Release performance and sustained-release efficacy of emamectin benzoate-loaded polylactic acid microspheres
2018-03-07YlNMingmingZHUXinyanCHENFuliang
YlN Ming-ming, ZHU Xin-yan, CHEN Fu-liang
Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, P.R.China
1.Introduction
Emamectin benzoate is a novel macrocyclic lactone insecticide isolated through fermentation of the naturally occurring avermectin molecule produced by the soil microorganism Streptomyces avermitilis Kim & Goodfellow(Ioriatti et al.2009; Li et al.2010).It is stable in aqueous solutions of pH 5-8 (at 20°C) but easily loses stability under UV light (Zhu et al.2011).Application of this insecticide is limited by its quick degradation when applied to agricultural soils.However, microsphere formulations of emamectin can protect from UV degradation and decrease soil adsorption.Microspheres (solid spherical matrices) or microcapsules (inner core and outer shell) are created by uniformly dispersing or encapsulating a pesticide’s active ingredient into a biodegradable carrier polymer via physical and chemical methods (Zhang 2004; Zhou et al.2011).With the degradation of the carrier, the active ingredient can be fully released for its insecticidal function (Lu 2006;Sun et al.2008).Emamectin benzoate microspheres are encapsulated inside a polymer carrier, such as gelatin or polylactic acid, for soil application.Soil adsorption is reduced as a result of the pesticide-soil direct contact, prolonging insecticidal durability and improving root knot nematodes prevention.Drug-loaded polylactic acid microspheres have been historically prepared using an emulsificationsolvent evaporation method (Bodmeier and Mcginity 1987).This method yielded a higher drug loading delivery system that allowed for faster drug release.Furthermore,the pH during drug preparation could also significantly alter drug release rates.Another study prepared an emamectin benzoate-microcystis sustained-release formulation to explore its release kinetics (Wu 2011).The Ritger-Peppas equation (Mt/M0=ktn) was used to perform linear fitting.Currently, microspheres are mainly utilized in the medical field for applications in pharmaceutical drug release systems.Reports regarding pesticide applications are limited and few studies investigating release models of pesticide-loaded microspheres have been reported.In the present study, release curves of emamectin benzoateloaded polylactic acid microspheres in media under three different pH levels were examined.A zero order kinetics model (Q=a+bt), the first-order kinetics model(Q=a(1-ebt)), and the Higuchi model were applied to fit the experimental data, and the proper kinetics model for our microspheres was established based on a previous study(Wu et al.2007).The influence of particle size on release behavior was observed for future use as a reference for practical applications.Three percent emamectin benzoate microemulsion and distilled water were used as controls.The sustained-release efficacy of the emamectin benzoate-loaded polylactic acid microspheres on the diamondback moth (Plutella xylostella L.) was tested for toxicity effects.
2.Materials and methods
2.1.Agents and equipment
The chemical agents used in experiments were as follows:70% emamectin benzoate technical material (TC) (Hebei Weiyuan Biochemical Limited Co., Ltd., China), polylactic acid (injection molding grade, Mr=200 000, Shenzhen Guanghua Weiye Co., Ltd., China), methylene dichloride(analytical grade, Shanxi Xilong Chemical Factory, China),methanol, acetonitrile (chromatographic grade, Fisher,USA), potassium biphthalate (analytical grade, Beijing Chemical Reagent Company, China), NaOH, KH2PO4,borax and HCl (analytical grade, Beijing Chemical Factory,China), gelatin (Amresco, USA), P.xylostella, cabbage seed(Jingfeng 1, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Science), and emamectin benzoate 30ME (Institute of Plant Protection, Chinese Academy of Agricultural Sciences).
The instruments used in experiments were as follows:S10-3 constant temperature magnetic stirrer (Shanghai Sile Instrument Co., Ltd., China), 79-1 magnetic stirrer with heating (Changzhou Guohua Electric Appliance Co.,Ltd., China), Agilent 1220 Infinity HPLC System (Agilent Technologies, USA), BT-9300H Laser Particle Size Distribution Analyzer (Dandong Bettersize Instrument Co.,Ltd., China), AA-200 electronic balance (0.1 mg, Denver Instrument Company, USA), SHB-IIIA vacuum pump(Zhengzhou Greatwall Scientific Industrial and Trade Co.,Ltd., China), 120-mesh screen (Shangyu Shashai Company,China), and HI 2221 pH/ORP Temperature Tester (Hanna Instrument, Italy).
2.2.Test method
Preparation for emamectin benzoate-loaded polylactic acid microspheresOrganic phase: A certain amount of polylactic acid was dissolved in 20 mL of methylene chloride.After dissolution, emamectin benzoate was added to the organic solvent at a drug:polylactic acid ratio of 1:4 and stirred until completely dissolved.Water phase:Distilled water of 160 mL was mixed with 2% (w/v) sodium dodecyl benzene sulfonate and 0.5% (w/v) gelatin.The organic phase was slowly added into the water phase, and the system was emulsified by high-speed mixing at 30°C.Then, the emulsion was heated to 40°C until the methylene chloride had completely evaporated.After suction filtration, washing, and drying, the emamectin benzoateloaded polylactic acid microspheres were obtained.Four formulations of microspheres with different sizes, drug loadings, and encapsulation efficiencies were prepared and their drug release behaviors were compared.
Microsphere morphology and particle size distributionAn appropriate sample of emamectin benzoate-loaded polylactic acid microspheres was taken and fixed on a tape.After gold spraying using an ion plating apparatus under vacuum, the surface morphology of the microspheres was examined by scanning electron microscope.A laser particle size distribution analyzer was used to measure the particle size and distribution of the microspheres.Particle size and particle size distribution were characterized by volume median diameter (D50) and span, respectively.The smaller the span, the narrower the particle size distribution.Span=(D90-D10)/D50, and D10, D50, and D90are the particle diameters when the microspheres volumes were 10, 50,and 90%, respectively.
Drug loading measurementsHPLC was used to measure drug loading.Microspheres (30 mg, accuracy of up to 0.2 mg)were mixed with a solution (methanol:acetonitrile=1:1, v/v)and brought to a final volume of 10 mL.The solution was dissolved by ultrasound sonication for 20 min followed by filtration through 0.45-μm screen.
The HPLC conditions were as follows:
Column: waters sunfire-C18(150 mm×4.6 mm, 5 μm);mobile phase: methanol:acetonitrile:ammonium solution=45:45:10 (v/v) with the ammonium solution prepared by mixing ammonium with water at a ratio of 1:300(v/v).Flow rate: 1.0 mL min-1, column temperature: 30°C,wavelength: 245 nm, injection volume: 20 μL.
Buffer solution preparationThe following stock solutions were prepared with distilled water: 0.1 mol L-1potassium biphthalate, 0.1 mol L-1NaOH, 0.1 mol L-1KH2PO4,0.025 mol L-1borax, and 0.1 mol L-1HCl.Three buffer solutions were prepared by mixing the stock solutions at the following ratios (Liu 2012) :
pH 5: 250 mL potassium biphthalate (0.1 mol L-1)+113 mL NaOH (0.1 mol L-1), the final volume was adjusted to 500 mL using distilled water.
pH 7: 500 mL KH2PO4(0.1 mol L-1)+291 mL NaOH(0.1 mol L-1), the final volume was adjusted to 1 000 mL using distilled water.
pH 9: 250 mL borax (0.025 mol L-1)+23 mL HCl(0.1 mol L-1), the final volume was adjusted to 500 mL using distilled water.
Release rate measurementsRelease rates were determined using the dialysis bag method (Kong 2012).At room temperature, microsphere samples were enclosed in a semipermeable membrane tube that was placed in a flask containing 50 mL of media and covered with aluminum foil.A 5-mL aliquot of the media was removed at fixed intervals and replaced with equal amounts of fresh media.The 5-mL aliquot was then transferred into a 10-mL volumetric flask and dissolved into the mobile phase.Emamectin benzoate was then detected by HPLC and its concentration was calculated by the following formula:
采用氧化镁半熔法处理样品、热水浸提时发现,因氧化镁颗粒较细且带有电荷,其在水中不易凝聚,形成的沉淀体积较大,对高铼酸根有一定的吸附作用,故试验尝试采用加入凝聚剂的方式来凝聚沉淀以减少其对铼的吸附。选取2g氧化镁和铼标准溶液为试验对象,按照实验方法,分别以氨水、硝酸铵、聚乙烯醇和氢氧化钠为凝聚剂进行试验,以铼的测定值除以理论值计算铼回收率,结果见表2。
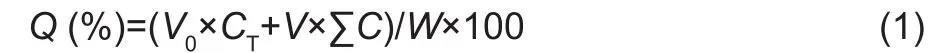
Where, Q is the cumulative release percentage (%), CTis the measured active ingredient concentration in the released medium solution at the release time point (mg mL-1), C is the measured active ingredient concentration in the released medium solution before the release time point (mg mL-1),W is the total mass of the encapsulated active ingredient(mg), V0is the total volume of the released medium solution(mL), and V is the volume of the aliquot (mL).
Emamectin benzoate microsphere release detection at different pH levelsEmamectin benzoate-loaded polylactic acid microspheres (No.1 in Table 1, (50±0.2) mg) were suspended in various buffers at pH 5, 7, and 9.Release conditions were room temperature, no light and no vibration.Microsphere detection was performed using the same procedure described above.At days 3, 6, 10, 20, 30, 45,and 60, 5-mL aliquots of the medium were collected and replaced with an equal volume of fresh medium as previously described (Han 2010).Concurrently, emamectin benzoate with the same concentration was used as a control to detect the degradation rate of the drug.The emamectin benzoate mass fraction released from microspheres was detected by HPLC and further used to correct the accumulated release rate.The following formula was used:
Corrected accumulated release rate (%)=Accumulated release rate of microspheres/(1-Degradation rate)×100
Modeling the microsphere release curvesThe zero order kinetics model (Q=a+bt), the first-order kinetics model(Q=a(1-ebt)), and the Higuchi model were applied to fit the release curves of the polylactic acid microspheres in buffers with different pH levels.Q is the accumulated release rate of emamectin benzoate, t is the release time, and a and b are the intercept and slope of the equation, respectively.
lndoor toxicity test for wettable powder containing emamectin benzoate-loaded polylactic acid microspheresAfter secondary processing, the emamectin benzoate-loaded polylactic acid microspheres were made into a wettable powder containing 5% microspheres.The microspheres were uniformly distributed in distilled water,yielding an emamectin benzoate solution at concentrations of 25, 50, and 100 mg L-1.The solution was then sprayed onto potting cabbage leaves at a dosage of 60 mL pot-1.At days 7, 15, 30, 50, and 60 after spraying, the cabbage leaves were cut (5 cm×5 cm) and placed in culture dishes.Ten P.xylostella individuals at 2nd instar (indoor-reared for>5 years and fed on commercial cabbage-leaves) were fed(25°C, relative humidity 50-80%, light period 16 h L:8 h D)in the culture dishes.The dishes were capped and covered with preservative film.Mortality rates were calculatedafter 48 h.Meanwhile, a microemulsion containing 3%emamectin benzoate was used as a positive control at a dosage of 50 mg L-1.Each process was repeated three times.Distilled water was used as a negative control.The Abbott formula was applied to correct the mortality rate of the treatment groups:
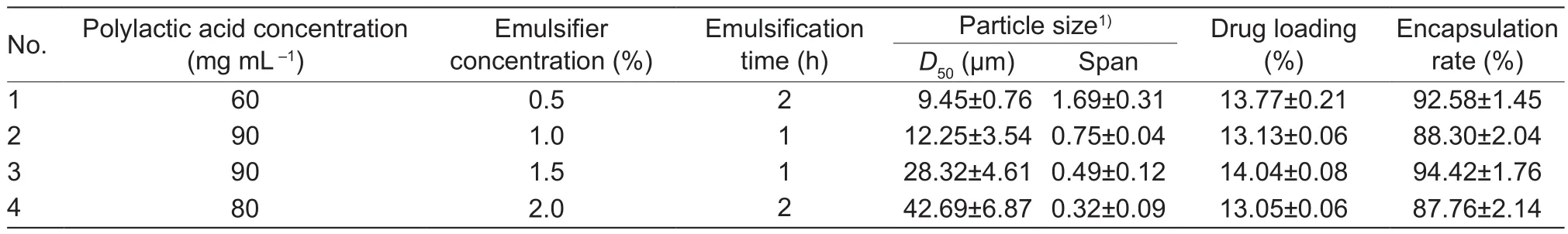
Table 1 Quality indicators of different emamectin benzoate microspheres

3.Results
3.1.Morphology and particle size distribution
The scanning electron microscope images of emamectin benzoate-loaded polylactic acid microspheres have a uniform distribution, no adhesion, and a rounded shape with a smooth surface (Fig.1).
Particle size distribution of microspheres has some influence on the release property of pesticide formulations(Table 2 and Fig.2).The D50of the emamectin benzoateloaded polylactic acid microspheres was 8.42 μm and the microsphere span range was 1.72, indicating that the microspheres particle size had a narrow distribution.
3.2.Effect of buffer pH level on the emamectin microsphere release rate
Drug release curves of emamectin benzoate showed that different pH levels affected dissolution (Fig.3).The results indicated that emamectin benzoate-loaded polylactic acid microspheres were highly capable of undergoing sustained release and the entire degradation process was relatively slow.The curves showed that in the acidic and neutral media, the release process could be divided into two stages:an initial burst release stage and a sustained release stage.During the initial release, release rates were relatively fast with 40% of the encapsulated drug being released.Thereafter, the release rate decreased and the release was sustained during the designated sustained release stage.The release rate in the alkaline medium was significantly slower than those in the acidic or neutral media.In the pH 5 medium, the accumulated release rate increased to about 60% by day 60, while the accumulated release rate increased to 50% in the pH 7 medium.In the pH 9 medium,the degradation rate of emamectin benzoate relatively was slow at 80%, giving a release rate of only 26% at day 60.
3.3.Microsphere size influence on drug release
Emamectin benzoate-loaded polylactic acid microspheres with different sizes were prepared by changing the components and emulsification time.Microsphere particles were evaluated for quality using the following indicators:particle size distribution, drug loading percentage, and encapsulation efficiency rate (Table 1).
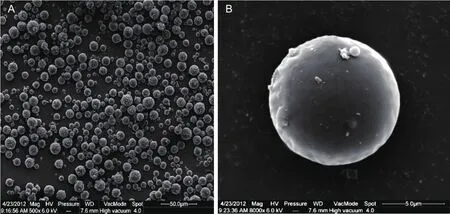
Fig.1 Scanning electron microscope images of emamectin benzoate-loaded polylactic acid microspheres.A, multiple microspheres shown at 500× magnification.B, individual microsphere shown at 8 000× magnification.

Table 2 The particle size of emamectin benzoate-loaded polylactic acid microspheres
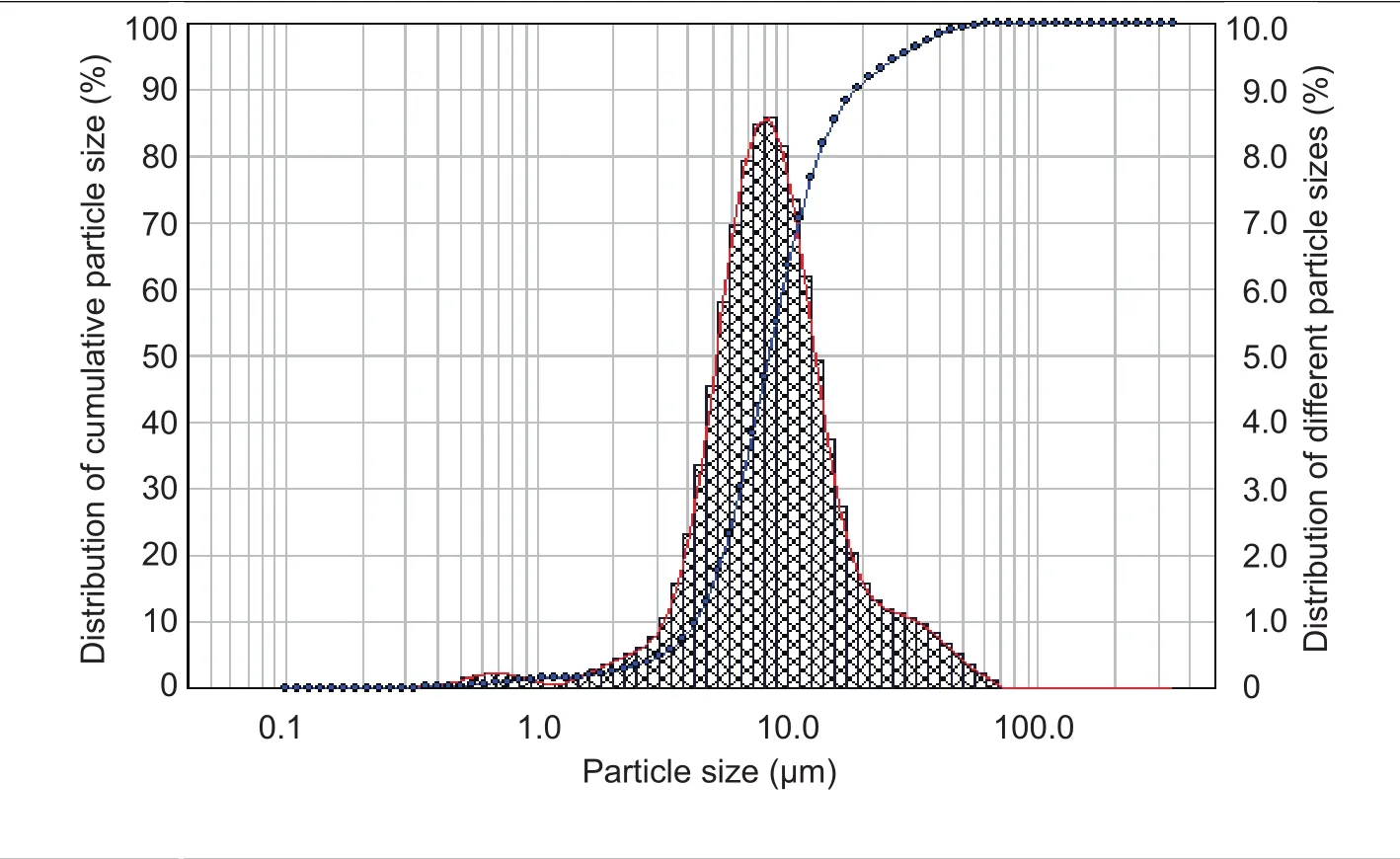
Fig.2 The particle size distribution of emamectin benzoate-loaded polylactic acid microspheres.

Fig.3 Dissolution curve of drug release in different solutions of emamectin benzoate microsphere.Q is the cumulative release percentage.Error bars indicate SD.
The release curves of four microspheres of different sizes are shown in Fig.4.The dissolution curves showed that with increasing particle size, the release rate gradually decreased.Microsphere No.1 (D50=9.45 μm) exhibited an accumulated release rate of 50% at day 20 and 60% at day 60.However, microsphere No.4 (D50=42.69 μm) had a release rate of 15% at day 20 and only 20% by day 60.
3.4.Release curve modeling
The emamectin benzoate degradation rate was very high in the alkaline buffer with pH=9, greatly influencing release rate detection.Thus, in the present study, release curve modeling was only performed in buffers with pH levels of 5 and 7.
Modeling the emamectin benzoate microsphere release curves in acidic mediumBy using the zero order kinetics,first order kinetics, and Higuchi models, the release curves in an acidic buffer at pH 5 were fitted (Fig.5).
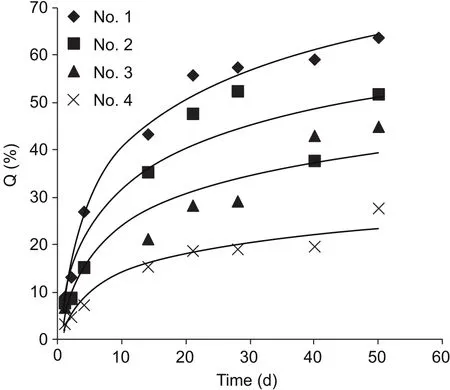
Fig.4 Drug release dissolution curve for various emamectin benzoate microsphere particle sizes.Q is the cumulative release percentage.
The zero order kinetics model gave a Q=11.408+0.867t and R2=0.8694, the first-order kinetics model gave a Q=60.271(1-e-0.045t) and R2=0.9771, and the Higuchi model gave a Q=8.6618t1/2-6.332 and R2=0.9495.
Modeling emamectin benzoate microsphere release curves in neutral mediumBy using the zero order kinetics,first order kinetics, and Higuchi models, the release curves in a neutral buffer at pH 7 were fitted (Fig.6).
The zero order kinetics model gave a Q=12.923+0.795t and R2=0.9157, the first-order kinetics model gave a Q=-55.390×(1-e-0.054t) and R2=0.968, and the Higuchi model gave a Q=8.002t1/2-3.605 and R2=0.929.
Under each of the two different pH conditions, R was obtained from the three kinetics equations (Table 3).Comparison of R values revealed that in the acidic and neutral media, the first-order kinetic model was superior to the zero order kinetics and Higuchi models.These findings thus indicated that the first-order kinetic model was more appropriate for release fitting.
3.5.Sustained-release efficacy of wettable powder containing 5% emamectin benzoate-loaded polylactic acid microspheres on P.xylostella toxicity
The indoor toxicity test of the wettable powder (Table 1, No.1)and microemulsion were measured (Table 4).At 7 days after spraying at a 50 mg mL-1microsphere spraying dosage,the mortality of P.xylostella at 48 h was 55.18%, lower than that of the microemulsion control (82.76%).However,when the concentration was increased to 100 mg mL-1, the mortality of P.xylostella at 48 h was 75.87%, similar to the mortality observed in the microemulsion control.As the spraying time increased, the efficacy of the microspheres improved.At 15 days after spraying, the mortalities at 48 h using the three concentrations were all higher than that of the microemulsion.At day 60, the mortality at 48 h using a concentration of 100 mg mL-1still remained greater than 50%.
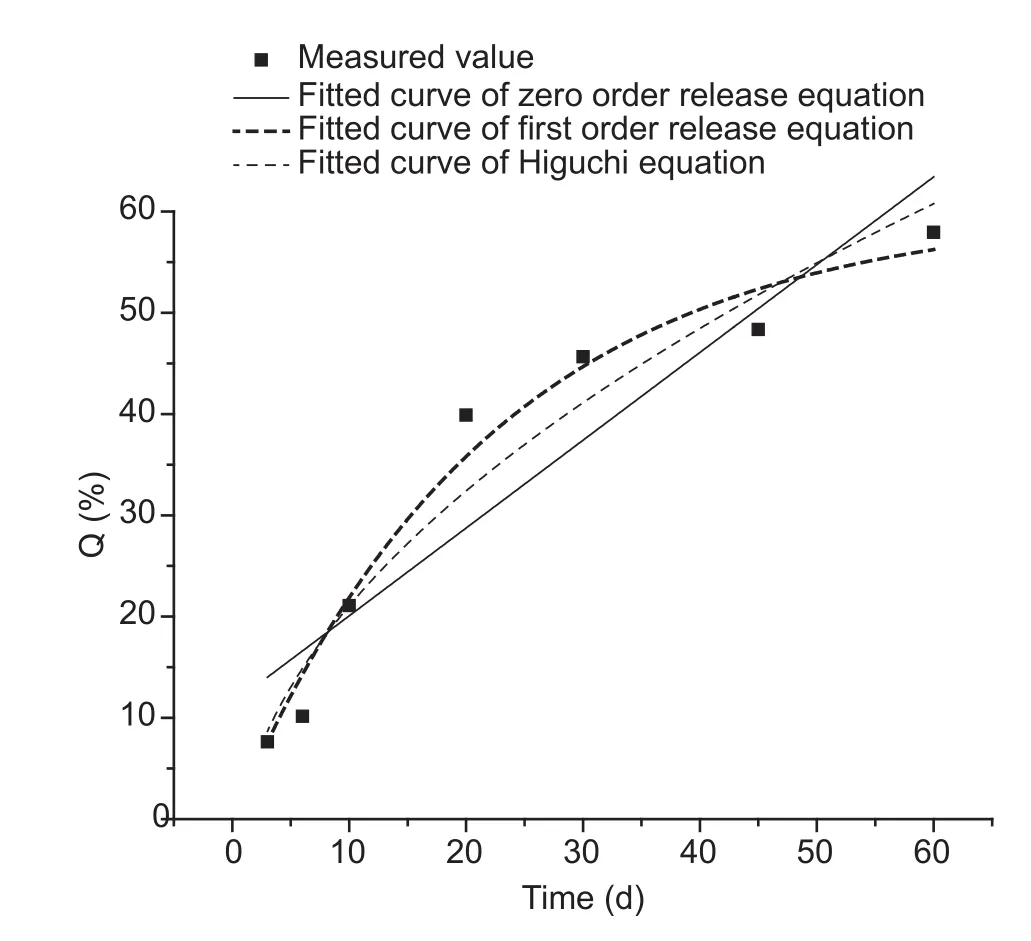
Fig.5 Fitted curves of three model equations in acid solution of emamectin benzoate microsphere.Q is the cumulative release percentage.

Fig.6 Fitted curves of three model equations in neutral solution of emamectin benzoate microsphere.Q is the cumulative release percentage.

Table 3 Comparison of R value under different solutions and models
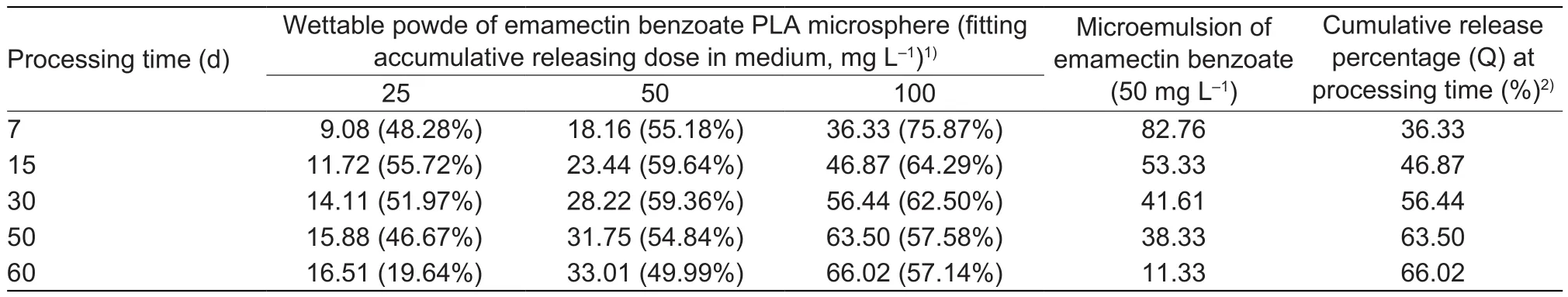
Table 4 The correlation experimental results between accumulative releasing rate of emamectin benzoate polylactic acid (PLA)microsphere and the corrected mortality of Plutella xylostella at 48 h in the laboratory
Combined with the sustained release kinetics study, when the accumulated release rate was greater than 35 mg L-1(i.e.,the accumulated released dosage efficacy at 36.33 mL L-1was 75.87%, Table 4), the microspheres achieved high efficacy with long-term persistence.The accumulated release rate of 100 mg L-1microspheres at day 60 reached the standard value, but those of dosages of 25 and 50 mg L-1did not, indicating that these were not appropriate concentrations in preventing and controlling harmful insects in the field.
4.Discussion
Microsphere-associated drug release is a complicated process that is accomplished through three main stages(Huang 2011; Xing et al.2011).First, the effective component is released via an initial burst.Prior to the initial burst, the component was first absorbed onto the surface of the microsphere, but during the burst, the component first diffused into the medium, thereby resulting in its accumulation and a high release rate within a short time.Second, the existence of water induces the polylactic acid microsphere to swell (Reeve et al.1993), and the drug diffuses out through the microsphere’s pores.Third,degradation of the carrier materials promotes drug release(Fu et al.2003; Barakat et al.2008).The release rate of the encapsulated drug is influenced by several factors, including drug properties, polymer degradation rate and particle size.
The release curves show that the release rate of emamectin benzoate from the polylactic acid microspheres was relatively slow, with an accumulated release rate of about 60% by day 60.This may be due to the fact that the degradation of polylactic acid carrier material is slow (about 3-6 months), depending on its polylactic acid molecular weight (MW) and structure (Sinclair 1973; Zhang et al.2008).In the present study, the applied polylactic acid was of an industrial injection molding grade, and the MW was relatively high (about 200 000 Da), thereby leading to a slow degradation.Furthermore, the carrier molecules are entangled with emamectin benzoate, making it difficult for the drug to reach the surface of the microspheres (Nie et al.2004; Fu 2008).Based on makeup of the microsphere formulation, the release rate was very slow and the release period was long, prolonging the persistence of emamectin benzoate.Only by increasing drug loading or the spraying dosage at the initial stage could long-term insecticidal efficacy be achieved.
The release rate was also affected by particle size.Generally, using microspheres with a smaller median size results in faster release rates.This is due to the larger specific surface that increases the area for drug diffusion,ultimately increasing the release rate (Dong 2012).The release rates of emamectin benzoate differed with various pH levels.It has been shown that the accelerating effect of hydrogen ions on polylactic acid microspheres is stronger than that of hydroxyl ions (Tsuji and Ikarashi 2004).Another study reported that the degradation rates of polylactic acid in acid and alkaline media are higher than that in neutral buffer(Ma et al.2004).However, in the present study, we found that the release rate of emamectin benzoate at the mildly acidic pH 5 was only slightly higher than that at a neutral pH 7 but higher than that at pH 9.Emamectin benzoate was stable in solutions within the pH range of 5.2-8.0 and has a degradation rate of 8.6% in a light-protected,pH 5 buffer.The emamectin was hardly degraded in the pH 7 buffer, whereas, in the pH 9 buffer, the degradation rate at day 60 was 80.9%.The detected release rate of emamectin benzoate in the pH 9 buffer at day 60 was only 26%, possibly because the degradation rate of the drug was higher than its release rate.Furthermore, we observed that the microspheres developed in our study were not appropriate for applications in slightly alkaline water or soil.For practical field applications, due to the combined impacts of natural factors such as wind, light, and microorganisms, the release rate of the drug encapsulated in the microspheres may be even higher than in our experimental findings.Microspheres drug release is an intricate process that can be influenced by various factors.Furthermore, the release mechanism is also very complex and thus requires further exploration.
The results of the efficacy testing showed that compared to the rapid-acting insecticidal efficacy of a microemulsion,microspheres possess a sustained-release effect with longer-term efficacy.Our calculations showed that with a 50% drug efficacy, only a single microsphere spray (100 mg L-1) was required for treatments of 60 days.The persistence period of the microemulsion (50 mg L-1) was only 15 days,thereby requiring the spray be applied for four times.Therefore, the microsphere dosage was half that of the microemulsion, the spray frequency was three times less,creating a dramatic decrease in application and labor costs.
The results of the release kinetics indicated that the microspheres with accumulated release rate of greater than 35 mg mL-1possessed better fast-acting properties and persistence.Thus, this value could be considered for the treatment threshold of emamectin benzoate-loaded microspheres.
5.Conclusion
Emamectin benzoate-loaded polylactic acid microspheres possessed significant sustained release properties, but were not appropriate for application under alkaline conditions.Release kinetics model fitting showed that in buffers of pH 5 and 7, the emamectin benzoate microsphere release was in accord with the first-order kinetics model.The result of indoor toxicity testing demonstrated that microspheres were faster acting and longer persisting compared to conventional formulations, exhibiting a threshold dose of 35 mg mL-1.
Acknowledgements
The study was supported by the National Key Research and Development Program of China (2016YFD0200502,2017YFD0200301).
Barakat N S, Elbagory I M, Almurshedi A S.2008.Controlledrelease carbamazepine granules and tablets comprising lipophilic and hydrophilic matrix components.The American Association of Pharmaceutical Scientists, 9, 1054-1062.
Bodmeier R, Mcginity J M.1987.The preparation and evaluation of drug-containing poly(dl-lactide) microspheres formed by solvent evaporation method.Pharmaceutical Research, 4,465-471.
Dong D W.2012.Preparation and characterization of melamineformaldehyde microcapsules filled with acetamiprid.MSc thesis, Beijing University of Chemical Technology, China.(in Chinese)
Fu L Y.2008.Research of the synthesis and the release rate of pesticide slowrelease substrate.MSc thesis, Heilongjiang University, China.(in Chinese)
Fu X D, Gao Y L.2003.Test for in vitro release rate of microspheres and correlation of its in vivo-in vitro.Chinese Journal of New Drugs, 12, 608-611.(in Chinese)
Han W Q.2010.Study on preparation of polylactic acid and poly lactic acid microspheres.MSc thesis, Beijing University of Chemical Technology, China.(in Chinese)
Huang B B, Zhong J B, Zhang X X, Yang F J, Wu Z J, Wu G.2011.Effect of ratio of core material to wall material on quality of microsphere of spinosad.Agrochenicals, 50,714-717.(in Chinese)
Ioriatti C, Anfora G, Angeli G, Civolani S, Schmidt S, Pasqualini E.2009.Toxicity of emamectin benzoate to Cydia pomonella (L.) and Cydia molesta (Busck) (Lepidoptera:Tortricidae): Laboratory and field tests.Pest Management Science, 65, 306-312.
Kong L E.2012.The microencapsulation of three different pesticides and research of their controlled release properties.MSc thesis, Chinese Academy of Agricultural Sciences.(in Chinese)
Li W, Lu F S, Guo W T.2010.Preparation and characterization of emamectin benzoate microcapsules.Chinese Journal of Applied Chemistry, 27, 1381-1385.(in Chinese)
Lu Y.2006.Study proceedings of gelatin microsphere.The Science and Technology of Gelatin, 26, 57-68.(in Chinese)
Ma X Y, Shi S X, Xia Y Z.2004.Preparation and degradation of polylactide and its copolymer.Journal of Beijing University of Chemical Technology (Natural Science Edition), 31,51-56.(in Chinese)
Nie Q, Chen P, Wu C.2004.Study on controlled release of the mixture of polylactide and drug.Chemical World, 45,20-21, 16.(in Chinese)
Reeve M S, Mccarthy S P, Gross R A.1993.Preparation and characterization of (R)-poly(β-hydroxybutyrate)-poly(εcaprolactone) and (R)-poly(β-hydroxybutyrate)-poly(εlactide) degradable diblock copolymers.Macromolecules,26, 888-894.
Sinclair R G.1973.Slow-release pesticide system.Polymers of lactic and glycolic acids as ecologically beneficial, costeffective encapsulating materials.Environmental Science& Technology, 7, 955-956.
Sun R X, Shi J J, Guo Y C.2008.Study of factors influencing diameter of gelatin microsphere.The Science and Technology of Gelatin, 28, 119-124, 128.(in Chinese)
Tsuji H, Ikarashi K.2004.In vitro hydrolysis of polylactide crystalline residues as extended-chain crystallites III.Effects of pH and enzyme.Polymer Degradation and Stability, 85,647-656.
Wu C, Zhang Y, Xu L.2007.Study on the release kinetics of OPC’s microcapsule.Chemistry and Adhesion, 29,172-175.(in Chinese)
Wu Y K.2011.Preparation and evaluation of emamectin benzoate microcystis controlled release formulation.MSc thesis, Shanghai Normal University, China.(in Chinese)
Xing C C, Chen S H, Sun M.2011.Study on the release-kinetics of microcapsule of synthetic capsaicin.Chemical World, 52,228-231, 243.(in Chinese)
Zhang M, Cui C N, Song J.2008.Analysis of the factors influencing degradability and degradation mechanism of polylactic acid.Packaging Engineering, 29, 16-18, 46.(in Chinese)
Zhang Y.2004.The review of microspheres for slowrelease pesticides.Herald of Medicine, 23, 843-844.(in Chinese)
Zhou X H, Liao L W, Zhang Z X.2010.Progress on preparation and its modification of microspheres for slowrelease pesticides.Agrochenicals, 49, 862-867, 873.(in Chinese)
Zhu J, He Y, Gao M, Zhou W, Hu J, Shen J, Zhu Y C.2011.Photodegradation of emamectin benzoate and its influence on efficacy against the rice stem borer, Chilo suppressalis.Crop Protection, 30, 1356-1362.
猜你喜欢
杂志排行
Journal of Integrative Agriculture的其它文章
- Characteristic analysis of tetra-resistant genetically modified rice
- A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis
- Characterization of GhSERK2 and its expression associated with somatic embryogenesis and hormones level in Upland cotton
- GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean
- Molecular cloning and functional characterization of a soybean GmGMP1 gene reveals its involvement in ascorbic acid biosynthesis and multiple abiotic stress tolerance in transgenic plants
- Responses of the antioxidant system to fluroxypyr in foxtail millet(Setaria italica L.) at the seedling stage
