Responses of the antioxidant system to fluroxypyr in foxtail millet(Setaria italica L.) at the seedling stage
2018-03-07GUOMeijunWANGYuguoYUANXiangyangDONGShuqiWENYinyuanSONGXiGUOPingyi
GUO Mei-jun, WANG Yu-guo, YUAN Xiang-yang, DONG Shu-qi, WEN Yin-yuan, SONG Xi-e, GUO Ping-yi
Key Laboratory of Crop Chemical Regulation and Chemical Weed Control of Shanxi Province/Agronomy College, Shanxi Agricultural University, Taigu 030801, P.R.China
1.lntroduction
Foxtail millet (Setaria italica L.), a member of the Poaceae family, is one of the most important traditional Chinese cereal crops and is extensively cultivated in China.It has been recognized as having potential to health benefits and to reduce the risk of various diseases, especially cancer and cardiovascular diseases (Anand et al.2008).In addition,foxtail millet is widely used as a nourishing gruel or soup for pregnant women in North China, and the daily consumption of foxtail millet in Shanxi Province is 118 g per person per day(Fujita et al.1996; Zhang et al.1997).However, foxtail millet production in China is limited by some factors, including weed infestations.Weeds caused the yield reduction of foxtail millet up to 55.56% (Zhou et al.2012).Foxtail millet grows slowly under the cool conditions of spring, allows broadleaf weeds to start growing after foxtail emergence,thus reducing the biomass of foxtail millet (William et al.2009).Consequently, broadleaved weeds have become an increasing problem in many foxtail millet fields, and manual weeding with intensive labor costs, is the main method of weed control for this crop.Although chemical weed control is more efficient and economical compared to the manual weed control (Pornprom et al.2010), there few suitable herbicides are registered for weed control in foxtail millet fields.
Fluroxypyr (4-amino-3,5-dichloro-6-fluoro-2-pyridyloxyacetic acid), a pyridine herbicide, has been used for broadleaf weed control in cereals, olive trees, and croplands (Carvalho et al.2008; Hellou et al.2009; Sdiri et al.2013).Previous studies have demonstrated that the proposed label dose of fluroxypyr has a desirable effect with regard to controlling broadleaf weeds and is relatively safe for maize (Zea mays) and winter wheat (Triticum aestivum) (Liu 2014).In terms of wheat metabolic and physiological characteristics, the application of fluroxypyr does not cause any phytotoxic phenomena due to the presence of a large group of substances that converts herbicides molecules into non-toxic metabolites (Nicolae and Aurlian 2015).Nonetheless, there are significant differences in the sensitivity of crop varieties to fluroxypyr (Stevan et al.2010), and plant tolerance to fluroxypyr also depends on the metabolic rate, antioxidant activity, and fluroxypyr dosage(William et al.2009).Presently, there is a scant evidence indicating whether fluroxypyr is safe for use on foxtail millet.
Moreover, research involving herbicide treatments on foxtail millet is very rare.The rational application of monosulfuron, monosulfuron plus propazine, 2,4-D, and prometryn at the pre-emergence stage can control weeds effectively in foxtail millet but readily results in phytotoxicity(Tian et al.2010).It is also reported that sethoxydim at the post-emergence stage can be used for grassy weed control in Zhangzagu 5 (Xie et al.2014).Additionally, the application of 22.5 g ai ha-1of tribenuron-methyl at the post-emergence stage was found to be relatively safe for Zhangzagu 10 and did not affect its yield or grain quality (Ning et al.2015).However, grain yield loss in foxtail millet by broadleaved weeds have became increasing problem in many foxtail millet growing areas in China, and broadleaved weeds are 4-5 folds to grass weeds (Zhou et al.2010).Therefore,there is a need to explore potential herbicides for controlling broadleaf weeds in foxtail millet field.
To address the above issue, the present study was carried out to investigate the safety and physiological mechanisms of the post-emergence herbicide fluroxypyr tolerance of high-quality conventional Jingu 21 and high-yield hybrid Zhangzagu.The Jingu 21, one of the main conventional varieties of foxtail millet in China, has been widely grown in the major foxtail millet production regions of northern China for 30 years.The hybrid foxtail millet “Zhangzagu”series, one parent of which was derived from Jingu 21, is also popular, with a large production area in China owing to its high stress resistance, yield, and nutritional value(Dong et al.2014).Presently, there is little knowledge about the effect of the fluroxypyr on the mechanism of plant physiology and molecular biology for Zhangzagu hybrid millet.Thus, the objective of this study were to perform a comprehensive investigation of the fluroxypyr impact on the growth, physiological processes between conventional and hybrid foxtail millet when exposed to varied rates of fluroxypyr application, and to achieve a better understanding of the physiological metabolism for the fluroxypyr-induced oxidative stresses in foxtail millet seedlings, thereby providing a theoretical basis for the field application and cultivation of foxtail millet tolerance to herbicide.
2.Materials and methods
2.1.Greenhouse experiment
The seeds of hybrid foxtail millet (Setaria italica L.) cultivars,i.e., Zhangza 3, Zhangza 5, and Zhangza 10, were supplied by the Zhangjiakou Academy of Agricultural Sciences of Hebei Province, China and Jingu 21 were provided by the Shanxi Academy of Agricultural Sciences, China.Fluroxypyr(20% emulsifiable concentrate (EC)) was provided by Dow AgroSciences Co.(Jiangsu, China).
The experiment was designed as a randomized complete block design with three replications.Twenty seeds were sown in each plastic pot of 130 mm in diameter.The pots were filled with a 1:2 mixture of sand and loam soil with 49.18 g kg-1organic matter, 72.51 mg kg-1total nitrogen,19.1 mg kg-1available phosphorus, and 111.25 mg kg-1rapidly available potassium at Shanxi Agricultural University.The seeds were covered with 1 cm of the 1:2 sand/soil mixture, and each pot was carefully watered (natural light, 28°C/(16±3)°C).After their emergence, foxtail millet seedlings were thinned and maintained at 10 uniform plants per pot.After growth at five-leaf stage, these plants were treated with 0, 0.5, 1, 2, and 4 L ai ha-1fluroxypyr, and the recommended effective dosages by the manufacturer were 1 L ai ha-1(for winter wheat) (Eskandar et al.2007).Herbicides were applied using a laboratory pot sprayer equipped with a nozzle fan nozzle (ZYQB-7), previously calibrated to deliver 450 L ha-1.Physiological parameters of foxtail millet seedlings were determined 15 days after treatment (DAT) (Fluroxypyr’s half-life period is only 11 days,and not affect the subsequent crop after application 15 days).
2.2.Determination of chlorophyll
Pigment contents of the leaves were evaluated according to the method proposed by Lichtenthaler (1987).The fresh leaves of plants (0.1 g) were extracted in 10 mL acetone (80%, v/v) and stored in the dark for 24 h.The supernatants were collected in order to measure the pigments content.
2.3.Assays of enzyme activities
The second and third upper leaves of the plants from the glasshouse trial (0.1 g) were homogenized at 4°C in 2 mL Na-phosphate buffer (pH 7.0), containing 0.1 mmol L-1ethylene diamine tetraacetic acid (EDTA) and 1% polyvinyl pyrrolidone (PVP) (w/v).The homogenate was centrifuged at 10 000 r min-1for 15 min at 4°C in a refrigerated centrifuge.The supernatant was used for measuring the activities of superoxide (SOD), peroxidase (POD), catalase (CAT),and ascorbate peroxidase (APX) with a 756C-UV-VIS-spectrophotometer (Shanghai Spectrum Instruments Co.,Ltd., China).
The SOD activity was measured using the method of Beauchamp and Fridovich (1971) by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT).The supernatant extract (20 μL) was mixed with 5 mL of reaction buffer solution consisting of 50 mmol L-1phosphate buffer (pH 7.8), 13 mmol L-1L-methionine, 0.075 mmol L-1NBT, 0.1 mmol L-1EDTA, and 0.002 mmol L-1riboflavin.The test tubes were illuminated below 4 000 lx for 15 min at 25°C.A blank sample containing only reaction solution (no enzyme), was kept in the dark; the control tube, containing only the reaction solution (no enzyme), was kept in the light.The absorbance of the irradiated solution was measured at 560 nm, and a non-irradiated complete reaction mixture was served as a control.A total of 1 U of SOD activity was defined as the amount of enzyme, required to inhibit 50% of the initial reduction of NBT based on comparison with tubes lacking enzyme under light conditions.
The POD activity was assayed according to the method of Castillo et al.(1984), by estimating the determination of guaiacol oxidation by H2O2at 470 nm.The 3 mL reaction mixture contained 3 mL of 100 mmol L-1Na-phosphate buffer(pH 6.0), 19 μL of guaiacol, 28 μL of 30% H2O2, and 20 μL of supernatant.Changes in the absorbance of the reaction solution were recorded for 3 min at 470 nm (extinction coefficient: 26.6 mmol L-1cm-1).
CAT activity was determined at 240 nm continuously for 3 min following H2O2decomposition as described by Aeobi (1974).The reaction mixture contained of 2.7 mL of 50 mmol L-1Tris-HCl (pH 7.0), 50 μL of 200 mmol L-1H2O2, and 20 μL of the supernatant (extinction coefficient:39.4 mmol L-1cm-1).
The activity of APX was determined according to the method of Nakano and Asada (1981).The reaction mixture contained 3 mL of 50 mmol L-1sodium phosphate buffer(pH 7.0), 0.4 mL of 0.3 mmol L-1EDTA, 1 mL of 0.9 mmol L-1ascorbate, and 0.1 mL of the supernatant.After incubation for 5 min at 25°C, the above reaction mixture was added to 0.5 mL of 0.25 mmol L-1H2O2.The decrease in absorbance at 290 nm (extinction coefficient: 2.8 mmol L-1cm-1) was recorded for 1 min.
The glutathione reductase (GR) activity was measured according to Halliwell and Foyer (1978).Fresh leaves of the plants from the glasshouse trial (0.1 g) were homogenized with 2 mL Tris-HCl (pH 7.5, containing 1% PVP and 0.1 mmol L-1EDTA) in an ice bath.The homogenate was centrifuged at 10 000 r min-1for 15 min at 4°C.The reaction mixture contained of 3 mL of Tris-HCl (pH 7.5), 3 mmol L-1MgCl2,0.5 mmol L-1oxidized glutathione (GSSG) and 0.15 mmol L-1nicotinamide adenine dinucleotide phosphate (NADPH).After incubation for 5 min at 25°C, the supernatant (150 μL)was added to the above reaction mixture and the change in absorbance was calculated at 340 nm for 3 min.
2.4.Determination of lipid peroxidation
MDA concentration was evaluated according to the method of Heath and Packer (1968).The second and third upper leaves of the plants from the glasshouse trial (0.1 g) were homogenized in 5 mL 0.1% trichloroacetic acid (TCA).The homogenate was mixed with 5 mL 0.5% thiobarbituric acid(TBA), boiled for 15 min, cooled quickly and centrifuged for 10 min at 4 000 r min-1.The supernatant was used to measure the MDA concentration.The absorbance of the supernatant was measured by subtracting the non-specific absorbance at 600 nm from the absorbance at 532 nm.The MDA concentration was calculated using an extinction coefficient of 155 mmol L-1cm-1.
2.5.Determination of the superoxide generation rate
The superoxide generation rate was determined as described by Elstner and Heupel (1976).The second and third upper leaves of the plants from the glasshouse trial (0.1 g) were homogenized with 2 mL of 65 mmol L-1Na-phosphate buffer (pH 7.8).The homogenate was centrifuged at 10 000 r min-1for 10 min.The supernatant was collected to determine the superoxide production rate.The 1 mL supernatant was mixed with 1 mL of 65 mmol L-1Na-phosphate buffer (pH 7.8) and 0.2 mL of 10 mmol L-1hydroxylammonium chloride.After incubation for 20 min at 25°C, 1 mL of the above reaction mixture was added to 1 mL of 17 mmol L-14-aminobenzene sulphonic acid and 1 mL of 7 mmol L-1α-naphthylamine, mixed, and then incubated for 30 min at 30°C.The absorbance of the lower pink waterphase was monitored at 530 nm.
2.6.Measurement of the H2O2 content
The hydrogen peroxide levels were determined according to Zhang et al.(2012).Leaf tissues (0.1 g) from the glasshouse trial were homogenized in an ice bath with 5 mL chilled acetone.The homogenate was centrifuged at 4 000 r min-1for 15 min, and 1 mL of the supernatant was added to 0.1 mL of 20% TiCl4concentrated hydrochloric acid and 0.2 mL of concentrated ammonia water and then centrifuged for 10 min at 10 000 r min-1.The precipitate was dissolved in 3 mL of 1 mol L-1H2SO4.The absorbance of the supernatant was calculated at 410 nm.
2.7.Estimation of the total, reduced and oxidized ascorbic acid contents
The estimation of ascorbate acid/dehydroascorbate (AsA/DHA) was carried out according to Jiang and Zhang(2001).Leaf tissues from the glasshouse trial (0.1 g) were homogenized in 2 mL of 5% chilled sulfosalicylic acid in an ice bath and centrifuged at 10 000 r min-1for 15 min at 4°C.The supernatant (100 μL) was recovered and neutralized by the addition of 24 μL of 1.84 mol L-1triethanolamine,5 mL Na-phosphate buffer (pH 7.5, containing 2.5 mmol L-1EDTA) and 50 μL of 10 mmol L-1DTT for 10 min at 25°C to reduce DHA to AsA.The above reaction was mixed with 50 μL of 0.5% n-ethylmaleimide to remove DTT, 200 μL of 10% trichloroacetic acid (TCA), 44% phosphoric acid, and 4% 2,2-bipyridine, and 100 μL of 3% FeCl3for 60 min at 40°C.The change in absorbance was measured at 525 nm.This method was used to measure the total ascorbic acid content and to measure the amount of AsA used per volume of distilled water to replace DTT and n-ethylmaleimide.A standard curve prepared using AsA and DHA was used to calculate the amounts of total ascorbic acid, reduced DHA(total DHA-reduced AsA) and AsA.
2.8.Estimation of the total, reduced and oxidized glutathione contents
The estimation of reduced glutathione/oxidized gluthione(GSH/GSSG) was carried out according to Nagalakshmi and Prasad (2001).Leaf tissues from the glasshouse trial(0.1 g) were homogenized in 2 mL 5% chilled sulfosalicylic acid in an ice bath and centrifuged at 10 000 r min-1for 15 min at 4°C.A 100-μL aliquot of the supernatant was removed and neutralized by the addition of 24 μL 1.84 mol L-1triethanolamine and 50 μL 2-vinylpyridine for 60 min at 25°C for GSSG reductase to mask GSH via derivatization and to allow for the determination of GSSG alone.The above reaction was mixed with 2.7 mL 50 mmol L-1Naphosphate buffer (pH 7.5, containing 2.5 mmol L-1EDTA),20 μL 10 mmol L-1nicotinamide adenine dinucleotide phosphate (NADPH) and 80 μL 12.5 mmol L-15,5´-dithiobis-(2-nitrobenzoic acid) DTNB for 10 min at 25°C.A 20-μL aliquot of glutathione reductase (50 U mL-1) was added and the change in absorbance at 412 nm was monitored for 3 min.This method was used to measure GSSG and the total glutathione used in isopycnic distilled water to replace 2-vinylpyridine.A standard curve prepared using GSSG and GSH was used to calculate the amounts of total glutathione,reduced GSH (total GSH-oxidized GSSG) and GSSG.
2.9.Statistical analysis
The data (mean±SE) were analyzed using the data processing system (SAS 8.0) program package according to a two-factor randomized complete block design to compare different herbicide dosages and different varieties.Duncan’s test (P<0.05) was used to determine the significant differences among the treatments.Simple correlation coefficients were calculated based on the treatment means.
3.Results
3.1.Effect of fluroxypyr on plant height and chlorophyll of foxtail millet
Compared with the control, fluroxypyr treatment at 2.0 and 4.0 L ai ha-1decreased the plant height by 10.63 and 18.81%for Zhangzagu 3, and 13.02 and 21.20% for Zhangzagu 5,respectively (Fig.1-A).However, there were no significant differences between the two treatments in Zhangzagu 3 and Zhangzagu 5.For Zhangzagu 10 and Jingu 21, the reduction in plant height (15.42 and 15.52%, 13.89 and 21.97%, respectively) was significant at 2.0 and 4.0 L ai ha-1compared with the control, respectively.
The contents of chlorophyll in leaves of foxtail millet declined with increasing fluroxypyr concentrations (Fig.1-B).Although both the hybrid millet and Jingu 21 showed similar trends, the decreasing chlorophyll contents in each variety were not identical (Fig.2).At 2.0 and 4.0 L ai ha-1, the chlorophyll content of Zhangzagu 5 significantly decreased to 24.56 and 38.95%, respectively.Moreover, Zhangzagu 3 and Zhangzag 10 showed a similar trend, in which significant differences were found after 15 d of exposure to fluroxypyr at 0.5 to 4 L ai ha-1.Compared with the hybrid millet,chlorophyll contents significantly decreased 19.06, 20.47,25.41, and 39.30% compared with the control, respectively.
3.2.Effect of fluroxypyr on MDA content and oxygen metabolism of foxtail millet
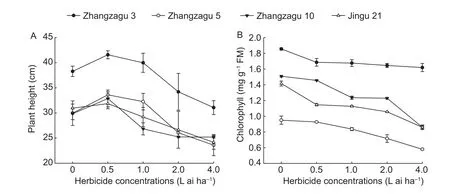
Fig.1 Effect of fluroxypyr on the plant height (A) and the contents of chlorophyll (B).Plants were treated with fluroxypyr for 15 days after treatment.FM, fresh mass.Values are the mean±SE.

Fig.2 Effect of fluroxypyr dosages on foxtail millet seedling.Representative photos of foxtail millet at 15 days after fluroxypyr treatment.A, Jingu 21.B, Zhangzagu 3.C, Zhangzagu 5.D, Zhangzagu 10.0, 0.5, 1.0, 2.0, and 4.0 are five test dosages (unit,L ai ha-1) of fluroxypyr, respectively.
As shown in Table 1, increasing dosages of fluroxypyr increased MDA content, and H2O2contents and thegenerating rate in all the varieties; however, these effects were more pronounced in Jingu 21 compared with Zhangzagu.The MDA content with dosages at ≥0.5 L ai ha-1showed significant differences from the control in terms of Jingu 21, Zhangzagu 3, and Zhangzagu 10.In Zhangzagu 5, fluroxypyr significantly increased the MAD content at dosages 2 and 4 L ai ha-1.On the other hand, no significant differences in the treatments were observed foramong the three Zhangzagu cultivars(Zhangzagu 3, Zhangzagu 5, and Zhangzagu 10), and the generationin Jingu 21 was significantly increased(28.83%) at 4 L ai ha-1compared to the control.Meanwhile,the H2O2content of Jingu 21 showed significantly increased as fluroxypyr increased from 1 to 4 L ai ha-1.The trends of H2O2content between Zhangzagu 3 and Zhangzagu 10 were similar, being significantly higher than the control at 4 L ai ha-1, though no significant differences in H2O2content(P<0.05) was found between Zhangzagu 5 and the control.
3.3.Effect of fluroxypyr on antioxidant enzyme activity of foxtail millet
Compared to the control, the activities of antioxidant enzymes increased with increasing dosages of fluroxypyr;however, the effects were more pronounced in Zhangzagu compared with Jingu 21 (Table 2).A significant increase in SOD activity at dosages≥0.5 L ai ha-1was found for Zhangzagu (Table 2), and the SOD activity in Jingu 21 significantly increased at 1 L ai ha-1.In Zhangzagu and Jingu 21, fluroxypyr significantly increased the POD activity at dosages≥0.5 L ai ha-1.On the other hand, the CAT activity was similar between Zhangzagu 3 and Zhangzagu 5,significantly increasing at dosages≥0.5 L ai ha-1compared to the control.Meanwhile, the CAT and GR activities of Jingu 21 significantly increased at 1 and 2 L ai ha-1, and the APX activity significantly increased at dosages≥1 L aiha-1.In Zhangzagu 3, the changes in APX and GR activities were similar; and were significantly higher than those of the control at dosages≥1 L ai ha-1.Additionally, the APX activity was a significantly increased between Zhangzagu 5 and Zhangzagu 10 with dosages≥2 L ai ha-1.The GR activity of Zhangzagu 5 was significantly higher at 2 L ai ha-1, and
the GR activity of Zhangzagu 10 was significantly higher at dosages≥1 L ai ha-1.

Table 1 Effect of different concentrations of fluroxypyr on malondialdehyde (MDA) and reactive oxygen species (ROS) of foxtail millet1)

Table 2 Effects of different concentrations of fluroxypyr on superoxide (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) activities of foxtail millet1)
The relationship between the protective enzymes and damage index was similar among the four varieties (Table 3).In Jingu 21 and Zhangzagu cultivars, a significant positive correlation was found for SOD, POD, CAT, APX, and GR,indicating that the five types of antioxidant enzymes showed a good coordination.However, a correlation for the damage index and protective enzymes differed among varieties,which explains the differences in the internal tolerant mechanism among varieties.
3.4.Effect of fluroxypyr on ascorbic acid metabolism of foxtail millet
Zhangzagu cultivars and Jingu 21 showed similar AsA levels in the presence of fluroxypyr, which induced a progressive increase in these activities at low concentration, but a decrease at higher concentrations (Table 4).In Jingu 21 and Zhangzagu 10, fluroxypyr treatment showed similar changes in terms of AsA and DHA contents, no significant differences were obtained with dosages at 0-4 L ai ha-1.The total AsA content significantly increased 59.12% at 1 L ai ha-1in Jingu 21 and 34.45% in Zhangzagu 10 at 2 L ai ha-1.Compared with the control, the AsA content was significantly increased by 40.72% in Zhangzagu 3, and by 67.08% in Zhangzagu 5 at 2 L ai ha-1, respectively.On the other hand, AsA/DHA ratio was not affected by fluroxypyr treatments among four varieties.This result shows that AsA can be used as an index of plant antioxidant capacity and that a high AsA content alleviates the damage caused by ROS.
3.5.Effect of fluroxypyr on GSH metabolism of foxtail millet
As shown in Table 5, the GSH content and total GSH content of Jingu 21 were similar at 1 to 4 L ai ha-1, and significant compared to the control.In Zhangzagu 3, the changes in the GSH, GSSG, and total GSH contents were similar and significantly higher than those of the control at 4 L ai ha-1.In Zhangzagu 5, no significant effects were obtained between the GSH and total GSH contents at 0.5-4 L ai ha-1; however,the GSSG content was significant at dosages≥0.5 L ai ha-1compared with the control.On the other hand, fluroxypyr significantly increased the GSH content, total GSH content and the GSH/GSSG ratio in Zhangzagu 10 at dosages≥1 L ai ha-1.
Table 3 Correlation coefficient between malondialdehyde (MDA) content, content, H2O2 content and protective enzymes

Table 3 Correlation coefficient between malondialdehyde (MDA) content, content, H2O2 content and protective enzymes
1) SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; GR, glutathione reductase.* and ** represent significantly differences at P<0.05 and P<0.01, respectively.
?

Table 4 Effects of different concentrations of fluroxypyr on ascorbate acid (AsA), dehydroascorbate (DHA), and total ascorbate contents and AsA/DHA ratio of foxtail millet1)

Table 5 Effects of different concentrations of fluroxypyr on reduced glutathione (GSH), oxidized glutathione (GSSG), and total glutathione (GSH+GSSG) contents, and GSH/GSSG ratio of foxtail millet1)
4.Discussion
The safety of herbicide use on crops may be demonstrated through analysis of agronomic traits (height, leaf area,leaf color, plant growth speed, biomass, yield, etc.) and physiological variations (protective enzyme activities, lipid peroxidation, etc.) (Zhao et al.2010; Qian et al.2011; Yuan et al.2013).In this study, fluroxypyr treatment inhibited the growth of foxtail millet, as reflected by reduced plant height(Figs.1 and 2-A).Guo et al.(2010) also demonstrated that the herbicide fluroxypyr affected the growth of rice (Oryza sativa).Fluroxypyr-inhibited growth was closely linked to the generation of ROS in leaves.In addition, cultivar-dependent differences in plant height reduction due to fluroxypyr were observed at a dosages of ≥2 L ai ha-1(the Zhangzagu hybrid)and ≥1 L ai ha-1(Jingu 21).
On the other hand, several symptoms related to the growth change were observed.One observation was the chlorosis of leaves with fluroxypyr.Previous studies have demonstrated that the reduction of chlorophyll content might be a good indicator for monitoring damage to the plant growth and development (Zhou 2003; Song et al.2007; Yin et al.2008).In this study, it has been shown that chlorophyll was sensitive to fluroxypyr spray because treatment with fluroxypyr at 4 L ai ha-1might active in the chloroplast electron-transport system and disturb the photosynthesis.Furthermore, difference in chlorophyll reduction might be attributed to varietal properties of herbicide tolerance.Our results demonstrate that Zhangzagu hybrid millet had a greater tolerance mechanism than that of Jingu 21 at herbicide high levels.
Previous studies have demonstrated that herbicides trigger oxidative stress by leading to lipid peroxidation (MDA as a result of lipid peroxidation reaction) and producing ROS,e.g.,and H2O2(Song et al.2007).Our results showed a significant increase in MDA concentration in the fluroxypyrtreated samples compared to the controls.In general, an increase in the rate of the lipid peroxidation occurs under stress conditions, resulting in increased ROS accumulation.Recent studies have demonstrated that organic toxic substances, such as herbicides, are able to induce intracellular ROS production in plants (Wang and Zhou 2006;Song et al.2007; Yin et al.2008).In this study, fluroxypyr at low concentrations did not induce a significant increase inand H2O2contents; however, high concentrations caused a remarkable increase, demonstrating that within the scope of tolerance, ROS production is tightly controlled by enzymatic or non-enzymatic mechanisms (Mittler 2002).
Under normal circumstances, an equilibrium exists between ROS production and antioxidant defense, yet ROS accumulation altered the balance between ROS production and scavenging capacities under herbicide stress, which will ultimately result in lipid peroxidation.To address fluroxypyr-induced oxidative stress, plants have multiple strategies to confer their tolerance to herbicide-induced toxicity, and prevention of oxidative damage to cell has been suggested as one of the mechanisms of tolerance.The antioxidant machinery is composed of enzymes (e.g., SOD,CAT, POD, GR and APX) and non-enzymatic components(e.g., glutathione, ascorbate).The increased activities of antioxidant enzymes, such as SOD, CAT, POD, GR, and APX, reflects not only the degree of toxicity but as well the ability to tolerate the stress.SOD is the first line of defense against ROS damage, catalyzing the dismutation of superoxide radical to O2and H2O2.Our study revealed an important stimulation of SOD activity after fluroxypyr application, which may be a response to the accumulation of ROS and especially superoxide anion.Indeed,increased SOD activity might be attributed to elevated production of superoxide, thus resulting in activation of existing enzyme pools or up-regulated gene expression(Lei and Hong 2009).POD, CAT, APX, and GR are the key enzymes for eliminating H2O2.POD, which utilizes phenolic compounds as substrates to decompose H2O2,is widely present in different tissues of plants (Yang et al.2008).The increased POD activity in leaves exposed to lower fluroxypyr concentrations may be a response to H2O2accumulation.However, the decreased POD activity after exposed to higher levels of fluroxypyr in tissues indicated capacity to control the oxidative stress.Similarly, we found a large increase in CAT activity, which is invoked by H2O2accumulation, at lower fluroxypyr concentrations.Whereas,lower CAT activity was found at high concentrations, which was due to the high level of superoxide/H2O2and reduced enzymes capacities, particularly those of heme-containing enzymes, such as catalases and group III peroxidases(Lei and Hong 2009).APX is part of the ascorbateglutathione cycle and is also responsible for eliminating hydrogen peroxide (Sarvajeet and Narendra 2010).Our study revealed an important stimulation of APX activity in fluroxypyr-treated plants, and this stimulation may be a response to H2O2accumulation and/or may be related to the activation of the ascorbate-glutathione cycle.GR plays an important role in catalyzing the reduction of oxidized glutathione to reduced glutathione.Our results indicate that GR activation in response to fluroxypyr, suggesting that this enzyme most likely plays a role in detoxifying the high level of ROS caused by fluroxypyr-triggered oxidative stress in foxtail millet.In accordance with our results, Guo et al.(2010) showed several antioxidative enzymes such as SOD, POD, CAT, and APX activities were increased at low concentration of herbicide and decreased at higher concentrations, dealing with fluroxypyr-induced oxidative stress in rice (Oryza sativa).Additionally, previous studies have reported higher activities of SOD, CAT, and POD in Zhangzagu 5 than in conventional variety under sethoxydim stress at the seedling stage (Xie et al.2014).Similar results were obtained for hybrid maize, with higher antioxidant enzyme activities under herbicide stress (Ma 1999; Li 2004).In the Zhangzagu hybrid and Jingu 21, there was a significant positive correlation between protective enzymes and MDA,and H2O2contents; however, the correlation coefficient between MDA and CAT, POD, GR, and APX of the Zhangzagu hybrid were higher than those of Jingu 21.Results demonstrated that, under herbicides stress,the Zhangzagu hybrid had better coordination between protective enzymes and MDA content, and the activities of SOD, CAT, POD, GR, and APX were more efficiently scavenge ROS in Zhangzagu hybrid than those in Jingu 21,which can protecte cells against ROS toxicity.These results suggest that the tolerance of foxtail millet is associated with the accumulation of ROS and the capability of detoxification lipid peroxidation under herbicide stress and that Jingu 21 is more sensitive to fluroxypyr than Zhangzagu hybrid millet.
In addition to the antioxidant enzyme system, plants possess non-enzymatic antioxidant defense systems,including ascorbate, glutathione, phenolic compounds,anthocyanins and tocopherols, which act in concert to scavenge ROS and protect against oxidative damage (Faller and Fialho 2009).AsA and GSH are important antioxidants against ROS.AsA can directly eliminate O2-., OH·, and1O2and is also a peroxidase substrate.Meanwhile, AsA also plays a pivotal role in protecting activities of enzymes,which contain prosthetic transition metal ions (Mehlhorn et al.1996).In this study, treatment with fluroxypyr at≥1 L ai ha-1significantly increased the AsA content in the Zhangzagu hybrid.However, the AsA content in Jingu 21 decreased at dosages of ≥1 L ai ha-1, possibly because of the herbicide tolerance of this cultivar or to the degration of the herbicide into non-phytotoxic products (Athar et al.2008).Additionally, GSH also plays an important role in several growth and development related events in plants,which is an important antioxidant in the cellular milieu and responsible for maintaining the normal reduced state of cells to inhibit the effects of ROS under oxidative stress(Rennenberg and Brunold 1994).Some researchers have reported that a high GSH/GSSG ratio is necessary to improve the capacity of resistance to oxidation in plants(Rennenberg and Brunold 1994).In this study, the AsA and GSH contents, AsA/DHA ratio, and GSH/GSSG ratio were slightly higher in hybrid millet than those in Jingu 21 at a relatively low dose of herbicide but with a remarkable effect at high levels.This finding may be due to the higher operation efficiency of the AsA-GSH cycle under herbicides stress, and the greater capacity of Zhangzagu hybrid against ROS damage.In concordance with our results, Wang et al.(2015) reported induced AsA and GSH activities in rice resistant to cold, and that AsA/DHA and GSH/GSSG ratios were increased improving resistance in plants under cold water stress.Thus, the Zhangzagu hybrid can maintain high rates of protection mechanisms to eliminate or reduce ROS caused fluroxypyr-induced oxidative damage 15 DAT,to protect they continue to grow.
5.Conclusion
Our data suggested that the hybrid millet (Zhangzagu) is more tolerance than the conventional variety Jingu 21,when treated with higher dosages.To cope with fluroxypyrinduced oxidative stress, the Zhangzagu hybrid effectively reduced the level of ROS by increasing antioxidative enzymes activities and antioxidant contents, thereby alleviating herbicide damage to biological membranes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31301269), the National Key Technologies R&D Program of China (2014BAD07B01),the Scientific and Technological Project in Shanxi Province,China (20150311016-2), the Science and Technology Key Research Project in Shanxi Province, China (2015-TN-09), the Key Research and Development General Project in Shanxi Province, China (201603D221003-2), and the Program for the Top Young Innovative Talents of Shanxi Agricultural University, China (TYIT201406).
Aeobi H.1974.Catalase.In: Bergmeyer H U, ed., Methods of Enzymatic Analysis.Verlag Chemie/Academic Press,Weinheim, Germany.pp.673-680.
Anand P, Kunnumakara A B, Sundaram C.2008.Cancer is a preventable disease that requires major lifestyle changes.Pharmaceutical Research, 25, 2097-2116.
Athar H R, Khan A, Ashraf M.2008.Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat.Environmental and Experimental Botany, 63,224-231.
Beauchamp C, Fridovich I.1971.Superoxide dismutase:improved assays and an assay applicable to acrylamide gels.Analytical Biochemistry, 44, 276-287.
Carvalho C P, Salvador A, Navarro P, Monterde A, Martínez-Jávega J M.2008.Effect of auxin treatments on calyx senescence in the degreening of four mandarin cultivars.HortScience, 43, 747-752.
Castillo F I, Penel I, Greppin H.1984.Peroxidase release induced by ozone in Sedum album leaves.Plant Physiology,74, 846-851.
Dong B D, Liu M Y, Jiang J W.2014.Growth, grain yield, and water use efficiency of rain-fed spring hybridmillet (Setaria italica) in plastic-mulched and unmulched fields.Agricultural Water Management, 143, 93-101.
Elstner E F, Heupel A.1976.Inhibition of nitrite formation from hydroxylammonium chloride: A simple assay for superoxide dismutase.Analytical Biochemistry, 70, 616-620.
Eskandar Z, Mohsmmad A B, Saeid S.2007.Broadleaved weed control in winter wheat (Triticum aestivum L.) with post-emergence herbicides in Iran.Crop Protection, 26,746-752.
Faller A L K, Fialho E.2009.The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking.Food Research International, 42, 210-215.
Fujita S, Sugimoto Y, Yamashita Y, Fuwa H.1996.Physicochemical studies of starch from millet (Setaria italica Beauv.).Food Chemistry, 55, 209-213.
Guo L W, Jing C, Ling T, Hong Y.2010.Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa).Ecotoxicology, 19, 124-132.
Halliwell B, Foyer C H.1978.Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography.Planta, 139, 9-17.
Heath R L, Packer L.1968.Photoperoxidation in isolated chloroplasts I.Kinetics and stoichiometry of fatty acid peroxidation.Archives of Biochemistry and Biophysics,125, 189-198.
Hellou J, Leonard J, Cook A, Doe K, Dunphy K, Jackman P, Tremblay L, Flemming J M.2009.Comparison of the partitioning of pesticides relative to the survival and behaviour of exposed amphipods.Ecotoxicology, 18,27-33.
Jiang M Y, Zhang J H.2001.Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings.Plant and Cell Physiological, 42, 1265-1273.
Lei J, Hong Y.2009.Prometryne-induced oxidative stresss and impact on antioxidant enzymes in wheat.Ecotoxicology and Environmental Safety, 72, 1687-1693.
Li Y B.2004.The study on mechanism and forecast of crop heterosis.Journal of Jilin Normal University, 2, 83-85.(in Chinese)
Lichtenthaler H K.1987.Chlorophylls and carotenoids:Pigments of photosynthetic biomembranes.Methods Enzymol, 148, 350-382.
Liu L C.2014.Study 20% fluroxypyr control broadleaf weeds in maize field.Modernizing Agriculture, 417, 4-5.(in Chinese)
Ma Y T.1999.Relationship between peroxidase and heterosis in maize.Journal of Xi’an United University, 2, 11-15.(in Chinese)
Mehlhorn H, Lelandais M, Korth H G, Foyer C H.1996.Ascorbate is the natural substrate for plant peroxidases.FEBS Letters, 378, 203-206.
Mittler R.2002.Oxidative stress, antioxidants and stress tolerance.Trends in Plant Science, 7, 405-410.
Nagalakshmi N, Prasad M N V.2001.Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus.Plant Science, 160, 291-299.
Nakano Y, Asada K.1981.Hydrogen peroxide is scavenged by ascrobate specific peroxidase in spinach chloroplasts.Plant and Cell Physiology, 22, 867-880.
Nicolae I, Aurelian P.2015.Aspects of winter wheat physiology treated with herbicides.Agriculture and Agricultural Science Procedia, 6, 52-57.
Ning N, Yuan X Y, Dong S Q, Wen Y Y, Gao Z P, Guo M J, Guo P Y.2015.Grain yield and quality of foxtail millet (Setaria italica L.) in response to Tribenuron-Methyl.PLoS ONE,10, e0142557.
Pornprom T, Sukcharoenvipharat W, Sansiriphun D.2010.Weed control with pre-emergence herbicides in vegetable soybean (Glycine max L.Merrill).Crop Protection, 29,684-690.
Qian H.2011.Enantioselective phytotoxicity of the herbicide Imazethapyr on the response of the antioxidant system and starch metabolism in Arabidopsis thaliana.PLoS ONE, 6,e19451.
Rennenberg H, Brunold C.1994.Significance of glutathione metabolism in plants under stress.Progress in Botany,55, 142-156.
Sarvajeet S G, Narendra T.2010.Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.Plant Physiology and Biochemistry, 48, 909-930.
Sdiri S, Navarro P, Salvador A.2013.Postharvest application of a new growth regulator reduces calyx alterations of citrus fruit induced by degreening treatment.Postharvest Biology and Technology, 75, 68-74.
Song N H, Yin X, Chen G F, Yang H.2007.Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils.Chemosphere, 68, 1779-1787.
Stevan Z K, Avishek D, Jon S, Leo D C.2010.Tolerance of winter wheat (Triticum aestivum L.) to pre-emergence and post-emergence application of saflufenacil.Crop Protection,29, 148-152.
Tian B H, Wang J G.2010.Study on selection of suitable herbicides for hybrid millet.Journal of Hebei Agricultural Sciences, 14, 46-47.(in Chinese)
Wang G J, Wang J Y, Ma D R, Miao W, Zhao M H, Chen W F.2015.Responses of antioxidant system to cold water stress in weedy and cultivated rice with different chilling sensitivity.Scientia Agricultura Sinica, 48, 1660-1668.(in Chinese)
Wang M E, Zhou Q X.2006.Effect of herbicide chlorimuronethyl on physiological mechanisms in wheat (Triticum aestivum).Ecotoxicol Environ Safety, 64, 190-197.
William E M, Eric N J, Dan J U.2009.Tolerance of foxtail millet to combinations of bromoxynil, clopyralid, fluroxypyr, and MCPA.Weed Technology, 23, 94-98.
Xie L L, Guo P Y, Yuan X Y.2014.Effect of sethoxydim on physiological characteristics of hybrid millet Zhangzagu 5 seedling.Journal of Shanxi Agricultural Sciences, 42,223-226.(in Chinese)
Yang J H, Gao Y, Li Y M, Qi X H, Zhang M F.2008.Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon.Scientia Horticulturae, 118, 200-205.
Yin X L, Jiang L, Song N H, Yang H.2008.Toxic reactivity of wheat (Triticum aestivum) plants to herbicide isoproturon.Journal of Agricultural and Food Chemistry, 56, 4825-4831.
Yuan X Y, Guo P Y, Qi X, Ning N, Wang H, Wang H F, Wang X, Yang Y J.2013.Safety of herbicide Sigma Broad on Radix Isatidis (Isatis indigotica Fort.) seedlings and their photosynthetic physiological responses.Pesticide Biochemistry Physiology, 106, 45-50.
Zhang Y P, Jia F F, Zhang X M, Qiao Y X, Shi K, Zhou Y H,Yu J Q.2012.Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima.Acta Physiologiae Plantarum, 34, 713-720.
Zhang Z W, Qu J B, Xu G F, Song L H, Wang J J, Shimbo S.1997.Maize and foxtail millet as substantial sources of dietary lead intake.Science of the Total Environment,208, 81-88.
Zhao R, Guo P Y, Yuan X Y, Wang J Y, Han M Q.2010.Effect of paraquat on the antioxidative enzyme activities and lipid peroxidation in opium poppy (Papaver somniferum L.).Journal of Plant Diseases and Protection, 117, 55-59.
Zhou H Z, Liu H X, Bo K Y, Jia H Y, Lv P.2012.Study on prediction model of millet yield loss caused by weeds in summer season millet field.Journal of Agricultural, 2,12-15.(in Chinese)
Zhou H Z, Ren Z Q.2010.Problems and development trend of chemical weeding in millet field.Journal of Hebei Agricultural Sciences, 14, 56-58.(in Chinese)
Zhou Q X.2003.Interaction between heavy metals and nitrogen fertilizers applied in soil-vegetable systems.Bulletin of Environmental Contamination and Toxicology,171, 338-344.
杂志排行
Journal of Integrative Agriculture的其它文章
- Characteristic analysis of tetra-resistant genetically modified rice
- A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis
- Characterization of GhSERK2 and its expression associated with somatic embryogenesis and hormones level in Upland cotton
- GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean
- Molecular cloning and functional characterization of a soybean GmGMP1 gene reveals its involvement in ascorbic acid biosynthesis and multiple abiotic stress tolerance in transgenic plants
- Light interception and radiation use efficiency response to tridimensional uniform sowing in winter wheat
