Characteristic analysis of tetra-resistant genetically modified rice
2018-03-07HUWenbinDENGXiangyangDENGXiaoxiangDENGLihuaXIAOYoulunHEXingjianFUXiqinXIAOGuoying
HU Wen-bin, DENG Xiang-yang DENG Xiao-xiang, DENG Li-hua XIAO You-lun, HE Xing-jian, FU Xi-qin, XIAO Guo-ying
1 Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, P.R.China
2 State Key Laboratory of Hybrid Rice/Hunan Hybrid Rice Research Center, Changsha 410125, P.R.China
3 University of Chinese Academy of Sciences, Beijing 100049, P.R.China
4 Hunan Plant Protection Institute, Hunan Academy of Agricultural Sciences, Changsha 410125, P.R.China
5 Sericultural Research Institute of Hunan Province, Changsha 410125, P.R.China
1.Introduction
Rice is one of the most important food crops in the world that feeds more than half of the world’s population and over 60% of Chinese population (Lou et al.2014; Muthayya et al.2014).With development of breeding techniques, the yields of new rice varieties are greatly increased as well.However,the diseases, insects, and weeds are still serious limiting factors for full achievement of yield potential in rice.Even though adopting conventional pest management, the annual loss of rice yield reaches 10% by insects and diseases, and more than 5% by weeds (Deng et al.2014a).At present,the dominant method for farmers to manage insects,diseases, and weeds in the field are spraying pesticides and selective herbicides, with only some obstinate weeds being removed manually.However, the manual weeding is becoming more and more diseconomy because of the continuous increase of labor costs.The chemicals control not only increases the economic cost in rice production,but also causes the insecticide residues that deteriorate the ecological environment.Breeding resistant rice is the most economical and effective way to reduce the harms of them (Zhang 2007; Zheng et al.2016).In order to reduce the harms of diseases on rice, many disease-resistant genes were discovered in rice, and were widely applied(Pradhan et al.2015; Chen et al.2016; Wang et al.2016).Compared to the successful application of disease-resistant genes, the practical insect- and herbicide-resistant genes from rice were not found except brown planthopper (BPH)resistant gene (Hu et al.2016) and imidazolinone-resistant gene (Webster and Masson 2001).Particularly, the genes resistant to rice borers, which harm rice seriously, have not been found in rice till now (Chen et al.2009; Visalakshmi et al.2014).And the application of imidazolinone-resistant gene also brings seriously weed resistance and herbicide residues (Heap 2014; Bundt et al.2015).As a result, the introduction of borer- and herbicide-resistant genes through biotechnique can effectively make up for the deficiency in rice germplasm (Dunwell 2014) .
The glyphosate-resistant gene encoding 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) is the most widely applied herbicide-resistant gene in the world,and has been successfully applied in soybean, corn, cotton,and other crops (Bonny 2016), thus has attracted increasing attention in the genetically modified rice research.In the 1990s, Monsanto Company (USA) took the lead in developing a glyphosate-resistant rice variety, Roundup Ready Rice(Edwards 2001), with the Epsps gene.Chhapekar et al.(2015) successfully transformed the optimized Cp4-Epsps gene into IR64 by Agrobacterium-mediated transformation,and the glyphosate tolerable concentration of transformants was up to the 1% solution of commercial glyphosate.Zhao et al.(2011) transformed the new EPSPS-encoding gene G6 into the rice variety Xiushui 11, and the transformants could endure the 8 g L-1glyphosate solution.Although the application area of glyphosate-resistant crops is the largest,the study of herbicide-resistant rice with glufosinate-resistant gene was historically earlier and more successful than that of glyphosate-resistant gene (Christou et al.1991).In 1996,Chinese researcheres obtained the glufosinate-resistant rice Jingyin 119 and Zhongbai 4 (Huang et al.1997).Subsequently, such glufosinate-resistant transformants were developed by use of rice restorer line R187, Xiushui 04,D68, and E32 (Xiao 2009).Fu et al.(2001) transformed glufosinate-resistant gene into the male sterile line Peiai64S via Agrobacterium mediation, and gained the first glufosinate-resistant rice male sterile line.Utilizing herbicide-resistant restorer line to breed hybrid rice, it is advantageous not only to the control of weeds in F1hybrid field, but also to the removal of male sterile plants mixed in F1hybrid by spraying herbicides; when producing hybrid rice seed with herbicide-resistant male sterile line, it facilitates the removal of paternal plants by spraying herbicide and harvest of hybrid seeds by combine as well (Xiao 2009).Deng et al.(2014b) introduced Bar (Bialaphos-resistant gene from Streptomyces hygroscopicus) and Epsps genes into photoperiod-sensitive genic male sterile (PGMS) line 7001S, and obtained the glufosinate and glyphosateresistant transgenic rice, aiming to realize the alternate use of glyphosate and glufosinate in a field and to reduce the risk of weed resistance.As for herbicide-resistant rice developed till now, three glufosinate-resistant rice varieties have been approved for commercial cultivation (http://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=17&Crop=Rice).
The Bt (Bacillus thuringiensis) gene is the most effective borer-resistant gene for application so far, in which the type of Cry1 protein is studied thoroughly and shows strong toxic on insects from Lepidoptera, so it has been widely applied in the borer-resistant rice.In 1989, Yang et al.(1989) successfully transformed the primordial Bt gene from B.thuringiensis into the japonica rice Taibei 309 by electroporation, and obtained the first transgenic Bt rice.Previously, the transgenic Bt rice had low level of protein expression and poor toxicity because the Bt genes were directly from B.thuringiensis without codon optimization.Wünn et al.(1996) transferred the optimized Cry1Ab gene into IR58, and obtained the transformants on which the mortality of striped rice borer and yellow rice borer were as high as 100%.Deng et al.(2014a) transferred the codon-optimized Cry1Ca#gene into R893; the content of Cry1C protein increased greatly to the highest level of 36.07 μg g-1(fresh weight), and the mortality of the rice leaf roller reached 100% in 4 days feeding.To avoid the borer resistance produced due to the long-term use of a same gene, Cheng et al.(1998) transferred the optimized Cry1Ab and Cry1Ac genes into Nipponbare, and obtained three transformants with the lethality of striped rice borer and yellow rice borer reaching 100 and 97%, respectively.Tu et al.(2000) transferred the Cry1Ab and Cry1Ac fusion gene into Minghui 63; the transformant and its hybrid combination had very remarkable resistance to rice borer in the field.In 2004, Iran initiated the commercial cultivation of transgenic Bt rice (http://www.isaaa.org/gmapprovaldatabase/event/default.asp?EventID=221).Although the transgenic Bt rice had been granted a safety certificate in China (http://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=17&Crop=Rice), it still remained unapproved for commercial planting.
The breeding of rice with herbicide and borer-resistance is an important trend in the research of transgenic rice.In 2001, Park et al.(2001) constructed binary vector of Pat-Bt,then transferred the Pat (Phosphinothricin acetyl transferase gene from Streptomyces viridochromogenes) and Bt genes into rice, and obtained the transgenic rice with glufosinate and insect resistances.Tang et al.(2006) transferred Cry1C* and Bar genes into rice restorer line Minghui 63 by Agrobacterium-mediated transformation, and obtained transformant T1c-19 with the single copy of target genes;T1c-19 and its hybrid combination showed resistance not only to glufosinate, but also to rice leaf rollers and striped rice borer in the field.Zhao et al.(2015) constructed a vector that containing Cry1Ac, Cry1Ig, and Epsps, and then transformed them into Xiushui 134, which generated transformants GAI-14 with the glyphosate resistance and the 100% lethality to cotton bollworm, striped rice borer,and rice leaf roller.Deng et al.(2014a) obtained transgenic rice line B1C893 containing Bar and Cry1Ca#genes by Agrobacterium-mediated transformation, which showed high resistance to glufosinate and the 100% lethality to rice leaf roller.Yang Y Y et al.(2014) transferred bivalent Bt and Epsps genes into Xiushui 134, and the transformant S21 prevented striped rice borer and rice leaf roller from field in two consecutive years.Weng et al.(2014) introduced Bar and Cry2Aa#genes into the PGMS line 4008S, and the transformants showed high resistance against glufosinate,rice leaf roller, and striped rice borer.
However, above-mentioned transgenic rice studies mainly focused on the improvement of the herbicide and borer-resistant traits, but paid less attention to resistance of other diseases and insects; these shortcomings needed to be overcome.In this study, through Agrobacteriummediated transformation, we introduced glufosinateresistant gene Bar and insect-resistant gene Cry1Ca#into the restorer line R106 with BPH resistance genes (Bph14 and Bph15) and bacterial bright (BB) resistance gene(Xa23), and produced three new rice lines with resistance to the herbicide, borer, BPH, and BB.
2.Materials and methods
2.1.Transformation of rice
The variety R106, an outstanding indica restorer line (Oryza sativa L.indica) of two-line hybrid rice, was provided by Prof.Qi Huaxiong (Food Crops Research Institute, Hubei Academy of Agricultural Sciences, China); it carried Bph14,Bph15, and Xa23 genes.The plant expression vector pC3300-Cry1Ca#contains glufosinate-resistance gene Bar and optimized insect-resistant gene Cry1Ca#(Fig.1)(Deng et al.2014a).The mature embryos of R106, used as explants, were transformed using Agrobacterium-mediated transformation, based on methods of Toki et al.(2006) and Lin and Zhang (2005).
2.2.PCR and Southern blot
Rice genomic DNA was isolated by the cetyltrimethyl ammonium bromide (CTAB) method (Murray and Thompson 1980).PCR primers and procedures were shown in Table 1.The 20 μL PCR system was composed of following ingredients: 2×PCR Mix 10 μL; 1 μL 10 μmol L-1forward and reverse primers, respectively; 1 μL 10-20 ng μL-1template DNA; 7 μL H2O.PCR products were detected by 2.0%agarose tris-acetate-EDTA (TAE) gel electrophoresis.
Genomic DNA (20 μg per sample) were digested by EcoRI and HindIII respectively, separated on a 1.0% agarose gel and transferred onto the nylon membrane by capillary action.The DIG DNA Labelling and Detection Kit (11093657910,Roche Pharmaceutical Co., Ltd., Switzerland) was used for probe preparation, membrane washing, and band detection.The probes for Southern blot were prepared by labeling the fragments of Bar gene with digoxigenin by PCR using the same primers as above (Table 1).
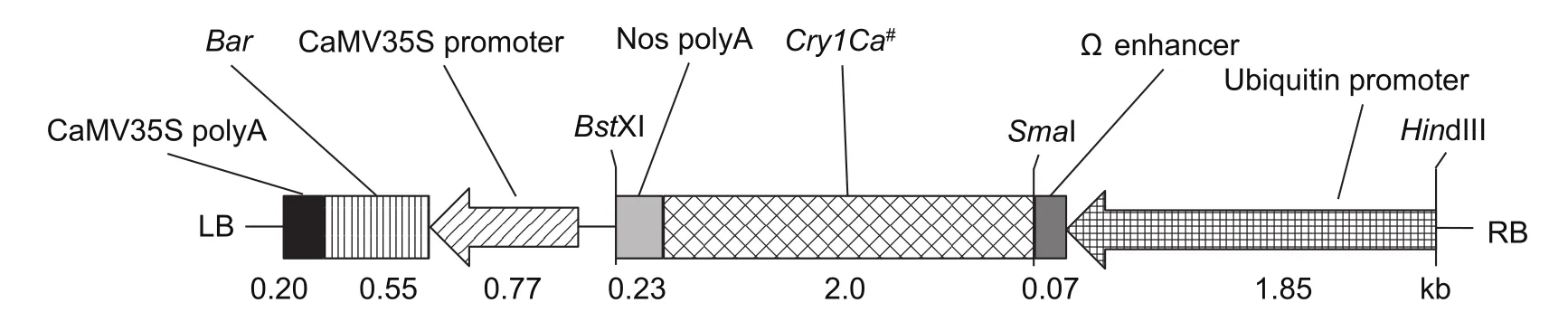
Fig.1 The T-DNA region of plasmid pC3300-Cry1Ca#.The Cry1Ca# gene was driven by ubiquitin promoter, enhanced expression by Ω enhancer and terminated by nopaline synthase (Nos) terminator; the Bar gene used as a selection marker was driven by CaMV35S promoter and terminated by CaMV35S polyA; LB, left border of T-DNA region; RB, right border of T-DNA region.

Table 1 Primers for molecular identification of target genes
2.3.Quantification of Cry1C and PAT proteins
The PAT protein of Bar gene in leaf, sheath, and stem of transformants at tillering stage in T2generation were quantified with the QualiplateTMKit for LibertyLink®PAT/Bar (EnviroLogix AP013, USA), and their Cry1C protein of Cry1C gene were quantified with the QualiplateTMKit for Cry1C (EnviroLogix AP007, USA).Approximately 20 mg tissue samples were ground in 1 mL extraction/dilution buffer; the extracts were then diluted to an appropriate concentration with dilution buffer.The enzyme-linked immunoabsorbent assay (ELISA) was carried out according to the manufacturer’s protocol.Absorbance at 450 nm were measured by microplate reader Tecan Infinite®200 PRO(Tecan Trading AG, Switzerland, hereinafter the same).The target protein concentrations were calculated based on the standard curve.Each group included three replicates.
2.4.Bioassay of glufosinate resistance
The marked leaves of transformants of T1generation at tillering stage were smeared with 1 g L-1glufosinate to validate the inheritance and expression of Bar gene.The results were observed and photographed after 7 days.The glufosinate resistances of three transformants of T3generation were determined: The plants of T3transformants were transplanted into basin with the diameter of 28 cm and height of 30 cm, 150 plants per basin; at the three-leaf plus one-leaflet stage, the plants of B1C106-1, B1C106-2, and B1C106-3 were sprayed by 0, 0.5, 1, 2, 4, 6, 8, and 10 g L-1glufosinate solution at 10 mL per basin respectively; for the R106 (CK), the concentrations of glufosinate solution decreased to 0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 g L-1,respectively due to its lack of glufosinate resistance; each group included three replicates; the results were observed and photographed after 7 days.
2.5.Bioassay of rice leaf roller resistance in laboratory and field
The insecticidal activities of B1C106-1, B1C106-2, and B1C106-3 of T3generation were analyzed with R106 as control, using the method previously reported by Yang et al.(2011).The fresh rice leaves of B1C106-1, B1C106-2,B1C106-3, and R106 at tillering stage were cut into segments about 10 cm.Four segments of rice fresh leaves with both ends wrapped in moistened filter papers and inoculated with 15 rice leaf rollers at first-instar larvae stage,were placed into a 20 cm×2.5 cm glass tube and put in a growth chamber at 28°C, 85% relative humidity, and 12 h illumination per day.Each group included three replicates.The damage to leaf tissues and the larval mortality were observed and photographed 5 days after feeding, and the corrected mortality was calculated.The corrected mortality(%)=(Larval mortality in transgenic plants-Larval mortality in control)/(1-Larval mortality in control)×100
The evaluation of rice leaf roller resistance of three transformants of T3generation was also carried out in the field.The plants of three transformants and R106 were transplanted in the field of Institute of Subtropical Agriculture,Chinese Academy of Sciences on June 3, 2014, with three plots per line, 50 plants per plot; all plants were under conventional management without spraying the insecticides against rice borers.At mature stage, the numbers of leaves,tillers, and plants injured by rice leaf rollers were observed and recorded.
2.6.Assessment of toxicity on silkworm
Approximately 0.1 g leaf of three transformants of T3generation and R106 were ground in 1 mL extraction/dilution buffer (EnviroLogix AP007, USA); then the extracts of Cry1C protein were obtained by centrifugation (5 000 r min-1for 5 min), and stored at 4°C.The assessment of toxicity on silkworm was carried out according to Ding et al.(2007):Four round cuts in 1 cm diameter from fresh mulberry leaves were painted evenly on their backs with 10 μL extracts of Cry1C protein, then dried in air until there were no obvious liquid on surface; then 30 second-instar larvae of silkworm race Liangguang 2 were put on; after 10 h, they were fed with unpainted fresh mulberry leaves; each group included three replicates.The mortality of silkworm was examined in 5 days and the corrected mortality were calculated according to Section 2.5.
2.7.Evaluation of BPH resistance
On September 15-20, 2015, brown plant hoppers were collected in the rice field of our institute, and propagated on rice susceptible variety TN1 in the green house.The seeds of three transformants of T4generation, BPH-resistant control R106, and BPH-susceptible control TN1 were germinated in Petri dish.At the one-leaf plus one-leaflet stage, 30 strong seedlings were chosen to be transplanted in a rectangular basin (25 cm×60 cm).At the three-leaf plus one-leaflet stage, they were subjected to 10 brown plant hoppers per plant.When 95% of seedlings of TN1 were wither, the BPH resistance were evaluated according to Liu et al.(2002): Resistance level=immune(IM) (resistance score=0) if dead seedling rate≤1.0%;resistance level=high resistance (HR) (resistance score=1)if dead seedling rate between 1.1-10.0%; resistance level=resistance (R) (resistance score=3) if dead seedling rate between 10.1-30.0%; resistance level=medium resistance (MR) (resistance score=5) if dead seedling rate between 30.1-50.0%; resistance level=medium susceptible(MS) (resistance score=7) if dead seedling rate between 50.1-70.0%; resistance level=susceptible (S) (resistance score=9) if dead seedling rate≥70.1%.
2.8.Evaluation of BB resistance
Fifteen representative strains FuJ, GD44, GDA15, GDA2,GDA7, HeN11, HNA1-4, PD, PX086, PX099, ScYc-6, YN1,YN11, YN18, and YN7 belonging to different BB pathotypes,were incubated in nutrient broth (NB) medium (3% beef extract, 5% peptone, 1% yeast extract) with shaking at 160 r min-1and 28°C for 24 to 36 h.The bacterial cell suspensions with concentrations of 3×108cfu mL-1were used to inoculate the rice plants by leaf-clipping method reported by Xiao et al.(2016).The plants of B1C106-1, B1C106-2, and B1C106-3 of T4generation, R106, and the control R9113 were planted in the rice field of Institute of Subtropical Agriculture on May 25, 2015, with three plots per line, 100 plants per plot; then each material was inoculated for 10 flag leaves at booting stage, and the LL (lesion length) was measured 21 days post-inoculation, then reaction of rice plant to BB was determined by the average LL as described by Huang et al.(2012): ≤1 cm, high resistance (HR); 1.1-3 cm, resistance(R); 3.1-5 cm, medium resistance (MR); 5.1-12 cm, medium susceptibility (MS); 12.1-20 cm, susceptibility (S); ≥20.1 cm,high susceptibility (HS).
2.9.Evaluation of agronomic traits
The three transformants of T3generation and receptor(R106) was sowed on November 15, 2014, and transplanted after 25 days in the field of Lingshui Experimental Station of our institute (Lingshui, Hainan Province, China).Each line contained three plots, and each plot contained 100 plants(both 20 cm space within a row and between rows).The field was managed under normal agricultural practice.At fully mature stage, the flag leaf length, plant height, effective panicles per plant, panicle length, spikelets per plant, grains per plant, seed set, and 1 000-grain weight were measured,and the theoretical yield was calculated from grains per plant, 1 000-grain weight, and planting density.
3.Results
3.1.ldentification of transformants
The rice variety R106 is an excellent restorer line, with desirable agronomic traits as well as resistance to BB and BPH, but the transformation of it is full of difficulties.After over one year work, three fertile transformants were obtained finally by Agrobacterium-mediated transformation.All transformants were resistant against glufosinate (Fig.2-A),and the results of PCR (Fig.2-B) and Southern blot(Fig.2-C) showed that the Cry1Ca#and Bar genes had been successfully integrated into the genome of R106, showing no deletions on the innate genes Bph14, Bph15, and Xa23 of R106.Among these transformants, there were found to be from different transformation events with single-copy insertion and were named as B1C106-1, B1C106-2, and B1C106-3 according to the order of plantlet regeneration.
3.2.Quantification of Cry1C and PAT proteins
The contents of Cry1C protein in leaf, sheath, and stem of three transformants at tillering stage were measured in T2generation by ELISA.In leaves, the contents of Cry1C protein ranged from 9.64 to 15.69 μg g-1, with an average content of 12.99 μg g-1; in sheaths, the contents of Cry1C protein ranged from 4.58 to 10.98 μg g-1, with an average content of 8.11 μg g-1; in stems, the contents of Cry1C protein ranged from 4.98 to 12.65 μg g-1, with an average content of 9.71 μg g-1; the contents of Cry1C protein among leaf, sheath, and stem were significantly different (P<0.01) in the descending order of leaf>stem>sheath (Fig.3-A).The contents of Cry1C protein in B1C106-1 ranged from 10.79 to 15.69 μg g-1, with an average of 12.95 μg g-1; in B1C106-2,that ranged from 4.58 to 10.35 μg g-1, with an average of 6.57 μg g-1; in B1C106-3, that ranged from 8.63 to 13.85 μg g-1, with an average of 11.30 μg g-1; and the contents of Cry1C protein in three transformants were significantly different(P<0.01) in trend of B1C106-1>B1C106-3>B1C106-2(Fig.3-B).The Cry1C protein could not be detected in R106(CK) at any stages and organs, so it was not shown in the Fig.3 (hereafter the same for PAT protein).

Fig.2 Identification of transgenic plants in T1 generation.A, identification of glufosinate resistance in T1 generation.B, PCR assay of enthetic genes Cry1Ca# and Bar and innate genes Bph14, Bph15, and Xa23 in T1 generation.C, Southern blot of Bar gene in T1 generation.M, DNA marker; +, plasmid pC3300-Cry1Ca#; 9311, the negative control of Bph14, Bph15, and Xa23 genes;R106, receptor of transformation; 1, 2, and 3, transformants B1C106-1, B1C106-2, and B1C106-3, respectively; EcoRI and HindIII,restriction enzyme to digest genomic DNA.

Fig.3 Quantification of Cry1C protein of three transformants at tillering stage in T2 generation.A, comparison of Cry1C protein contents among leaf, sheath, and stem in T2 generation.B, comparison of Cry1C protein contents among B1C106-1, B1C106-2,and B1C106-3 of T2 generation.Different capital or small letters mean significant difference at 0.01 or 0.05 levels, respectively;the values represent mean±SD.
The contents of PAT protein in leaf, sheath, and stem of three transformants of T2generation were measured by ELISA, and the results were shown in Fig.4.In leaves,the contents of PAT protein ranged from 37.36 to 46.43 μg g-1, with an average content of 42.28 μg g-1; in sheaths, the contents of PAT protein ranged from 12.87 to 18.37 μg g-1,with an average content of 15.85 μg g-1; in stems, the contents of PAT protein ranged from 23.88 to 28.22 μg g-1, with an average content of 26.09 μg g-1; and the PAT contents among three organs were significantly different(P<0.01) in descending order of leaf>stem>sheath(Fig.4-A).The contents of PAT protein in B1C106-1 ranged from 17.07 to 46.43 μg g-1, with an average of 28.54 μg g-1;in B1C106-2, that ranged from 12.87 to 44.32 μg g-1, with an average of 27.66 μg g-1; in B1C106-3, that ranged from 17.20 to 40.62 μg g-1, with an average content of 28.02 μg g-1; but there were no significant difference among average PAT protein contents of three transformants (Fig.4-B).
The profile of PAT protein contents among three transformants (Fig.4-B) was obviously different from that of Cry1C protein (Fig.3-B), although they were both constitutive expressed, and the reason needed further investigation.
3.3.Evaluation of resistance of three transformants to rice leaf roller
After feeding with leaves of R106 (negative control) for 3 days, the larvae of rice leaf roller grew visibly larger and acted normal, and the leaves were subjected to serious injuries; but on the leaves of three transformants, the larvae were under growth arrest and slow movements, and the leaves had no clear lesion (Fig.5-A).After 5 days, all larvae of rice leaf roller were dead on the leaves of B1C106-1 and B1C106-2, most of which only left brown marks because they have been dead for a relatively long period; only one on B1C106-3 remained alive, but it was under growth arrest,and could not move independently and its color grew dark.The results showed that the mortality of larval on B1C106-1 and BIC106-2 both reached 100% and on B1C106-3 was 97.37%, and there was no significant difference among them(Fig.5-B).In the field, the rolled leaves were found in three transformants, but all were only on the edge of plot, and no live insects were found on leaves.There were no significant differences on the number of injured plants, tillers, and leaves among three transformants (Table 2).The results of insect resistance in laboratory and field both showed that three transformants were highly resistance to rice leaf roller.

Fig.4 Quantification of phosphinothricin acetyl transferase (PAT) protein in three transformants at tillering stage in T2 generation.A, comparison of PAT protein contents among leaf, sheath, and stem in T2 generation.B, comparison of PAT protein contents among B1C106-1, B1C106-2, and B1C106-3 of T2 generation.Different capital or small letters mean significant difference at 0.01 or 0.05 levels, respectively; the values represent mean±SD.
3.4.Bioassay of toxicity of protein extracts on silkworm
The mulberry silkworm belongs to Bombycidae family of Lepidoptera order that domesticates for thousands of years, and the feeding technology of it is skillful.To assess the toxicity of CrylC protein on insects of Lepidopteran by using silkworms as substitute could evaded the difficulties of feeding insects of rice, such as rice leaf roller and rice borer.For evaluating lethality of CrylC protein on silkworms,the second-instar larvae of the silkworm were fed with mulberry leaves smeared with protein extracts from three transformants of T3generation.The results showed that the silkworms fed with mulberry leaves smeared with protein extracts of R106 (CK) grow normally, while those with extracts from B1C106-1, B1C106-2, and B1C106-3 died in large portion, and the average mortality of them were 90, 67.8, and 87.8%, respectively (Fig.6).The lethality of B1C106-1 and B1C106-3 was significantly higher than that of B1C106-2 (P<0.01), respectively, but the lethality of B1C106-1 and B1C106-3 showed no significant difference(Fig.6-B).There is significant positive correlation between the Cry1C protein content in leaf and the mortality of silkworm by means of Pearson’s correlation analysis (r=0.993).
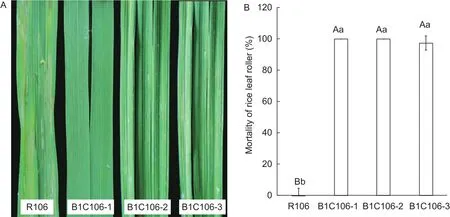
Fig.5 Evaluation of resistance of three transformants of T3 generation against rice leaf roller indoor.A, rice leaves gnawed by rice leaf roller.B, the mortality of rice leaf roller after inoculation for 5 dayson leaves of transgenic rice B1C106-1, B1C106-2 and B1C106-3, and control plant R106.Different capital or small letters mean significant difference at 0.01 or 0.05 levels, respectively;the values represent mean±SD.
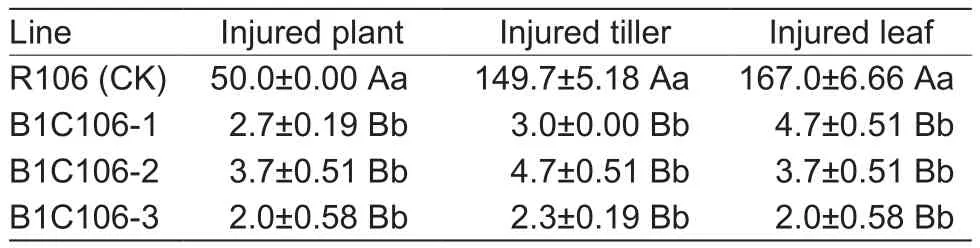
Table 2 The injuries on plants of three transformants of T3 generation caused by rice leaf rollers in the field (n=50)
3.5.Effect of different dose of glufosinate on three transformants
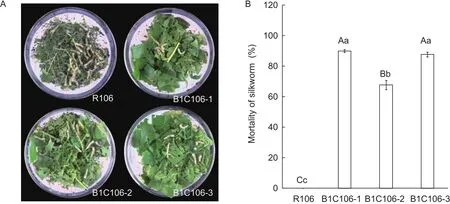
Fig.6 Evaluation of toxicity of protein extracts from leaves of three transformants of T3 generation on the second-instar silkworm.A, the mulberry leaves smeared with protein extracts fed on second-instar larvae of silkworm.B, the mortality of silkworm fed with mulberry leaves smeared with protein extracts from leaves of transgenic rice B1C106-1, B1C106-2, and B1C106-3, and control plant R106 for 5 days.Different capital or small letters mean significant difference at 0.01 or 0.05 levels, respectively.The values represent mean±SD.

Fig.7 Analysis on tolerance of three transformants against glufosinate in T3 generation.R106, no transgenic control; 1, 2, and 3,transformant B1C106-1, B1C106-2, and B1C106-3, respectively.
To find the tolerance dose of the transformants against glufosinate and guidelines of the safe application of herbicide, the effect of different dose of glufosinate on R106 and three transformants of T3generation was assessed(Fig.7).When the dose of glufosinate was not less than 0.016 g m-2, the R106 reacted rather quickly to glufosinate under above 25°C of daily mean temperature.The majority of the plants turned yellow after 3 days, and the mortality rose with the increase of dose of glufosinate.When the dose reached 0.065 g m-2, all of the R106 plants died.Although the integration of enthetic genes were different in B1C106-1,B1C106-2, and B1C106-3, the three transformants reacted in the same pattern to glufosinate: when the dose of glufosinate was less than 0.325 g m-2, the plants grew well without any adverse reaction; when the dose ranged from 0.325 to 0.975 g m-2, brown or yellow scars were left on leaves but the growth was rarely influenced; when the dose was over 0.975 g m-2, the mortality climbed as the dose increased.It showed that the introduction of Bar gene conferred transformants the tolerance to glufosinate as 15 times as the receptor, which facilitated the application of glufosinate in the paddy of transgenic rice.
3.6.Evaluation of BPH resistance of transformants
The results of BPH resistance showed that the control TN1 died completely after inoculation of BPH for 14 days, and its resistance scores was 9, while B1C106-1, B1C106-2,and B1C106-3 of T4generation and R106 grew normally with the exception of one to two dead plants, and their resistance scores were 1, their resistance levels were high resistance (Fig.8).The lines R106, B1C106-1, B1C106-2,and B1C106-3 contained Bph14 and Bph15 genes and were highly resistant to BPH, which illustrated that the integration of Cry1C#and Bar genes did not affect the resistance to BPH in transformants.
3.7.Evaluation of BB resistance of transformants
The resistance of rice against bacterial blight (Xanthomonas oryzae) was shown on Table 3.After inoculation of X.oryzae for 21 days, the susceptible control R9113 showed a range of response, such as high resistance to GD44 and YN18 pathotypes, and susceptibility to FuJ pathotype with the longest lesion length (19.88 cm).R106 and the three transformants had no lesion length longer than 1 cm, were high resistance to X.oryzae.There was no significant difference between R106 and three transformants on lesion length of X.oryzae, indicating that the integration of Cry1C#and Bar genes did not influence the resistance to X.oryzae in transformants.
3.8.Evaluation of agronomic traits
The agronomic traits of three transformants and R106(receptor) are listed on Table 4, that shows no significant difference among R106 (check) and three transformants on the flag leaf length, plant height, effective panicles per plant,panicles length, spikelets per plant, and 1 000-grain weight.Among the rest traits grains per plant, seed set, and theoretical yield, there are also no significant difference between R106 and B1C106-2, but these of R106 are significantly higher than that of B1C106-1 and B1C106-3 (P<0.01) respectively.That indicates the B1C106-2 retains the elite traits of receptor and is superior to the other two transformants.
4.Discussion
4.1.Pyramiding of enthetic and innate resistance genes

Fig.8 Evaluation of the resistance of three transformants of T4 generation against brown planthopper (BPH).A, bioassay of three transformants of T4 generation against BPH.B, the mortality of rice plant after BPH inoculation for 14 days.Different capital or small letters mean significant difference at 0.01 or 0.05 levels, respectively.Values represent mean±SD.
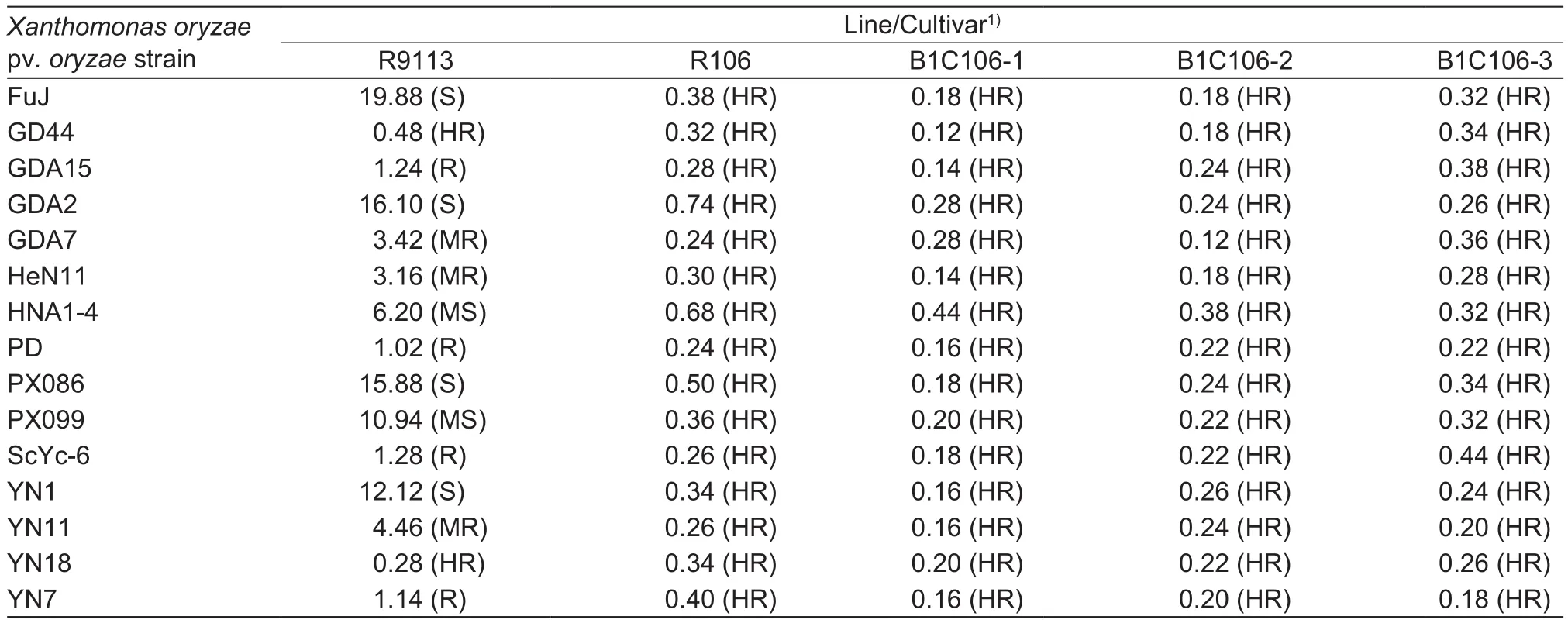
Table 3 The bacterial blight (BB) resistance (lesion length) of three transformants
Rice often suffers from diseases, insects, and weeds,which makes the yield potential could not be fully realized.Breeding rice varieties with high yield and high resistance is main tendency currently in rice research.Before the exploitation of marker-assisted selection (MAS), the rice varieties with multi-resistance were almost developed through phenotypic selections, which were time-consuming,laborious, and low efficiency.With the establishment and improvement of MAS, multiple gene stacking breeding becomes easier and quicker.Through combination of MAS and traditional breeding methods, Mao et al.(2011)obtained 16 rice lines with Xa23 and Bph20(t) genes by using the markers of C189 (Xa23) and BYL7 (Bph20(t))respectively, and these lines were highly resistant to BB and BPH.Jiang et al.(2015) stacked Pi2 and Xa23 genes into Guangzhan 63-4S, and obtained a new sterile line Hua1015S which was resistant against rice blast and BB.Xiao et al.(2016) stacked Xa23, Bph14, and Bph15 genes into restorer line Huazhan, and the improved Huazhan and its hybrid combinations were highly resistant to BB and BPH.Korinsaka et al.(2016) obtained 64 new lines with notable improvement on the tolerance against submergence and BPH through pyramiding Sub1 and qBbph12 genes into KDML105.
However, there is deficiency in the method of breeding multi-resistance rice variety by using conventional crossing and MAS strategies.First, some resistant genes are not optimal or not existing in rice, such as resistant genes against rice borers and herbicide-resistant genes.Secondly,the unwanted linkage of undesirable genes frequently accompanies with the stacking of resistance genes by sexualcrossing, and the separation of undesirable genes from tightly linked resistance genes needs a very large population and the possibility of picking out a plant free of unwanted linkage is very low.Because of the tightly linkage of resistant genes and unfavorable genes, the more the resistant genes stacked by sexual crossing, the more the unfavorable genes had to be brought in by linkage drag, that leaded to the varieties with multi-resistance were not excellent as a whole, such as low yield potential.Therefore, integration of genetic transformation, sexual hybridization, MAS, and phenotyping to breed multi-resistant rice become trending gradually.Datta et al.(2002) successfully stacked the Xa21 gene from line TT103, the chitinase gene from the TT-9 and Bt genes into one line, which showed highly resistance to BB and sheath blight as well as whose lethality to rice borer reached 100%.By combination of MAS and phenotyping,Jiang et al.(2004) pyramided Bt gene from Minghui 63/Bt and Xa21 gene from Minghui 63/Xa21 into one variety(Minghui 63/Bt&Xa21), and obtained resistances against rice borers and BB simultaneously.Through combination of backcross and MAS, Wei et al.(2008) pyramided Cry1Ab and Bar genes from Zhongguo 91 and Xa21 gene from Yujing 6 into elite rice lines, and all improved lines were resistant against rice borers, herbicide, and BB.Yan et al.(2013) successfully pyramided Xa7 and Xa21 genes from Huahui 20 and Cry1C* gene from T1c-19 into R207 by MAS and sexual hybridization, and the two improved lines from R207 and their hybrids with Jin23A were resistant against BB and rice borers.Wan et al.(2014) pyramided Cry1C*and Bar genes from T1c-19 and Bph14 and Bph15 genes from R022 by MAS and sexual hybridization into R022, and obtained a new restorer line KR022 resistant to BPH, rice borers and herbicide.
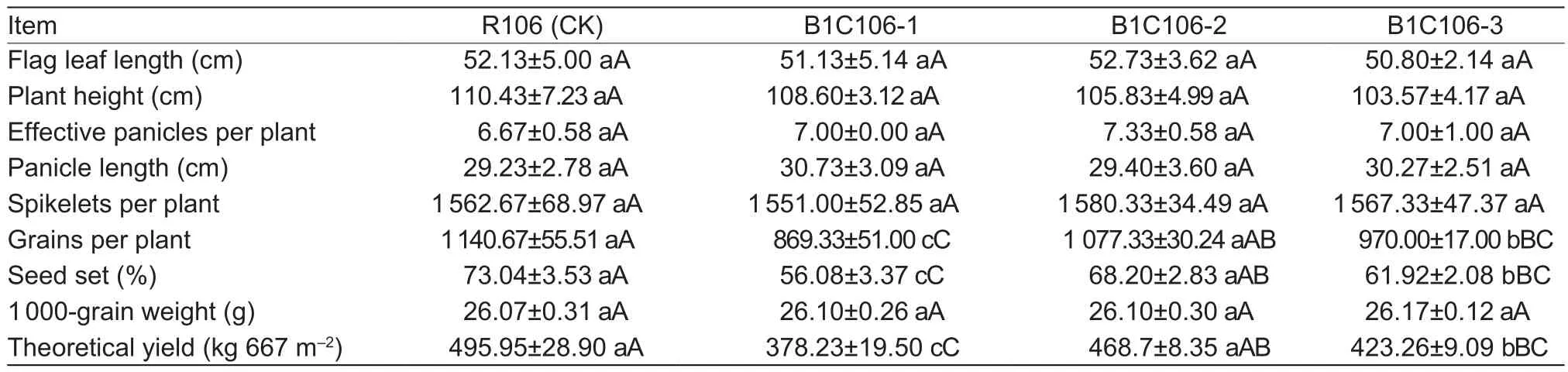
Table 4 Evaluation of transgenic lines on agronomic traits
In this study, in order to develop multi-resistant rice germplasm with high practicability, the Bar and Cry1Ca#genes were chosen as targeted genes to confer enthetic resistance to glufosinate and rice borer on rice, in which the resistance to glufosinate and rice borer are not existent in rice germplasm.Meanwhile, the restorer line R106 of two-line hybrid rice was taken as the receptor, for it has high combining ability, good agronomic traits (inheriting the majority of genome from 9113, a male parent of super hybrid rice), and highly resistance to BB and BPH (due to pyramided Bph14, Bph15, and Xa23 genes) (Li et al.2006a,b).After optimizing the composition of culture medium and adjusting combination and concentration of plant hormones,we overcame the difficulties of transformation of R106,and obtained the transformants with single-copy insertion showing four kinds of resistance to herbicide, borers, BB,and BPH, and these lines would play a significant role in further breeding of multi-resistant rice.
4.2.The feasibility of evaluating the Bt toxicity by using silkworm
The population of insects in rice field was obviously influenced by weather so there were great difficulty in obtaining a lot of larvae at the same developmental stage from the field; moreover, it was not easy to feed these insects in laboratory; both of them severely restricted the timeliness and veracity of bioassay of borer resistance in laboratory, and were troubling in the identification of borer resistance in transgenic rice.At present, the common methods for identification of rice borer resistance indoor mainly were collecting mature larvae and moths to spawn or eggs and hatched synchronously in order to obtain enough larvae at the same time, then inoculating larvae on rice for identification of rice borer resistance.However, we found a lot of problems in these methods: The larvae could not grow and develop uniformly in the field, so it was difficult to capture many healthy larvae at the same developmental stage; the larvae, pupa or moth of rice striped stem borer and rice leaf roller were easy to die after being collected, so it was hard to get enough larvae for trial; furthermore, the larvae of rice striped stem borer and rice leaf roller were small in size, so the operation of inoculation was difficult.Silkworm is an important model insect of Lepidoptera - An insect order that includes insects like rice striped stem borer and rice leaf roller.Silkworm is easy to breed and with high reproduction coefficients, and its development and growth habit is known well by human, so large numbers of its larvae at the same developmental stage can be easy to obtain.Silkworm is also an important insect for studying the insecticidal mechanism of Bt protein (Leonardi et al.1997; Nagamatsu et al.1998).Yang Y et al.(2014) studied the impact of Bt on silkworms,and found that the mulberry leaves loading with less than 1 800 pollen particulates cm-2of Bt rice containing Cry1C or Cry2A gene did not obviously affect on silkworms; but above 1 800 particulates cm-2, the silkworms became sensitive to the both kinds of protein.To the best of our knowledge,assessing the insect resistance of transgenic Bt rice with silkworms feeding on mulberry leaves smeared with Bt protein extracts had not been reported till now.
In this study, two approaches to evaluate Cry1C protein toxicity in laboratory were adopted that included bioassay of transgenic rice leaf by rice leaf roller and assessment of protein extracts from transgenic rice by silkworm; the former was the common method, but the latter was the first report.In the assessment of resistance of transformants to rice leaf roller indoor, nearly all the larvae feed with leaves of three transformants died; the difference of the mortality among three transformants was not significant, and the correlation between mortality and dosage could not be distinguished.The cause might be that the first-instar larvae of rice leaf roller were small in size, and the Cry1C protein amount of feeding in 5 days exceeded their threshold of tolerance.If the feeding time of rice leaf roller were shortened, that would make some larvae of rice leaf roller remained alive,but might magnify the slight difference of larvae in ages and weight leading to significant deviation of mortality.However, in the experiment with silkworms, the mortality of larvae of silkworms feed with protein extracts from three transformants differed from each other obviously, and the significant correlation between mortality and dosage was apparently observed.This is due to the fact that the larvae of silkworms had bigger size, similar weight and same instar to each other, and they were fed with mulberry leaves smeared Bt protein extracts for only 10 h, so the intake of Cry1C protein per unit weight of silkworms was less than that of rice leaf roller, then the experiment design could easily reveal significant positive correlation between the Cry1C protein content in leaves and the mortality of silkworms.The advantages of silkworm adoption were listed as follows:1) the silkworms were easy to feed, and large amounts of larvae at the same developmental stage with similar size and weight were obtained easily, which could meet the requirements of experiments with many groups and multiple batches, and guaranteed the high accuracy of experiments;2) the silkworms could be supplied almost throughout the year, so it broke the seasonal restrictions of capturing insects in the fields; 3) the fact that the larvae of silkworm were larger than the larvae of borer in size and weight and had stronger toxic resistance was favorable for studies on the doses effect.After the improvement and standardization for the protein extraction from leaves of transgenic rice, the feeding dose of protein extracts, the feeding process, and the selection of silkworm variety, the assay for evaluating the Bt protein toxicity with second-instar silkworm would be an important and useful method for future studies in this field.
It is worth noting that the silkworms naturally feed on mulberry leaves instead of rice leaves, and the insecticidal spectrum of different Bt proteins differ greatly.Before using this approach, it should be confirmed that the Bt protein is toxic to both borers and silkworms, and the correlation between mortality of borer and silkworm under different concentrations of Bt protein should be also assessed.Once the conditions are satisfied, the silkworms would be the excellent choice to assess the borer resistance of transgenic rice with high accuracy and convenience.
5.Conclusion
After genotyping, the stable heredity of two enthetic resistant genes Cry1Ca#and Bar and three innate resistant genes Bph14, Bph15, and Xa23 are proved, as well as the resistances of glufosinate, rice leaf roller, brown planthopper, and bacterial blight are verified by phenotyping,that demonstrated the successful development of tetraresistant genetically modified rice.The Cry1C and PAT protein contents in leaf, sheath, and stem descend in order:leaf>stem>sheath (P<0.01); the average contents of Cry1C protein among transformants show significant difference(P<0.01), but the average contents of PAT protein are not significant difference among transformants.It is primary validate that the silkworms can instead of rice borer to assesses the toxicity of Cry1C protein in rice plant.
Acknowledgements
This research was supported by the State Key Science and Technology Programme on Breeding of New Genetically Modified Organisms, China (2011ZX08001-003 and 2016ZX08001-003) and the Open Research Fund of State Key Laboratory of Hybrid Rice (Hunan Hybrid Rice Research Center), China.The author thanks for the help of Dr.Hui Wei from the National Renewable Energy Laboratory,Department of Energy, USA in revision of this manuscript.
Bonny S.2016.Genetically modified herbicide-tolerant crops, weeds, and herbicides: Overview and impact.Environmental Management, 57, 31-48.
Bundt A C, Avila L A, Pivetta A, Agostinetto D, Dick D P, Burauel P.2015.Imidazolinone degradation in soil in response to application history.Planta Daninha, 33, 341-349.
Chen H, Lin Y J, Zhang Q F.2009.Review and prospect of transgenic rice research.Science Bulletin, 54, 4049-4068.
Chen X J, Chen Y, Zhang L N, Xu B, Zhang J H, Chen Z X, Tong Y H, Zuo S M, Xu J Y.2016.Overexpression of OsPGIP1 enhances rice resistance to sheath blight.Plant Disease,100, 388-395.
Cheng X, Sardana R, Kaplan H, Altosaar I.1998.Agrobacteriumtransformed rice plants expressing synthetic CryIA(b) and CryIA(c) genes are highly toxic to striped stem borer and yellow stem borer.Proceedings of the National Academy of Sciences of the United States of America, 95, 2767-2772.
Chhapekar S, Raghavendrarao S, Pavan G, Ramakrishna C,Singh V K, Phanindra M L V, Dhandapani G, Sreevathsa R, Kumar P A.2015.Transgenic rice expressing a codonmodified synthetic CP4-EPSPS confers tolerance to broad-spectrum herbicide, glyphosate.Plant Cell Reports,34, 721-731.
Christou P, Ford T L, Kofron M.1991.Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos.Nature Biotechnology, 9, 957-962.
Datta K, Baisakh N, Thet K M, Tu J M, Datta S K.2002.Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight.Theoretical and Applied Genetics, 106, 1-8.
Deng L H, Deng X X, Wei S J, Cao Z C, Tang L, Xiao G Y.2014a.Development and identification of herbicide and insect resistant transgenic plant B1C893 in rice.Hybrid Rice, 29, 67-66.(in Chinese)
Deng L H, Weng L S, Xiao G Y.2014b.Optimization of Epsps gene and development of double herbicide tolerant transgenic PGMS Rice.Journal of Agricultural Science and Technology, 16, 217-228.
Ding S S, Yun W K, Hua L R, Hui M, Tao L Y.2007.Toxicity evaluation of twenty-five pesticides to Bombyx mori and observation of toxi symptoms.Science of Sericulture, 33,422-426.(in Chinese)
Dunwell J M.2014.Transgenic cereals: current status and future prospects.Journal of Cereal Science, 59, 419-434.
Edwards W J.2001.Weed control in Roundup ReadyTMrice.Proceedings of the California Weed Science Society, 53,133-135.
Fu Y P, Zhu Z G, Xiao H, Hu G C, Si H M, Yu Y H, Sun Z X.2001.Primary study on mechanization of seed production of hybrid rice by inducing Bar gene to Pei’ai 64S.Chinese Journal of Rice Science, 15, 97-100.(in Chinese)
Gao L J, Gao H L, Li R B, Li D Y, Zhou M, Yan Q, Zhou W Y,Zhang J, Deng G F.2010.Optimization and verification of molecular markers for rice bacteial blight resistance gene Xa23.Molecular Plant Breeding, 8, 660-664.(in Chinese)
Heap I.2014.Global perspective of herbicide-resistant weeds.Pest Management Science, 70, 1306-1315.
Hu J, Xiao C, He Y.2016.Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice.Rice, 9, 1-12.
Huang B, Xu J Y, Hou M S, Ali J, Mou T M.2012.Introgression of bacterial blight resistance genes Xa7, Xa21, Xa22 and Xa23 into hybrid rice restorer lines by molecular markerassisted selection.Euphytica, 187, 449-459.
Huang D N, Zhu B, Yang W, Xue R, Xiao H, Tian W, Li L, Dai S.1997.Identification of transgenic plants and transformation of antibacterial peptide B gene.Science in China (Series C), 27, 55-62.(in Chinese)
Jiang G H, Xu C G, Tu J M, Li X H, He Y Q, Zhang Q F.2004.Pyramiding of insect- and disease-resistance genes into an elite indica, cytoplasm male sterile restorer line of rice,‘Minghui 63’.Plant Breeding, 123, 112-116.
Jiang J F, Yang D B, Ali J, Mou T M.2015.Molecular markerassisted pyramiding of broad-spectrum disease resistance genes, Pi2 and Xa23, into GZ63-4S, an elite thermosensitive genic male-sterile line in rice.Molecular Breeding,35, 1-12.
Korinsaka S, Siangliwa M, Kotcharerk J, Jairin J, Siangliw J L,Jongdee B, Pantuwan G, Sidthiwong N, Toojinda T.2016.Improvement of the submergence tolerance and the brown planthopper resistance of the Thai jasmine rice cultivar KDML105 by pyramiding Sub1 and Qbph12.Field Crops Research, 188, 105-112.
Leonardi M G, Parenti P, Casartelli M, Giordana B.1997.Bacillus thuringiensis CrylAa δ-endotoxin affects the K+/amino acid symport in Bombyx mori larval midgut.The Journal of Membrane Biology, 159, 209-217.
Li J B, Wang C L, Xia M Y, Zhao K J, Qi H X, Wan B L, Zha Z P, Lu X G.2006a.Enhancing bacterial blight resistance of hybrid rice restorer lines through marker-assisted selection of the Xa23 gene.Acta Agronomica Sinica, 32, 1423-1429.(in Chinese)
Li J B, Xia M Y, Qi H X, He G C, Wan B L, Zha Z P.2006b.Marker-assisted selection for brown planthopper(Nilaparvata lugens Stål) resistance genes Bph14 and Bph15 in rice.Scientia Agricultura Sinica, 39, 2132-2137.(in Chinese)
Lin Y J, Zhang Q F.2005.Optimising the tissue culture conditions for high efficiency transformation of indica rice.Plant Cell Reports, 23, 540-547.
Liu G J, Fu Z H, Shen J H, Zhang Y H.2002.Comparative study on evaluation methods for resistance to rice planthoppers(homoptera: Delphacidae) in rice.Chinese Journal of Rice Science, 16, 52-56.(in Chinese)
Lou Y G, Zhang G R, Zhang W Q, Hu Y, Zhang J.2014.Reprint of biological control of rice insect pests in China.Biological Control, 68, 103-116.
Mao Z J, Liu P Q, Jiang L H, Li X.2011.Pyramiding of Xa23 and bph20(t) genes in rice varieties using molecular markerassisted selection.Journal of Southern Agriculture, 42,835-838.(in Chinese)
Murray M G, Thompson W F.1980.Rapid isolation of high molecular weight plant DNA.Nucleic Acids Research, 8,4321-4325.
Muthayya S, Sugimoto J D, Montgomery S, Maberly G F.2014.An overview of global rice production, supply, trade, and consumption.Annals of the New York Academy of Sciences,1324, 7-14.
Nagamatsu Y, Toda S, Koike T, Miyoshi Y, Shigematsu S,Kogure M.1998.Cloning, sequencing, and expression of the Bombyx mori receptor for Bacillus thuringiensis insecticidal CryIA(a) toxin.Bioscience Biotechnology & Biochemistry,62, 727-734.
Park S H, Park J, Smith R H.2001.Herbicide and insect resistant elite transgenic rice.Journal of Plant Physiology,158, 1221-1226.
Pradhan S K, Nayak D K, Mohanty S, Behera L, Barik S R, Pandit E, Lenka S, Anandan A.2015.Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna.Rice, 8, 30.
Tang W, Chen H, Xu C G, Li X H, Lin Y J, Zhang Q F.2006.Development of insect-resistant transgenic indica rice with a synthetic Cry1C* gene.Molecular Breeding, 18, 1-10.
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H.2006.Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice.The Plant Journal,47, 969-976.
Tu J M, Zhang G A, Datta K, Xu C G, He Y Q, Zhang Q F, Khush G S, Datta S K.2000.Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin.Nature Biotechnology, 18, 1101-1104.
Visalakshmi V, Satyanarayna N H, Jyothula D P B, Raju M R B, Murthy K V R.2014.Screening of rice germplasm for resistance to yellow stem borer scirpophaga incertulas walker.International Journal of Plant, Animal and Environmental Sciences, 4, 129-133.
Wan B L, Zha Z P, Li J B, Xia M Y, Du X S, Lin Y J, Yin D S.2014.Development of elite rice restorer lines in the genetic background of R022 possessing tolerance to brown planthopper, stem borer, leaf folder and herbicide through marker-assisted breeding.Euphytica, 195, 129-142.
Wang Y, Zhao J M, Zhang L X, Wang P, Wang S W, Wang H, Wang X X, Liu Z H, Zheng W J.2016.Analysis of the diversity and function of the alleles of the rice blast resistance genes Piz-t, Pita and Pik in 24 rice cultivars.Journal of Integrative Agriculture, 15, 1423-1431.
Webster E P, Masson J A.2001.Acetolactate synthaseinhibiting herbicides on imidazolinone-tolerant rice.Weed Science, 49, 652-657.
Wei Y P, Yao F Y, Zhu C X, Jiang M S, Li G X, Song Y Z, Wen F J.2008.Breeding of transgenic rice restorer line for multiple resistance against bacterial blight, striped stem borer and herbicide.Euphytica, 163, 177-184.
Weng L S, Deng L H, Lai F X, Xiao G Y.2014.Optimization of the Cry2Aa gene and development of insect-resistant and herbicide-tolerant photoperiod-sensitive genic male sterile rice.Czech Journal of Genetics and Plant Breeding, 50,19-25.
Wünn J, Klöti A, Burkhardt P K, Biswas G C G, Launis K, Iglesias V A, Potrykus I.1996.Transgenic indica rice breeding line IR58 expressing a synthetic CrylA(b) gene from Bacillus thuringiensis provides effective insect pest control.Nature Biotechnology, 14, 171-176.
Xiao G Y.2009.Recent advances in development of herbicide resistant transgenic hybrid rice in China.Rice Science, 16,235-239.
Xiao Y L, Li J J, Yu J H, Meng Q C, Deng X Y, Yi Z L, Xiao G Y.2016.Improvement of bacterial blight and brown planthopper resistance in an elite restorer line Huazhan of oryza.Field Crops Research, 186, 47-57.
Yan C Y, Liu Y, Mou T M.2013.Pyramiding Xa7, Xa21 and Cry1C* for the improvement of bacterial blight and borer resistance of hybrid rice Jinyou 207 by molecular markerassisted selection.Hybrid Rice, 28, 52-59.(in Chinese)
Yang H, Guo S D, Li J X, Chen X J, Fan Y L.1989.Transgenic rice plants produced by direct uptake of the δ-endotoxin protein gene from Bacillus thuringiensis into rice protoplasts.Scientia Agricultura Sinica, 22, 1-5.(in Chinese)
Yang H Y, You A Q, Yang Z F, Zhang F T, He R F, Zhu L L, He G C.2004.High-resolution genetic mapping at the Bph15 locus for brown planthopper resistance in rice (Oryza sativa L.).Theoretical and Applied Genetics, 110, 182-191.
Yang Y, Liu Y, Cao F Q, Chen X P, Cheng L S, Romeis J, Li Y H, Peng Y F.2014.Consumption of Bt rice pollen containing Cry1C or Cry2A protein poses a low to negligible risk to the silkworm Bombyx mori (lepidoptera: Bombyxidae).PLoS ONE, 9, e102302.
Yang Y Y, Mei F, Zhang W, Shen Z C, Fang J.2014.Creation of Bt rice expressing a fusion protein of Cry1Ac and Cry1I-Like using a green tissue-specific promoter.Journal of Economic Entomology, 107, 1674-1679.
Yang Z, Chen H, Tang W, Hua H X, Lin Y J.2011.Development and characterisation of transgenic rice expressing two Bacillus thuringiensis genes.Pest Management Science,67, 414-422.
Zhang Q F.2007.Strategies for developing Green Super Rice.Proceedings of the National Academy of Sciences of the United States of America, 104, 16402-16409.
Zhao Q C, Liu M H, Zhang X W, Lin C Y, Zhang Q, Shen Z C.2015.Generation of insect-resistant and glyphosate-tolerant rice by introduction of a T-DNA containing two Bt insecticidal genes and an EPSPS gene.Journal of Zhejiang University(Science B), 16, 824-831.(in Chinese)
Zhao T, Lin C Y, Shen Z C.2011.Development of transgenic glyphosate-resistant rice with G6 gene encoding 5-enolpyruvylshikimate-3-phosphate synthase.Agricultural Sciences in China, 10, 1307-1312.
Zheng S L, Chen B, Qiu X Y, Chen M , Ma Z Y, Yu X G.2016.Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China.Chemosphere, 144,1177-1192.
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection of illegal dyes in foods using a polyethersulfone/multi-walled carbon nanotubes composite membrane as a cleanup method
- Complete genome sequences of four isolates of Citrus leaf blotch virus from citrus in China
- The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep)
- Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears
- Estimation of irrigation requirements for drip-irrigated maize in a sub-humid climate
- Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system
