紫外光下氟取代H4W10O32作为一种高效催化剂催化双氧水氧化降解甲基橙
2017-11-28唐泽玉伏再辉汤森培张慎益张超刘亚纯尹笃林李建伟
唐泽玉+伏再辉+汤森培+张慎益+张超+刘亚纯+尹笃林+李建伟
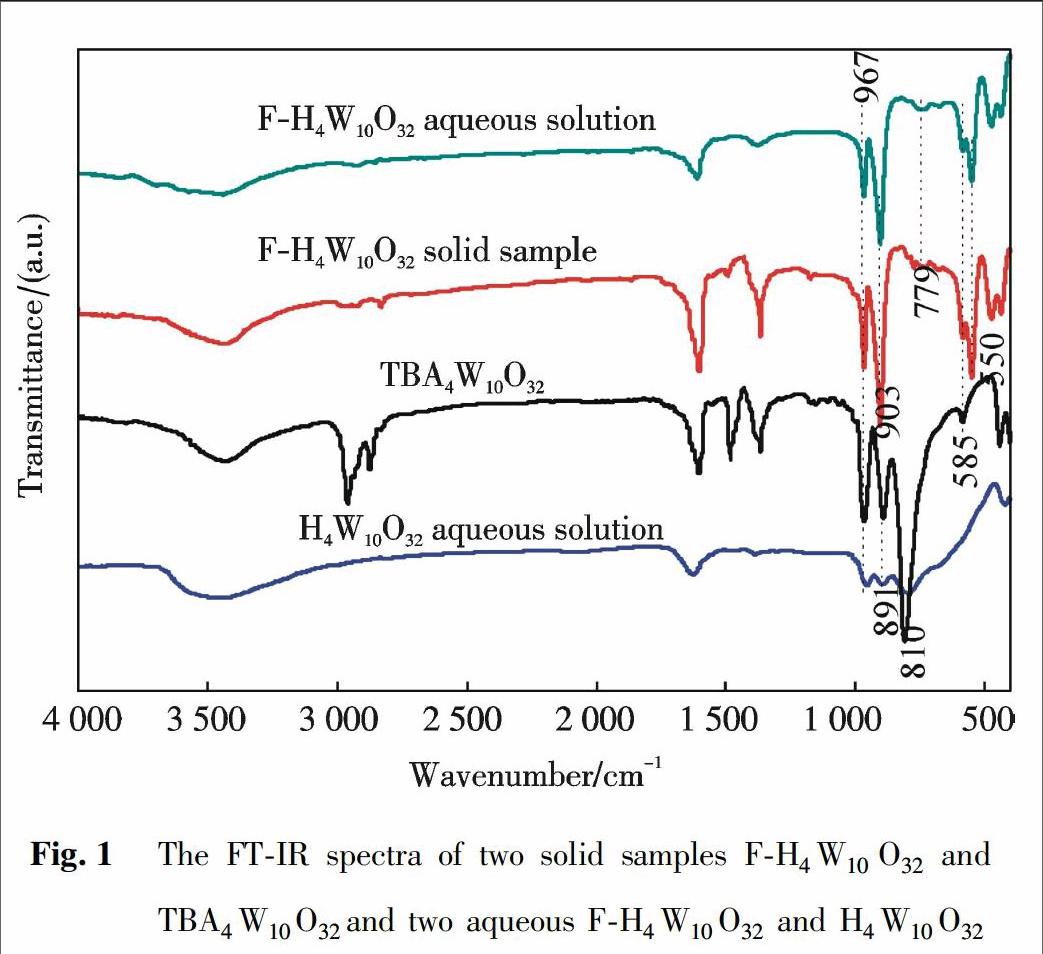
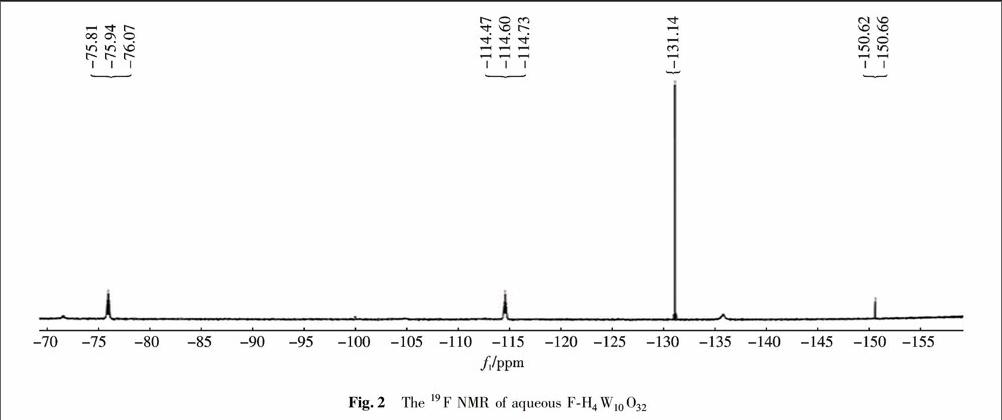

摘 要 以钨酸钠与氢氟酸为原料聚合得到氟取代的十聚钨酸(F-H4W10O32),并采用FT-IR,UV-vis和19F-NMR进行表征.由FT-IR和19F-NMR的表征结果得出,F原子可能通过取代W-Oc-W键的侧桥氧原子而被结合到十聚钨酸盐的框架中,显著改善了十聚钨酸盐的稳定性及其对水溶液中H2O2的活化能力.在紫外光照射下催化过氧化氢(H2O2)氧化降解的甲基橙(MO)实验显示出氟取代的十聚钨酸出色的光催化活性,其与亲本H4W10O32相比,降解速率增加到1.4~2.0倍.基于UV-Vis光谱表征,提出了一种合理的光催化氧化机理.
关键词 氟取代十聚钨酸;过氧化氢;甲基橙;光催化;氧化性降解
Fluorine-Substituted H4W10O32 as a Highly-Efficient Catalyst for Oxidative Degradation of Methyl Orange by Hydrogen Peroxide in Aqueous Solution Under Ultraviolet Light Irradiation
TANG Ze-yu, FU Zai-hui ,TANG Sen-pei, ZHANG Shen-yi,
ZHANG Chao, LIU Ya-chun, YIN Du-lin, LI Jian-wei(National and Local United Engineering Laboratory for New Petrochemical Materials & Fine Utilization of Resources,
Key Laboratory of Resource Fine-Processing and Advanced Materials of Hunan Province and Key Laboratory of
Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China),
College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China)
Abstract A fluorine-substituted decatungstate acid (F-H4W10O32) was conveniently synthesized via a polymerization of sodium tungstate with hydrofluoric acid and characterized by FT-IR, UV-Vis and 19F-NMR. And its photo-catalytic performance was evaluated using the oxidative degradation of methyl orange (MO) by hydrogen peroxide (H2O2) under ultraviolet light irradiation. Our results indicated that F atoms, as supported by FT-IR and 19F-NMR, are likely incorporated into the framework of decatungstate via replacing side-bridged oxygen atoms of W-Oc-W bonds, which can significantly improve the stability of decatungstate and its activation capacity to H2O2 in aqueous solution. As a result, it demonstrated an outstanding photo-catalytic activity for the oxidative degradation of MO by H2O2, achieving about 1.4~2.0 fold increase in degradation rate compared to the parent H4W10O32. Based on UV-Vis spectral characterizations, a reasonable photo-catalysis oxidation mechanism was proposed.
Key words fluorine-substituted decatungstate acid; hydrogen peroxide; methyl orange; photo-catalysis; oxidative degradation
中圖分类号 O64332 文献标识码 A 文章编号 1000-2537(2017)05-0059-12
During the past few decades, photo-catalytic oxidation technique has received extensive attention because of its potential applications in the synthesis of fine chemicals and especially environmental treatments[1-3]. Meanwhile, a large number of metal oxide and sulfide semi-conductors[4-6] and polyoxometalates[7-9] have been applied to effectively photo-catalyze the oxidative degradation of organic wastewater and the removal of waste gas. However, the photo-catalytic activity of most semiconductors is limited by their poor adsorption capacity for the organic dyes and the rapid recombination rate of their photo-generated electron-hole pairs. In general, the photo-catalytic performance of semiconductors can be markedly improved via the doping of various metal cations[10-11] and non-metal anions[12-23]. Among these doping agents, the doping of F- anion seems to be particularly effective and can exponentially increase the photo-catalytic activity of some semiconductors such as TiO2 [20,24-28], SrTiO3 [29], ZnWO4 [19, 30],Bi2WO6 [31],Bi2O3 [32] and BiPO4 [33]. The favorable effect of F- doping on the photo-catalytic activity of semiconductors may be originated from the following three reasons: i) the polarizability and dipole moment of semiconductor may be increased via F- anion replacing its lattice oxygen, which can lead to the improved adsorption capacity to organic contaminants in wastewater[33]. ii) F- doping can efficiently reduce the recombination of photo-generated electron-hole pairs on a semiconductor and thus improve the oxidative capacity of the photo-generated holes via lowering valence band edge[32]. And iii) F- doping may increase the surface hydroxyl density of semiconductor, which can efficiently induce the generation of hydroxyl radicals and improve the surface charge transfer efficiency[31, 34-35].endprint
Polyoxometalates have been extensively studied over the past decades with some of them possessing interesting applications in catalysis[36]. Among them, decatungstate anion (W10O324-) is one of the most promising examples, due to its high photo-catalytic activity in the conversion of organic compounds to the corresponding oxidative products under ultraviolet or visible light[37-44]. But, it can only be used in small amounts as a photo-catalyst for the purification of wastewater[45-47] because of its instability in water. In order to solve this problem efficiently, we are inspired by the above success examples of F-doped semiconductors and for the first time we tried to prepare an F-substituted decatungstate acid (F-H4W10O32) via a polymerization of sodium tungstate with hydrofluoric acid. Here, we report the initial results obtained from the synthesis of F-H4W10O32 and the effect of F doping on the stability of decatungstate acid in water and its photo-catalytic performance for the oxidative degradation of methyl orange (MO) by hydrogen peroxide (H2O2) under ultraviolet light irradiation.
1 Experimental
1.1 Materials and apparatus
Materials and regents used in this study were sodium tungstate (Na2WO4·2H2O), 40% hydrofluoric acid (HF), 30% hydrogen peroxide (H2O2), 12-phosphotungstic acid (H3PW12O40), tetra-n-butylammonium bromide (TBABr), acetonitrile (CH3CN) , concentrated hydrochloric acid (HCl), methyl orange (MO), all of which were of analytical grade without further purification. Distilled water was used throughout this experiment.
1.2 Characterization of samples
Liquid UV-Vis spectra of the samples in aqueous solution were recorded from 200 to 450 nm on UV-2450 spectrophotometer (Shimadzu, Japan) and their transmission FT-IR spectra were recorded from 400 to 4 000 cm-1on a Nicolet Nexus 510 P FT-IR spectroscopy using a KBr disk. 19F-NMR spectrum of the sample F-H4W10O32 was recorded on the Bruker Avance-500 superconductive Nuclear Magnetic Resonance, and its W and F contents measured on a sequential X-ray fluorescence spectrometer (XRF-1700, Shimadzu, Japan) with H3BO3 as a binder.
1.3 Preparation of catalysts
A preparation procedure of the F-substituted decatungstate acid is described as follows: Na2WO4·2H2O (001 mol) was dissolved with 10 mL H2O to obtain a solution under magnetic stirring. After adding 12 mL of 40% HF, the solution was heated to 70 ℃ and continuously stirred at this constant temperature for a sufficiently long time until it was completely evaporated to dryness. The resulting white solid was dissolved with a small amount of water, then filtered to remove insoluble NaF. Finally the filtrate obtained was evaporated to dryness to yield the product, marked as F-H4W10O32. The W and F content of F-H4W10O32 measured by XRF analysis was 92.75% and 2.16%, respectively, and the formula of as-prepared catalyst should be H4W10O30.75F2.5.endprint
