密度泛函理论方法研究铝-麦芽酚配合物的形态结构与水交换反应
2017-10-13张婧董绍楠侯晓霞毕树平
张婧,董绍楠,侯晓霞,毕树平
南京大学化学化工学院,南京 210023
密度泛函理论方法研究铝-麦芽酚配合物的形态结构与水交换反应
张婧,董绍楠,侯晓霞,毕树平*
南京大学化学化工学院,南京 210023
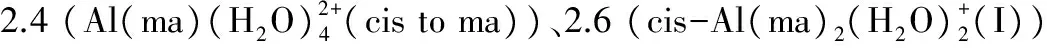
铝-麦芽酚配合物;密度泛函理论;铝形态结构;水交换反应;致毒机制
Received14 January 2017accepted31 May 2017

Keywords: aluminium(III)-maltolate complexes; density functional theory (DFT); the structures and speciation of aluminium; water-exchange reaction; toxicity mechanism
铝具有很强的神经毒性,被认为是导致阿尔兹海默病等神经退行性疾病的重要因素之一,其毒性大小与铝化合物的种类及形态有关[1]。麦芽酚(maltolate,简写为ma)是一种广泛用于食品添加剂的天然产物[2],易与水溶液中Al3+结合生成具有水溶性和脂溶性的电中性配合物[3]。铝-麦芽酚配合物在不同配比、pH等环境因素下具有多种形态,而Al3+的毒性与铝-麦芽酚配合物的赋存形态密切相关。麦芽酚配体增强了Al3+的膜通透性,进而提高了Al3+的生理活性和生物利用度[3]。Al3+进入细胞内作用于神经元、神经胶质细胞和DNA磷酸基团[3]等位点进而造成神经损伤[4]。国内外课题组[4-9]主要采用核磁共振、红外光谱、紫外光谱和质谱等实验方法对铝-麦芽酚体系进行研究,但由于现有实验条件的自身限制、准确解析和归属光谱的难度以及无法准确检测低浓度化合物的现状,使得这些研究难以区分铝-麦芽酚配合物的不同形态结构以及准确判断反应活性位点。量子化学计算方法为解决这一难题提供了新的思路[10]。本文采用密度泛函理论计算方法对铝-麦芽酚体系进行系统研究,主要工作从静态结构、动态水交换反应和不同铝形态与致毒机制的关联探讨3个方面进行:(1) 在超分子-极化连续(GP-SM-PCM)模型下优化铝-麦芽酚配合物静态结构得到键长、键角、NPA电荷和能量等参数,计算得到Al(ma)3配合物4种异构体的核磁共振、紫外和红外等光谱学数据并与文献实验值比较;(2) 模拟1∶1和1∶2铝-麦芽酚配合物各种可能位点的水交换反应,通过计算得到的相关结构-能量参数预测不同构型各位点的水交换速率,并与文献报道的实验值进行对比;(3) 探讨铝-麦芽酚配合物不同形态与转化以及水交换反应速率与Al3+生物有效性的关系和致毒机制。
1 原理与计算方法(Theory and calculation methods)
所有计算在Gaussian 03程序包中运行。所有结构均采用密度泛函理论(DFT)在B3LYP/6-311+G(d,p)基组水平下进行优化及频率计算。原子电荷由自然布居分析(NPA)得到,单点能采用MP2方法在相同基组水平下进行计算[10]。本文中所有构型的优化、频率和单点能计算均在0 K下进行,热力学计算在298.15 K和1 atm下进行。
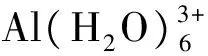

图1 铝-麦芽酚配合物的10种可能静态构型Fig. 1 Ten possible static configurations of the Al-maltolate complexes
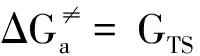

2 结果与讨论(Results and discussion)
2.1 铝-麦芽酚配合物的静态结构
图2为优化得到的1∶1、1∶2和1∶3铝-麦芽酚配合物10种静态构型。以赤道面左下角O原子为起点,逆时针编号1-4,赤道面上方O原子为编号5,下方O原子编号为6。计算所得的键长、键角、NPA电荷和能量等参数见表1,Al(ma)3配合物4种异构体的NMR、UV-vis、IR等数据和谱图分别见表2和3及图3和4。
(1)结构特征。以相同模型方法基组下优化得到的Al(H2O)63+团簇(Nm=12)为本底(Al-OH2键长1.916 Å),铝-麦芽酚1∶1配合物的平均Al-OH2键长(1.923 Å)稍有拉长,其中配体邻位Al-OH2键长为1.947 Å,对位平均键长为1.899 Å,表明麦芽酚配体增加了邻位水分子的不稳定性,同时稳定了对位水分子。1∶2配合物3个cis结构的Al-OH2键长(1.906~1.914 Å)与本底Al-OH2键长相差不大,分析可能原因为配体对其邻位活化与对位钝化效应相互抵消。1∶2配合物2个trans结构的Al-OH2键长(1.946~1.948 Å)和Al(H2O)63+团簇的Al-OH2键长(1.916 Å)相比明显增加,说明配体的加入一定程度上增强了配位水分子反应活性,分析原因为配体的邻位活化效应。由于Al(ma)3具有水溶性[3],故外层添加的6个水分子稳定在第二水化层并且与配位O原子形成氢键,4个构型的平均Al-O键长在1.934~1.939 Å之间,与Al(H2O)63+团簇相比增加了0.02 Å左右。通过分析1∶1、1∶2和1∶3铝-麦芽酚配合物不同形态结构的3个特征键角,发现所有形态均为六配位,其中赤道键角∠O1-Al-O2与∠O1-Al-O3在90°和175°左右,轴向键角∠O5-Al-O6在175°左右,构型为变形八面体。
(2)NPA电荷特征。如表1所示,随着配体数目的增加,铝-麦芽酚配合物的中心Al原子上正电荷逐渐减小(1.938 e→1.932 e→1.919 e),其中trans构型的1∶2配合物比1∶1配合物减少了0.01 e,1∶3构型比1∶1构型减少了0.019 e。分析原因可能为,随着配体的加入体系形成了更大的共轭体系导致电荷进一步分散。
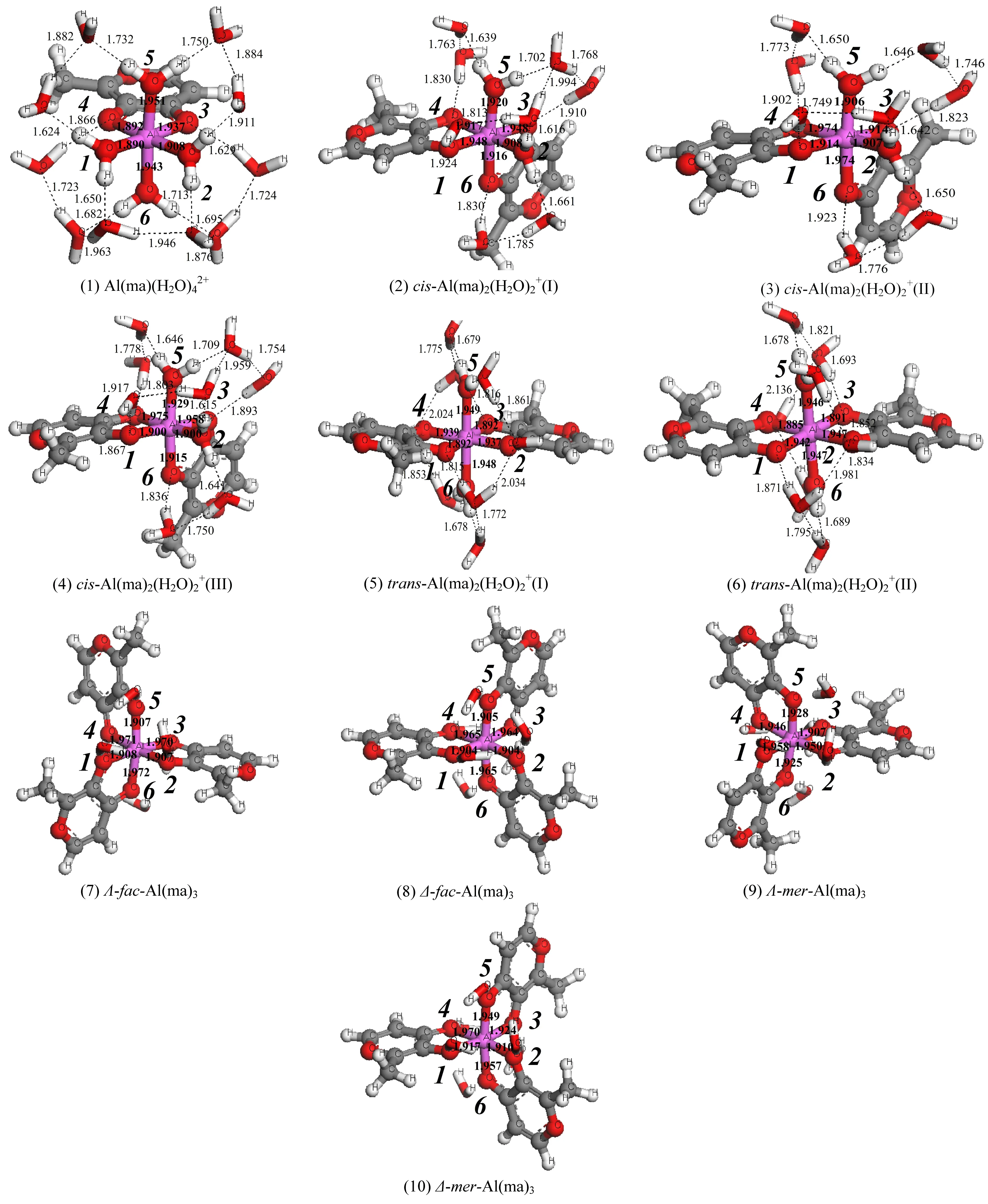
图2 铝-麦芽酚配合物优化得到的10种静态构型Fig. 2 The ten optimized static configurations of Al-maltolate complexes
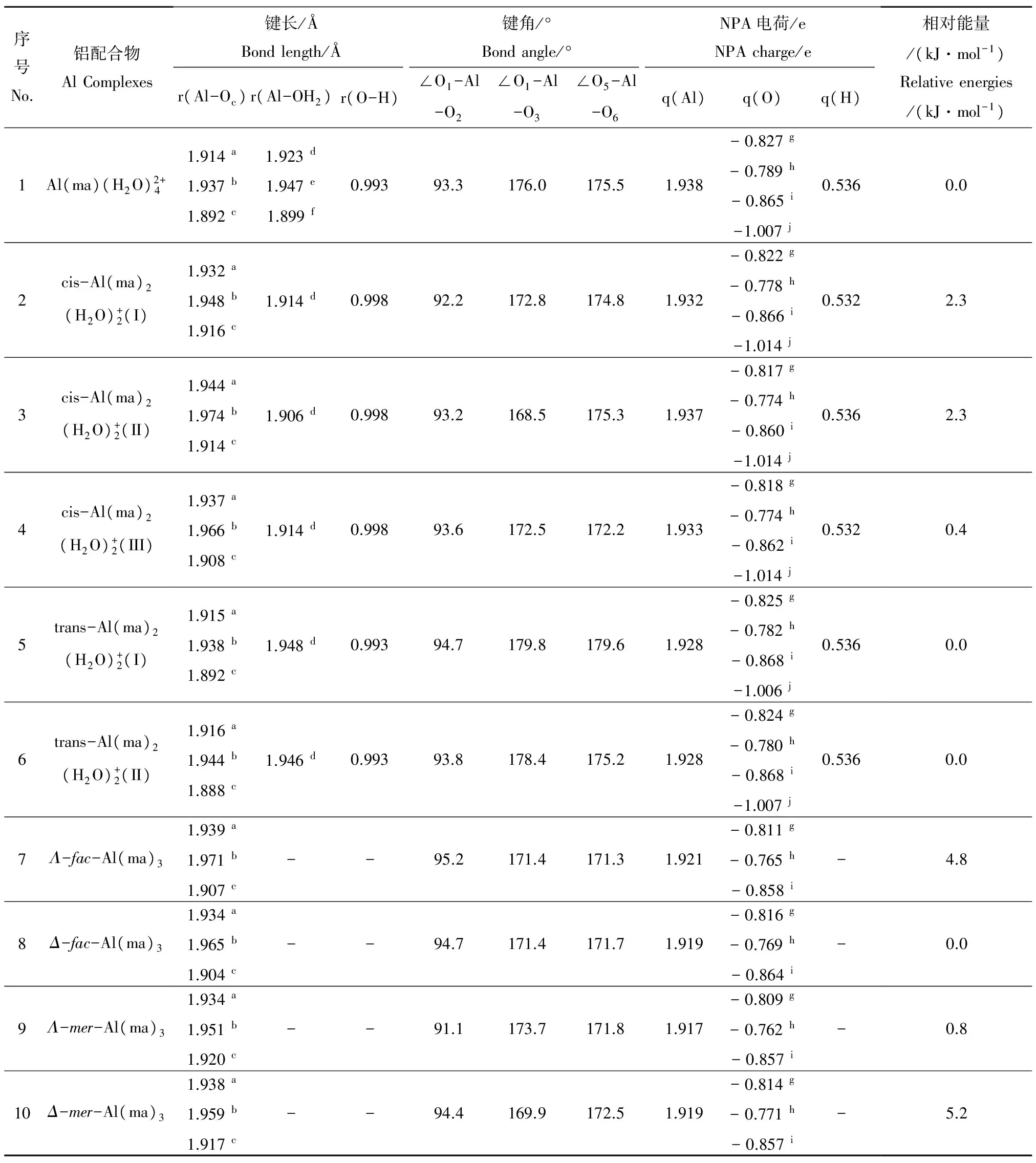
表1 铝-麦芽酚配合物10种静态构型的结构、NPA电荷和能量参数Table 1 Structural parameters, NPA charges and energies for ten static configurations of Al-maltolate complexes
注:∠O1-Al-O2和∠O1-Al-O3为赤道键角;∠O5-Al-O6为轴向键角;a铝与螯合O的平均键长;b铝与配体麦芽酚中4-吡喃酮O的(平均)键长;c铝与麦芽酚中3-羟基O的(平均)键长;dAl-OH2平均键长;eAl-OH2(cis)的平均键长;fAl-OH2(trans)的平均键长;g螯合O的平均NPA电荷;h4-吡喃酮O的(平均)NPA电荷;i3-羟基O的(平均)NPA电荷;j配位H2O中O的平均NPA电荷。
Note: ∠O1-Al-O2and ∠O1-Al-O3are the equatorial bond angles; ∠O5-Al-O6is the axial bond angle;athe average Al-O bond length between Al and chelated O;bthe (average) Al-O bond length between Al and 4-pyronate O;cthe (average) Al-O bond length between Al and 3-oxy O;dthe average Al-OH2bond length;ethe average Al-OH2(cis) bond length;fthe average Al-OH2(trans) bond length;gthe average NPA charge of chelated O;hthe (average) NPA charge of 4-pyronate O;ithe (average) NPA charge of 3-oxy O;jthe average NPA charge of O in H2O.
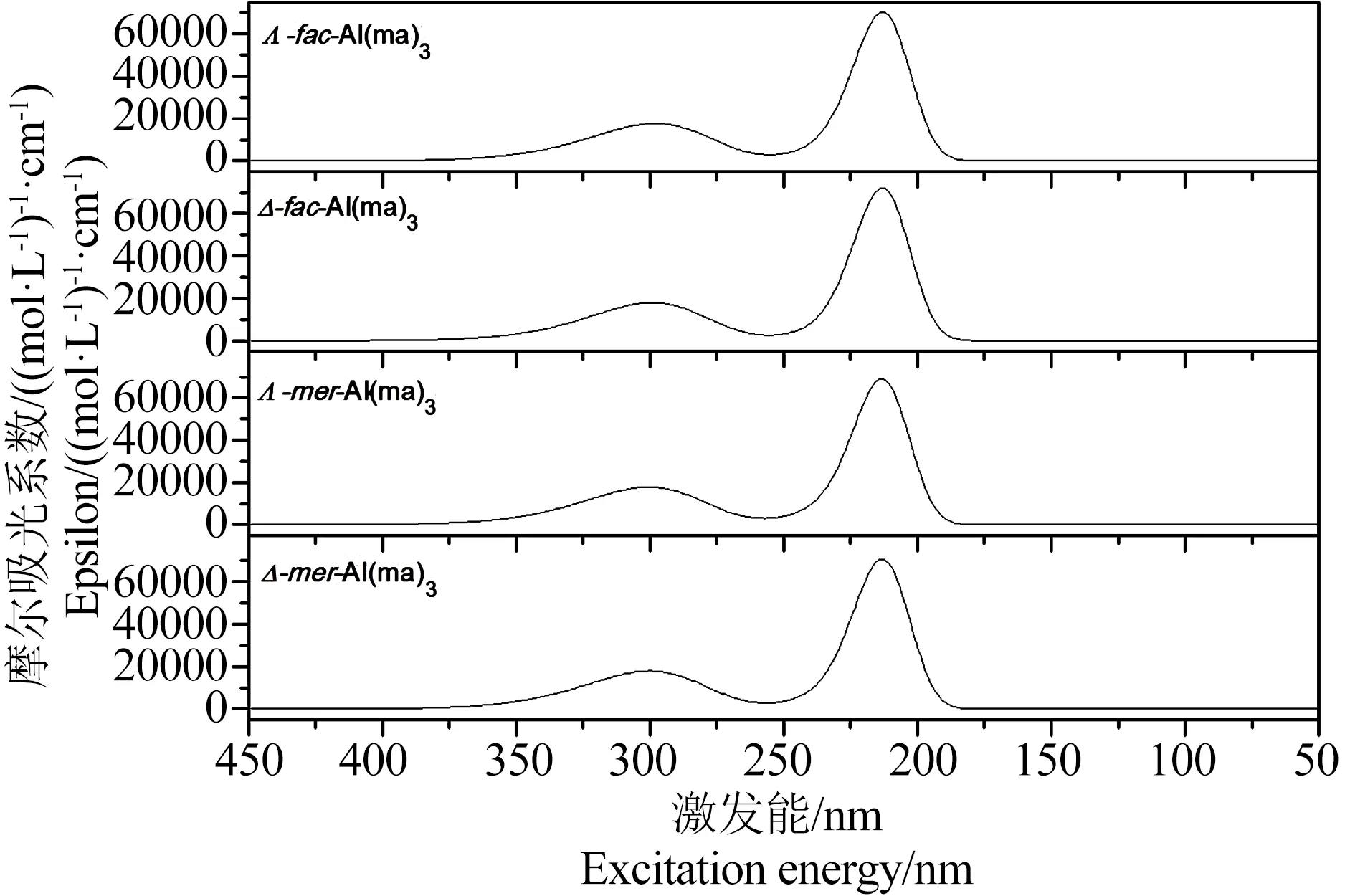
图3 Al(ma)3配合物在水溶液中的紫外吸收光谱图Fig. 3 The UV spectrums of Al(ma)3 complexes in aqueous solution

(4)光谱学特征。由表2中27Al NMR和1H NMR数据可以看出,计算得到的化学位移与实验值基本一致,说明GP-SM-PCM模型适合用于处理铝-麦芽酚配合物体系。如表3以及图3所示,Al(ma)3的4种同分异构体计算得到的紫外最大吸收波长~ 300 nm,与实验值305 nm相符。根据图4所示红外光谱图,选取特征Al-O振动、配体环中C=C振动以及C-H振动进行对比,以Λ-fac-Al(ma)3为例,文献中Al-O最强振动发生在410 cm-1和445 cm-1处,理论计算得到的Al-O振动主要在423~462 cm-1之间,两者基本相符。
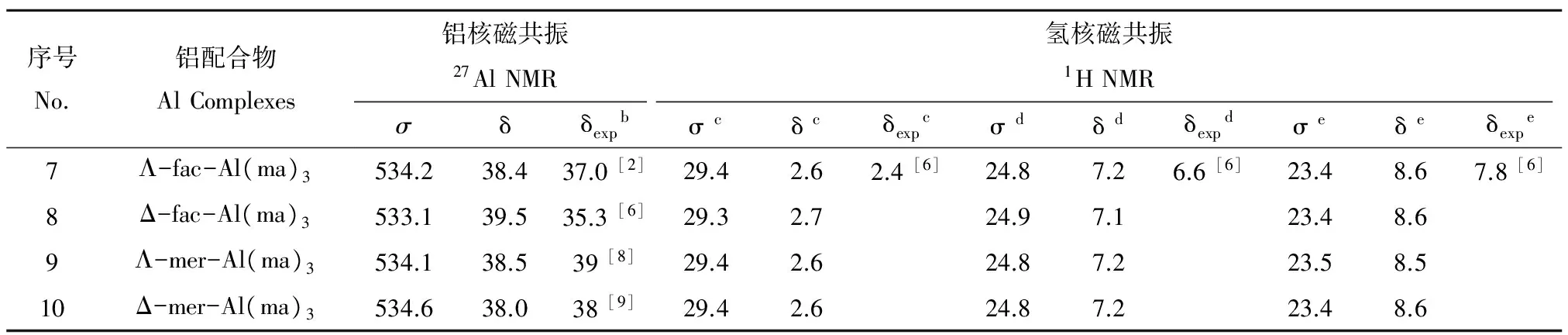
表2 1∶3铝-麦芽酚配合物的27Al NMR和1H NMR计算结果aTable 2 27Al NMR and 1H NMR shielding constants and chemical shifts (ppm) of Al(ma)3 a
注:a 27Al的Al(H2O)63+参比屏蔽常数为572.6 ppm (δ = 0 ppm),1H的四甲基硅烷(TMS)参比屏蔽常数为32.0 ppm(δ = 0 ppm);b实验中得到的27Al NMR数据无法区分4种异构体;c甲基中的H原子;d4-吡喃O相邻C原子上键合的H原子;e3-羰基O对位的C原子上键合的H原子。
Note:aReference shielding constants of Al(H2O)63+and tetramethyl silane (TMS) are 572.6 ppm (δ = 0 ppm) and 32.0 ppm (δ = 0 ppm) respectively;bThe isomers of Al(ma)3cannot be distinguished in the experiments;cH atom in the methyl group;dH atom is bonded to the C atom which is next to 4-pyronate oxygen;eH atom is in the para-position to 3-oxy oxygen.
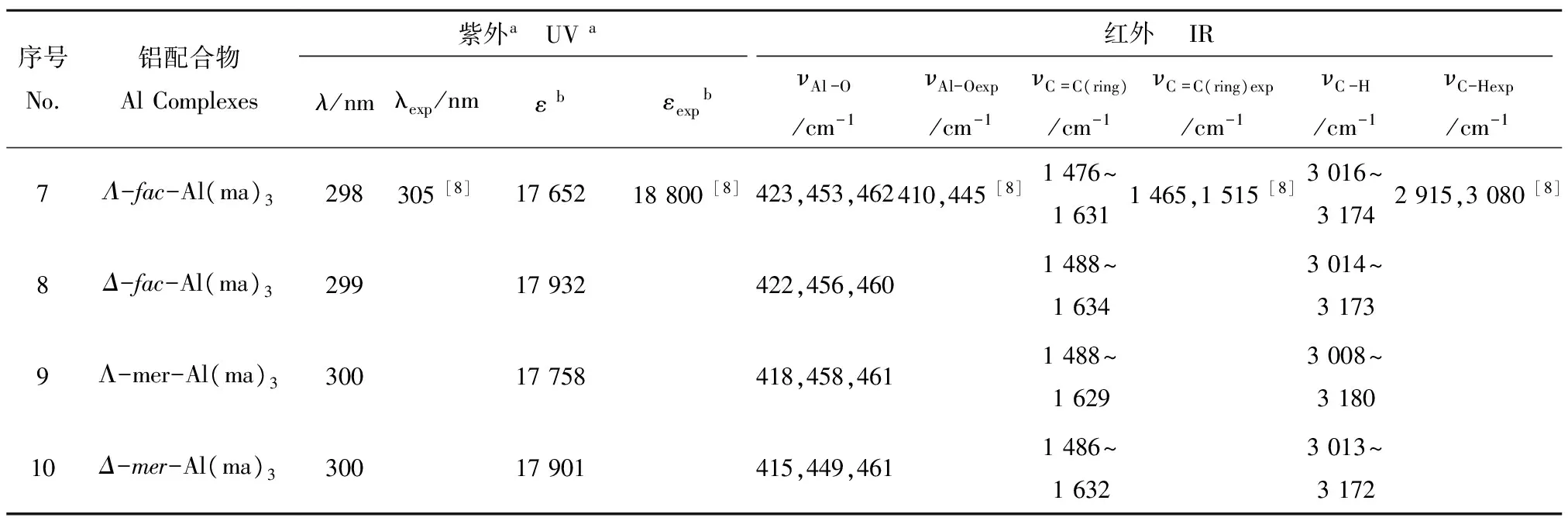
表3 1∶3铝-麦芽酚配合物的紫外和红外计算结果Table 3 The calculated ultraviolet spectral data and infrared absorptions of Al(ma)3
注:a文献[8]中检测紫外光谱时溶剂为饱和正辛醇的水溶液;b吸光度ε的单位为(mol·L-1)-1·cm-1。
Note:aThe solvent is water saturated with n-octanol for UV in reference [8];bthe unit for ε is (mol·L-1)-1·cm-1.
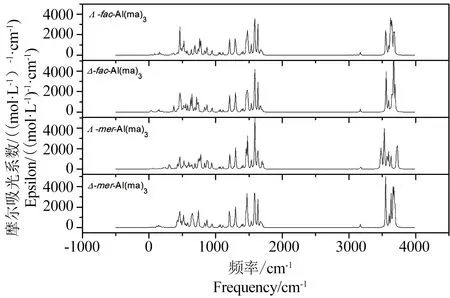
图4 Al(ma)3配合物的红外光谱图Fig. 4 The IR spectrums of Al(ma)3 complexes
2.2 铝-麦芽酚配合物的水交换反应
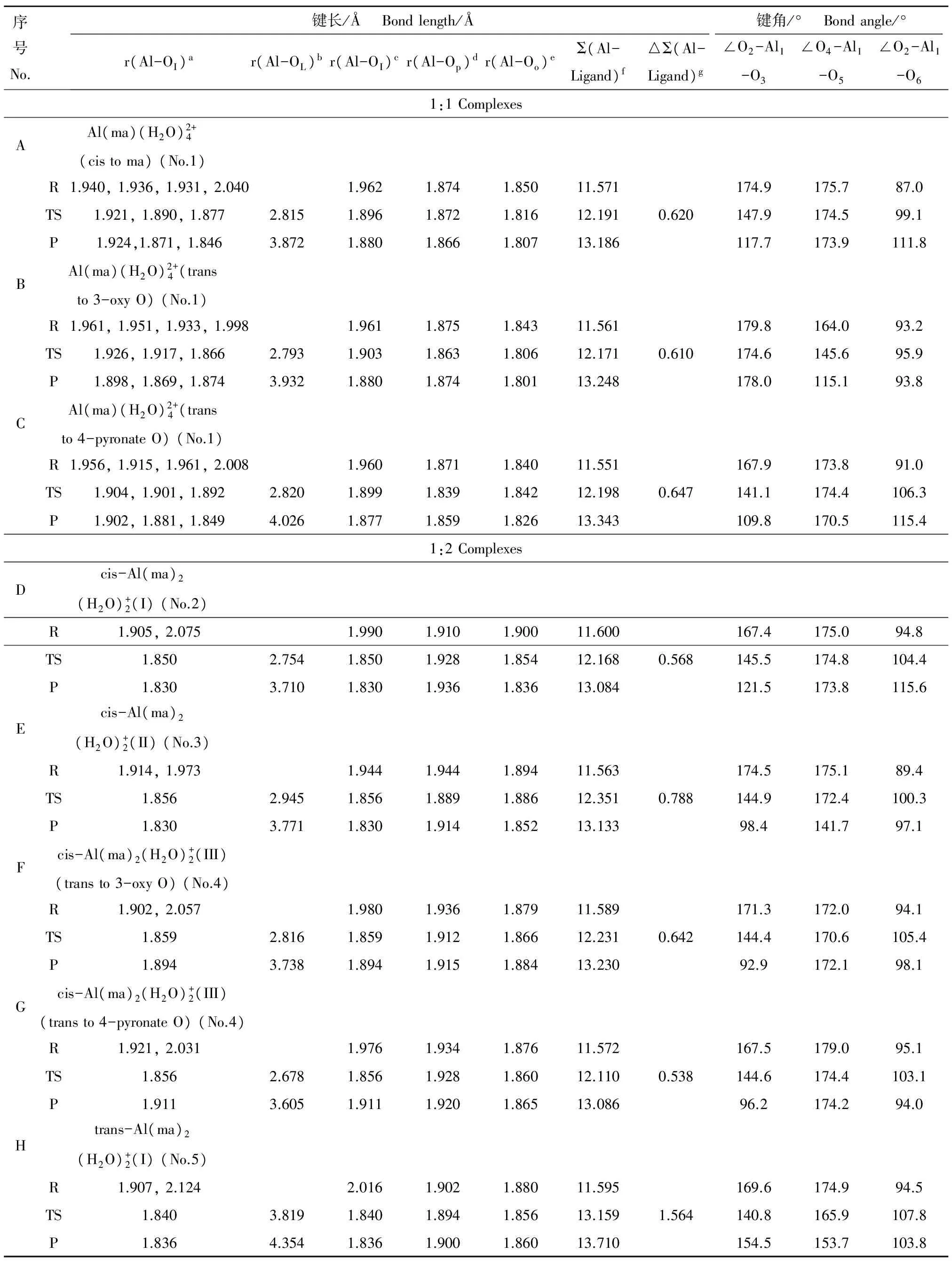
表4 1∶1/1∶2铝-麦芽酚配合物水交换反应的结构参数(Å)Table 4 Structural parameters for the water-exchange reaction of 1:1/1:2 Al-maltolate complexes (Å)
注:aAl与配位水中O原子的键长;bAl与离去水中O原子的键长;cAl与配位水中O原子的平均键长;dAl与配体麦芽酚中4-吡喃酮O (表示为Op)的(平均)键长;eAl与配体麦芽酚中3-羟基O (表示为Oo)的(平均)键长;fAl-O键长总和;g△Σ(Al-Ligand) = Σ(Al-Ligand)transition state- Σ(Al-Ligand)reactant。
Note:aThe Al-O bond length between Al and the O atom of coordinated water molecules;bthe Al-O bond length between Al and the O atom of leaving water molecule;cthe average Al-OH2bond length;dthe (average) Al-O bond length between Al and 4-pyronate O (Op);ethe (average) Al-O bond length between Al and 3-oxy O (Oo);fthe sum of Al-O bond length;g△Σ(Al-Ligand) = Σ(Al-Ligand)transition state- Σ(Al-Ligand)reactant.
以铝-麦芽酚配合物1∶1构型离去水位于配体邻位(cis to ma)的水交换反应为例,反应物中离去水和中心Al原子的距离为2.040 Å,轴向键角∠O4-Al1-O5为175.7°,赤道键角∠O2-Al1-O3与∠O2-Al1-O6分别为174.9°和87.0°,为一具有八面体结构的六配位化合物。随着水交换反应的进行,水分子逐渐离去到达过渡态,此时Al-OL距离为2.815 Å,且该水分子上O原子与另一配位水分子上H原子形成氢键(1.961 Å),虚频(-85.25 cm-1)下离去水分子在第一和第二水化层之间振动,水交换反应过程中轴向键角∠O4-Al1-O5基本不变化(175.7°→174.5°),赤道键角∠O2-Al1-O3明显减小(174.9°→147.9°),此时过渡态构型倾向于三角双锥结构。之后离去水分子进一步远离Al离子进入第二水化层形成五配位产物(Al-OL距离为3.872 Å),此时轴向键角∠O4-Al1-O5变化很小(174.5°→173.9°),赤道键角∠O2-Al1-O3与∠O2-Al1-O6分别变为117.7°和111.8°,产物为具有三角双锥结构的五配位配合物。8条水交换反应路径(A→H)的Δ∑(Al-Ligand)均为正值,说明铝-麦芽酚配合物1∶1/1∶2构型的水交换反应均属于解离活化机制[14]。
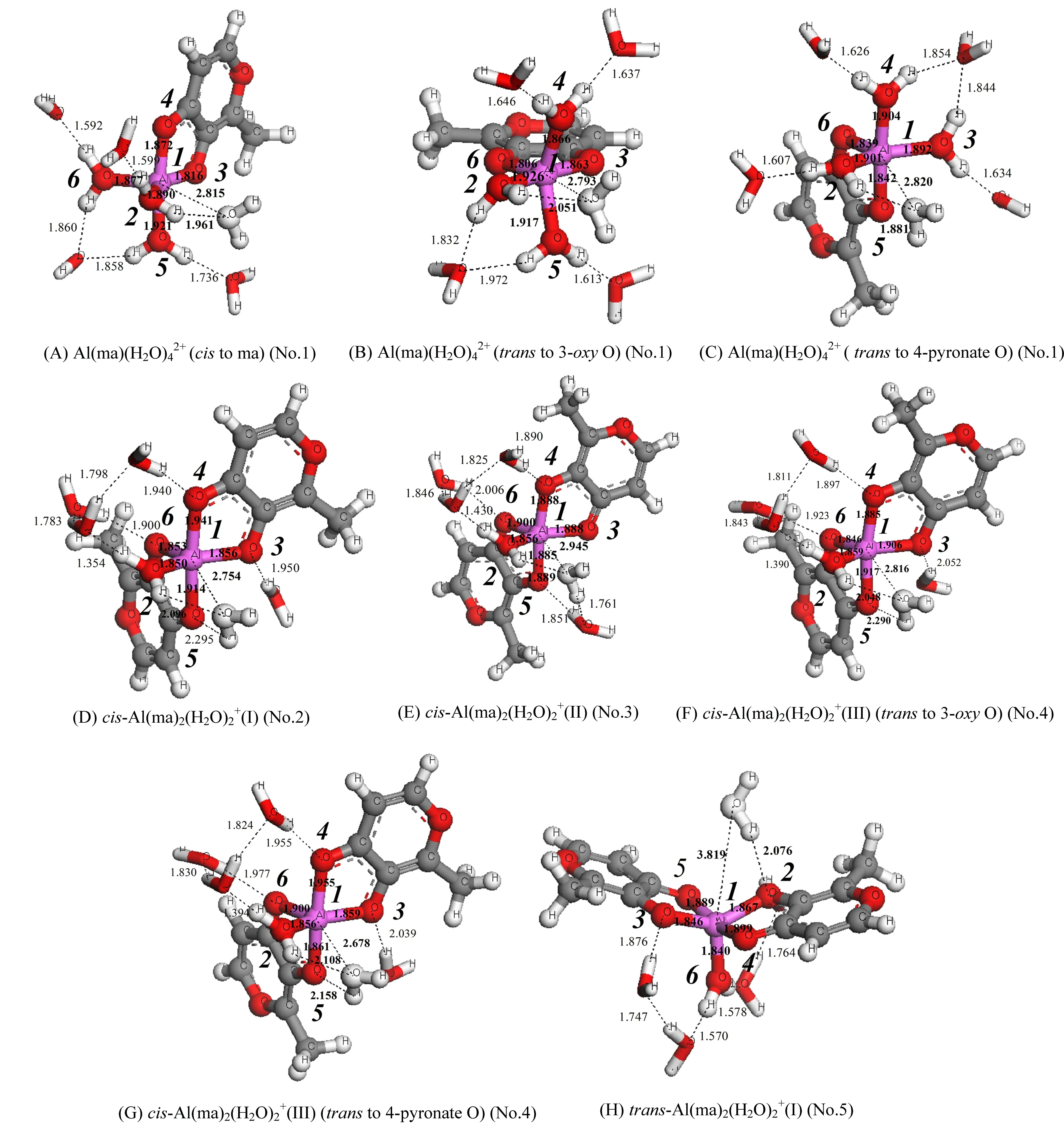
图5 1∶1/1∶2铝-麦芽酚配合物水交换反应过渡态构型图(离去水以白色标记)Fig. 5 The optimized transition state of the water exchange reaction for 1:1 and 1:2 Al-maltolate complexes (The leaving water molecules are white)
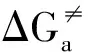
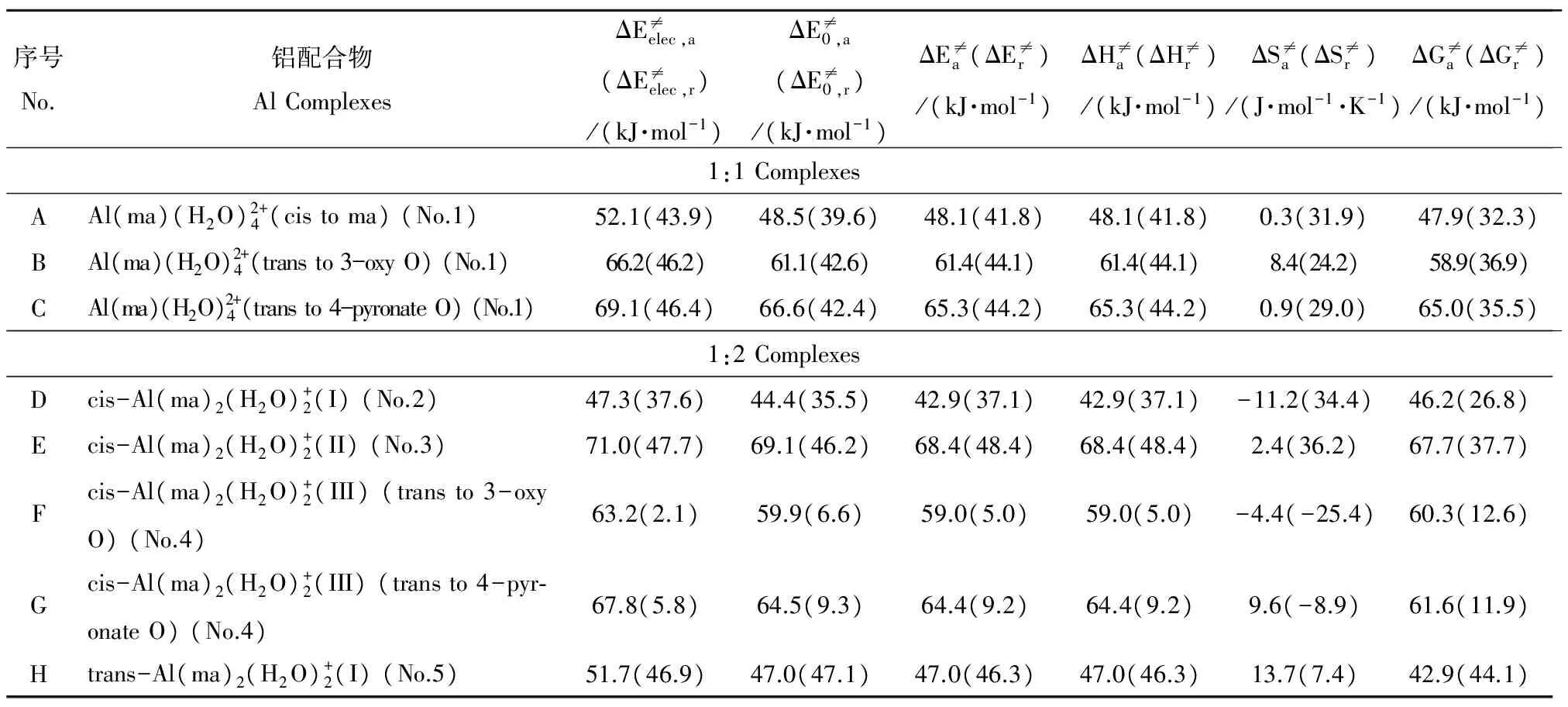
表5 1∶1/1∶2铝-麦芽酚配合物水交换反应的活化和反应能量参数Table 5 The active and reaction energies for the water-exchange reaction of 1:1/1:2 Al-maltolate complexes
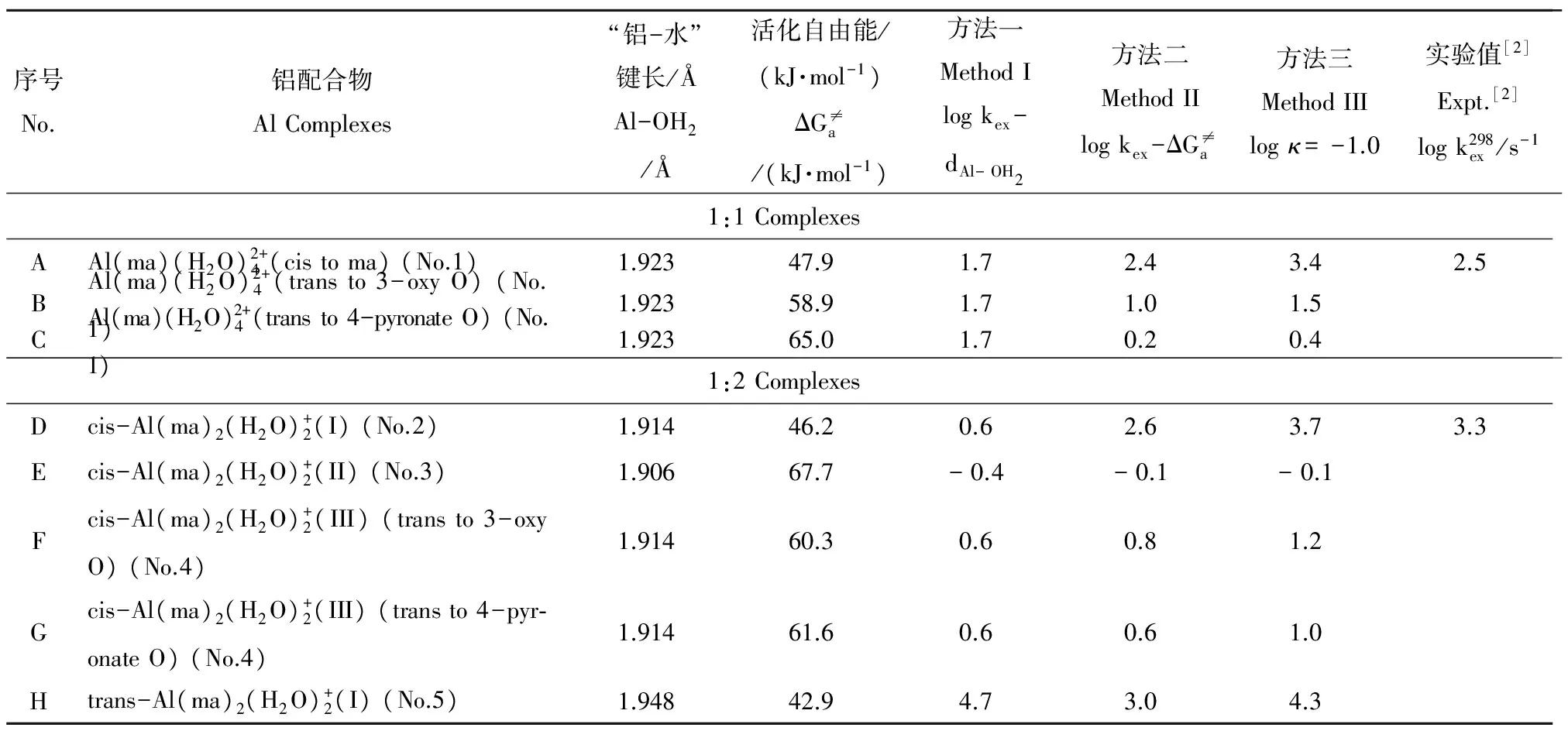
表6 3种方法预测铝-麦芽酚配合物的水交换反应速率logkex (s-1)Table 6 The predicted log kex (s-1) of Al-maltolate complexes by three methods
2.3 铝-麦芽酚配合物毒性与其形态结构之间关系的机理分析
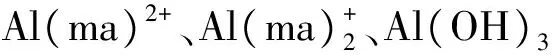
致谢:本文的数值计算在南京大学高性能计算中心的IBM刀片式集群机上完成。
[1] Song B, Sun Q, Li H K, et al. Irreversible denaturation of proteins through aluminum-induced formation of backbone ring structures [J]. Angewandte Chemie International Edition, 2014, 53: 6358-6363
[2] Yu P, Phillips B L, Olmstead M M, et al. Rates of solvent exchange in aqueous aluminium(III)-maltolate complexes [J]. Journal of the Chemical Society, Dalton Transactions, 2002, 43(10): 2119-2125
[3] Liang R F, Li W Q, Wang H, et al. Impact of sub-chronic Al-maltolate exposure on catabolism of amyloid precursor protein in rats [J]. Biomedical and Environmental Sciences, 2013, 26(6): 445-452
[4] Zhou Y Z, Harris W R, Yokel R A. The influence of citrate, maltolate and fluoride on the gastrointestinal absorption of aluminum at a drinking water-relevant concentration: A26Al and14C study [J]. Journal of Inorganic Biochemistry, 2008, 102(4): 798-808
[5] Tapparo A, Soldà L, Bombi G G, et al. Analytical validation of a general protocol for the preparation of dose-controlled solutions in aluminium toxicology [J]. Analyst, 1995, 120: 2425-2429
[6] Zambenedetti P, Tisato F, Corain B, et al. Reactivity of Al(III) with membrane phospholipids: A NMR approach [J]. BioMetals, 1994, 7(3): 244-252
[7] Tapparo A, Perazzolo M. N-octanl/water partition coefficients of the acetylacetonate and maltolate complexes of Al(III), Cr(III) and Fe(III) and of aluminum lactate [J]. International Journal Environmental Analytical Chemistry, 1989, 36(1): 13-16
[8] Finnegan M M, Lutz T G, Nelson W O, et al. Neutral water-soluble post-transition-metal chelate complexes of medical interest: Aluminum and gallium tris (3-hydroxy-4-pyronates) [J]. Inorganic Chemistry, 1987, 26(13): 2171-2176
[9] Finnegan M M, Rettig S J, Orvig C. A neutral water-soluble aluminum complex of neurological interest [J]. Journal of American Chemical Society, 1986, 108(16): 5033-5035
[10] Shi W J, Jin X Y, Dong S N, et al. Theoretical investigation of the thermodynamic structures and kinetic water-exchange reactions of aqueous Al(III)-salicylate complexes [J]. Geochimica et Cosmochimica Acta, 2013, 121(6): 41-53
[11] Bock C W, Markham G D, Katz A K, et al. The arrangement of first- and second-shell water molecules in trivalent aluminum complexes: Results from density functional theory and structural crystallography [J]. Inorganic Chemistry, 2003, 42: 1538-1548
[12] Wang J W, Rustad J R, Casey W H. Calculation of water-exchange rates on aqueous polynuclear clusters and at oxide-water interfaces [J]. Inorganic Chemistry, 2007, 46: 2962-2964
[13] Jin X Y, Yan Y, Shi W J, et al. Density functional theory studies on the structures and water-exchange reactions of aqueous Al(III)-oxalate complexes [J]. Environmental Science & Technology, 2011, 45: 10082-10090
[14] Rotzinger F P. Treatment of substitution and rearrangement mechanisms of transition metal complexes with quantum chemical methods [J]. Chemical Reviews, 2005, 105: 2003-2037
[15] 梁瑞峰, 牛侨. 麦芽酚铝的神经毒性研究[J]. 毒理学杂志, 2011, 25(6): 467-470
Liang R F, Niu Q. Neurotoxicity of aluminum maltolate [J]. Journal of Toxicology, 2011, 25(6): 467-470 (in Chinese)
[16] Liang R F, Li W Q, Wang X H, et al. Aluminium-maltolate-induced impairment of learning, memory and hippocampal long-term potentiation in rants [J]. Industrial Health, 2012, 50: 428-436
[17] Zhu M M, Li B F, Ma X, et al. Folic acid protected neural cells against aluminum-maltolate-induced apoptosis by preventing miR-19 downregulation [J]. Neurochemical Research, 2016, 41: 2110-2118
[18] Pikaar I, Sharma K R, Hu S, et al. Reducing sewer corrosion through integrated urban water management [J]. Science, 2014, 345(6198): 812-814
[19] Rauch W, Kleidorfer M. Replace contamination, not the pipes [J]. Science, 2014, 345(6198): 734-735
[20] Laird B D, Peak D, Siciliano S D. Bioaccessibility of metal cations in soil is linearly related to its water exchange rate constant [J]. Environmental Science & Technology, 2011, 45: 4139-4144
◆
InvestigationoftheConfigurationCharacteristicsandWater-ExchangeReactionsofAluminium(III)-MaltolateComplexesbyDensityFunctionalTheory
Zhang Jing, Dong Shaonan, Hou Xiaoxia, Bi Shuping*
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
10.7524/AJE.1673-5897.20170114005
2017-01-14录用日期2017-05-31
1673-5897(2017)3-261-12
X171.5
A
毕树平(1961-),男,分析化学博士,教授,博士生导师,主要从事环境分析化学和环境表界面电化学研究。
国家自然科学基金(No.21177054)
张婧(1991-),女,硕士研究生,研究方向为环境分析化学,E-mail: april_91@126.com;
*通讯作者(Corresponding author), E-mail: bisp@nju.edu.cn
张婧, 董绍楠, 侯晓霞, 等. 密度泛函理论方法研究铝-麦芽酚配合物的形态结构与水交换反应[J]. 生态毒理学报,2017, 12(3): 261-272
Zhang J, Dong S N, Hou X X, et al. Investigation of the configuration characteristics and water- exchange reactions of aluminium(III)-maltolate complexes by density functional theory [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 261-272 (in Chinese)
