proceedings of the National Academy of Sciences of USA,2011,108(30):12527-12532
[40]ZHAO P.Isolation andanalysis of fatty acid synthasis related genesGhKAR,GhHAD,GhENRin upland cotton[D].Beijing:China Agricultural University,2012
[41]LIU L,WANG Y M,ZHAO Y P,et al.Construction and function ofGhKARandGhENRexpression vector of fatty acid synthase gene in cotton[J].Cotton Science,2016,6:527-537
[42]LIU L.Gene expression profiling during fiber secondary cell wall development and functional verification ofGhKAR,GhHADandGhENRin cotton[D].Beijing:China Agricultural University,2015
[43]GUO G G.Basic Biochemistry[M].Beijing:Higher Education Press,2001:211-233
[44]WANG J Y,ZHU S G,XU C F.Biochemistry[M].Beijing:Higher Education Press,2004:265
[45]LI X F.Cloning and analysis of genes of fatty acid biosynthesis-related enzymes(KASI、FatB)and genetic transformation ofahFAD2BinArachishypogaeaL[D].Beijing:China Agricultural University,2010
[46]OHLROGGE J B,BROWSE J A.Lipid biosynthesis[J].Plant Cell,1995,7:957-970
[47]ZHAO L Q,LI R,LI W,et al.Cloning of delta-12 oleate desaturase geneFAD2-1 and construction of its ihpRNA and amiRNA interference vectors fromGossypiumhirsutum[J].Cotton Science,2011,23(2):189-192
[48]LIU Q,SURINDER P S,ALLAN G G.High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing[J].Plant Physiology,2002,129:1732-1743
[49]CRAIG W,LENZI P,SCOTTI N,et al.Transplastomic tobacco plants expressing a fatty acid desaturase gene exhibit altered fatty acid profiles and improved cold tolerance[J].Transgenic Research,2008,17:769-782
[50]CAI M,LI W H,WANG J,et al.Molecular cloning and expression analysis of stearoyl-ACP desaturase gene(GhSAD2)in upland cotton(GossypiumhirsutumL.)[J].Acta Botanica Boreali-Occidentalia Sinica,2016,9:1713-1720
[51]CASES S,SMITH S J,ZHENG Y W,et al.Identification of a gene encoding an acyl-CoA:diacylglycerol acyltransferase,a key enzyme in triacylglycerol synthesis[J].Proceedings of the National Academy of Sciences of USA,1998,95:13018-1323
[52]HOBBS D H,LU C,HILLS M J.Cloning of a cDNA encoding diacylglycerol acyltransferase fromArabidopsisthalianaand its functional expression[J].Febs Letters,1999,452:145-149
[53]LARDIZABAL K D,MAI J T,WAGNER N W,et al.DGAT2 is a new diacylglycerol acyltransferase gene family:purification,cloning,and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity[J].Journal of Biological Chemistry,2001,276:38862-38869
[54]WANG T T,MA X L,LI F L,et al.Advances in plant diacylglycerol acyltransferases and the coding genes[J].Guangdong Agriculture Science,2012,39(6):127-130
[55]JAKO C,KUMAR A,WEI Y D,et al.Seed-specific over-expression of anArabidopsiscDNA encoding a diacylglycerel acyltransferase enhances seed oil content and seed weight[J].Plant Physiology,2001,126:861-874
[56]ALAGNA F,AGOSTINO N,TORCHIA L,et al.Comparative 454 pyrosequencing of transcripts from two olive genotypes during fruit development[J].BMC Genomics,2009,10:399
[57]BOURGIS F,KILARU A,CAO X,et al.Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning[J].Proceedings of the National Academy of Sciences of USA,2011,108(30):12527-12532
[58]OAKES J,BRACKENRIDGE D,COLLETTI R,et al.Expression of fungal diacylglycerol acyltransferase2 genes to increase kernel oil in maize[J].Plant Physiology,2011,155:1146-1157
[59]SAHA S,ENUGUTTI B,RAJAKUMARI S,et al.Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferasc[J].Plant Physiology,2006,141:1533-1543
[60]SHOCKEY J M,GIDDA S K,CHAPITAL D C,et al.Tung Tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum[J].The Plant Cell,2006,18(9):2294-2313
[61]WURIE H R,BUCKETT L,ZAMMIT V A.Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids inHepG2 cells[J].Febs Journal,2012,279(17):3033-3047
[62]TAYLOR D C,ZHANG Y,KUMAR A,et al.Molecular modification of triacylglycerol accumulation by over-expression of DGAT1to produce canola with increased seed oil content under field conditions[J].Botany,2009,87:533-543
[63]PARCY F,VALON C,KOHARA A,et al.TheABSCISICACID-lNSENSITIVE3,FUSCA3,andLEAFYCOTYLEDON1 loci act in concert to control multiple aspects ofArabidopsisseed development[J].Plant Cell,1997(9):1265-1277
[64]TO A,VALON C,SAVINO G,et al.A network of local and redundant gene regulation governsArabidopsisseed maturation[J].Plant Cell,2006,18:1642-1651
[65]WESELAKE R J,TAYLOR D C,RAHMAN M H,et al.Increasing the flow of carbon into seed oil[J].Biotechnology Advances,2009,27:866-878
[66]BAUD S,MENDOZA M S,TO A,et al.WRINKLED1 specifies the regulatory action ofLEAFYCOTYLEDON2 towards fatty acid metabolism during seed maturation inArabidopsis[J].Plant Journal,2007,50:825-838
[67]MEINKE D W,FRANZMANN L H,NICKLE T C,et al.Leafy cotyledon mutants ofArabidopsis[J].Plant Cell,1994,6:1049-1064
[68]LI A Q,XIA H,WANG X J,et al.Cloning and expression analysis of peanut(ArachishypogaeaL.)LEC1[J].Acta Botanica Boreali-Occidentalia Sinica,2009,29(9):1730-1735
[69]TAN H L,YANG X L,ZHANG F X,et al.Enhanced seed oil production in canola by conditional expression of brassica napusLEAFYCOTYLEDON1 andLEC1-LIKEin developing seeds[J].Plant Physiology,2011,156:1577-1588
[70]MU J Y,TAN H L,ZHENG Q,et al.LEAFYCOTYLEDON1 is a key regulator of fatty acid biosynthesis inArabidopsis[J].Plant Physiology,2008,148:1042-1054
[71]LU Y P,LIU F Z,WAN Y S.In silico Analysis of peanut transcription factor geneWRI1[J].Molecular Plant Breeding,2012,3(10):363-370
[72]FOCKS N,BENNING C.Wrinkled1:a novel,low seed oil mutant ofArabidopsiswith efficiency in the seed-specific regulation of carbohydrate metabolism[J].Plant Physiology,1998,118:91-101
[73]RUUSKA S A,GIRKE T,BENNING C,et al.Contrapuntal network of gene expression duringArabidopsisseed filling[J].Plant Cell,2002,14:1191-1206
[74]LIU J,HUA W,ZHAN G M,et al.Increasing seed mass and oil content in transgenicArabidopsisby the overexpression of wri1-like gene fromBrassicanapus[J].Plant Physiology and Biochemistry,2010,48:9-15
[75]POUVREAU B,BAUD S,VERNOUD V,et al.Duplicate maizeWrinkled1 transcription factors activate target genes involved in seed oil biosynthesis[J].Plant Physiology,2011,156:674-686
[76]SHEN B,ALLEN W B,ZHENG P,et al.Expression ofZmLEC1 andZmWRI1 increases seed oil production in maize[J].Plant Physiology,2010,153:980-987
[77]LI Q,SHAO J H,TANG S H,et al.Wrinkled1 accelerates flowering and regulates lipid homeostasis between oil accumulation and membrane lipid anabolism inBrassicanapus[J].Front Plant Science,2015,6(192):1-15
[78]YANG Y,MUNZ J,CASS C,et al.2015,Ectopic expression ofWRlNKLED1 affects fatty acid homeostasis inBrachypodiumdistachyonvegetative tissues[J].Plant physiology,2015,169(3):1836-1847
[79]YANAGISAWA S.Dof domain proteins:plant-specific transcription factors associated with diverse phenomena unique to plants.Plant Cell Physiology,2004,45:386-391
[80]YANAGISAWA S,IZUI K.Molecular cloning of two DNA-binding proteins of maize that are structurally different but interact with the same sequence motif[J].The Journal of Biological Chemistry,1993,268:16028-16036
[81]WANG H W,ZHANG B,HAO Y J,et al.The soybeanDof-type transcription factor genes,GmDof4 andGmDof11,enhance lipid content in the seeds of transgenicArabidopsisplants[J].Plant,2007,52:716-729
[82]WU L S H,HONG G H H,HOU R F,et al.Classification of the single oleosin isoform and characterization of seed oil bodies in gymnosperms[J].Plant Cell physiology,1999,40:326-334
[83]ZHAO N,ZHANG Y,et al.Identification and expression of a stearoyl-ACP desaturase gene responsible for oleic acid accumulation inXanthocerassorbifoliaseeds[J].Plant Physiology and Biochemistry,2015,87:9-16
[84]DU L,LIU W T,YUAN G H,et al.Cross-resistance patterns to ACCase-inhibitors in American sloughgrass(BeckmanniasyzigachneSteud.)homozygous for specific ACCase mutations[J].Pesticide Biochemistry and Physiology,2016,126:42-48.
